Introduction
Ageratum conyzoides L. commonly known as billy goat weed is an alien weed species native of Central America and Mexico. As a member of Asteraceae family, the plant is herbaceous in habit, found throughout tropic and subtropic regions around the world including India.1 It is widespread across different agroecosystems and natural ecosystems2–4 owing to its wide ecological amplitude and adaptability.5Production of extensive numbers of seeds and its rapid spread to distant places helps in its encroachment to wider areas. A. conyzoides forms dense stands which out-compete the native species in terms of space and resource utilizations, affecting the biomass of native species. The loss of biomass or productivity results in disruption of the local ecosystem in terms of structure and functioning.6-10
Allelopathy is an interference mechanism in which plant release secondary metabolites into the environment that could have either inhibitory or stimulatory effect on the growth of nearby plants.6,11,12 Secondary metabolites such as alkaloids, flavonoids, phenolics, chromenes and essential oils have been identified from A.conyzoides13, some of which are considered as putative allelochemicals.14–16 Allelopathy is regarded as one of the reasons for imparting invasiveness by A. conyzoides.4,17 Water soluble phenolics as putative allelochemicals have been reported in number of studies in significant amount, deleteriously affected the early growth of rice, wheat, 14,18 chickpea19 and pea.20 The leaves were used by Chinese farmers to increase the soil fertility for paddy fields.18 Weed debris is reported to enrich the soil nutrients especially nitrogen.2,3 Enhanced phytotoxic effects are observed when conditions are extremely unfavourable for its growth.15
A.conyzoides is one of the weeds (locally known as “Bhuvanijhad”) that infest rice based (Oryza sativa L.) agroecosystems in Kumaun Himalayas. Rice is the main food crop of India21,22 adversely affected by this weed. In terraced rice cultivation especially in Himalayan belt, seeds are directly sown which develop together with weeds and could compete for space and nutrient resources. In addition, photochemicals released by weeds cause severe reduction in crop yield.
Therefore, the objectives of present study were to assess the phytotoxic effect of fresh and air (shade) dried aqueous extracts of leaves and roots of A. conyzoides against two rice varieties (Sava and Geru) and to assess the allelopathic tolerance potential of selected rice varieties.
Materials and Methods
Preparation of Extract
For preparation of fresh aqueous extract of leaf and root, green leaves and roots of A. conyzoides were collected from field and grounded to fine paste. 20 g paste of each plant part (leaf and root) was soaked in 200 ml distilled water in 1:10 w/v at room temperature for 48 hours. Soaked materials were occasionally shaked and the contents were filtered through Whatman filter paper No.1. The prepared stock solution was considered as 100% concentration and 50% concentration was prepared by diluting with distilled water. A treatment of distilled water was set as the control. For preparation of dry aqueous extract, the collected leaves and roots were air (shade) dried for 10 days and then grinded to fine powder by mortar and pestle. 100% and 50% concentration of each plant part (leaf and root) was prepared from method described for fresh aqueous extracts. The extracts prepared hereafter were named fresh leaf extract (FLE), fresh root extract (FRE), dry leaf extract (DLE) and dry root extract (DRE).
Seed Bioassay
An experiment was conducted in factorial arrangement form using a completely randomized design with three replicates. In this experiment, two varieties of rice viz. Sava and local landrace Geru were collected from local farmers and kept under two levels of extract concentrations treatment (50% and 100% concentration of extract) and distilled water as control. This experiment was carried out in the Department of Botany, DSB Campus, Kumaun University, Nainital (Uttarakhand), India.
Ten healthy, uniform sized seeds of both selected varieties were sterilized, washed with distilled water and were put in Petri dishes (with 9 cm diameter) with moistened filter paper in three replications. The Petri dishes were covered to prevent the loss of moisture by evaporation. Germination test were conducted under condition of 12h light/dark cycle with 14°C minimum and 24°C maximum temperature. Numbers of germinated seeds were recorded for upto 17 days. After 17 days the seedling were harvested. Germination percentage was determined by counting the number of germinated seeds every day divided by total number of tested seeds. Seed vigor index was calculated by:
SVI =

Inhibition percentage was calculated by:
Inhibition % =

Shoot and root length, fresh and dry (oven drying at 60°C) weight were recorded.
Statistical Analysis
The parameters measured were analyzed by Analysis of Variance (ANOVA) and if there any differences in the means were observed it was further analyzed by Duncan’s multiple range tests at level of 95%. Statistical analysis was performed using SPSS version 16.
Results
The statistical analysis showed that aqueous extracts obtained from both leaf and root of A. conyzoides significantly affected germination and seedlings length of rice varieties in laboratory bioassay. For leaf extract differences between varieties were significant only for shoot length while for root extracts, differences between varieties were significant for all observed parameters at p≤0.05 (Table 1).
Table 1: Analysis of Variance (ANOVA) for traits investigated for two varieties of rice in response to leaf and root extract of A. conyzoides.
|
Parameters |
Mean squares |
||||||
|
Df |
SL |
RL |
GP |
FW |
DW |
||
|
Leaf |
Extract |
1 |
23.62** |
25.99** |
16044.44** |
0.00ns |
0.00ns |
|
Varieties |
1 |
12.09* |
5.51ns |
900ns |
0.01** |
0.01** |
|
|
Treatment levels |
2 |
1.71ns |
5.32ns |
2905.55ns |
0.02ns |
0.00ns |
|
|
Root |
Extract |
1 |
10.67** |
14.56** |
1225.00* |
0.01** |
0.01** |
|
Varieties |
1 |
9.96** |
4.22* |
1225.00* |
0.001** |
0.00ns |
|
|
Treatment levels |
2 |
1.27ns |
4.65ns |
72.22ns |
0.00ns |
0.00ns |
|
|
**: Significant at p ≤ 0.01, *: Significant at p ≤ 0.05 and ns: Not significant. SL: Shoot length, RL: Root length, GP: Germination percentage, GR: Germination rate |
|||||||
Effect of Leaves on Seed Germination
FLE induced slight promotion (-5%) at 50% concentration in Sava variety, whereas 50% germination was inhibited at 100% concentration of Geru variety. In FLE the seed vigour index of Sava variety slightly increased (-8.2%) at both treatment levels except Geru variety (Table 1). Compared to control, DLE induced reduction in the germination percent of both rice varieties. The DLE had shown significant difference (p≤0.05) in germination percent of Sava variety. In both varieties germination as well as seed vigour was completely controlled in DLE of 100% concentration (Table 1).
Effect of Leaves on Seedling Growth
50% FLE concentration slightly promoted shoot length (-8.7%) and both shoot (-13.3%) and root length (-3.4%) at 100% concentration in Sava variety (Fig 1a). In Geru variety, both shoot and root length were inhibited with increasing concentrations (Table 2). Compared to control, DLE reduced both shoot and root length in both rice varieties (Fig 2a). The DLE had shown significant difference (p≤0.05) in both shoot and root length of Sava variety. Complete inhibition (100%) in seed growth was observed in both varieties at 100% DLE concentration (Table 2).
Table 2: Effect of A. conyzoides leaves on seed germination, growth and seed vigour index of two rice varieties
|
Variety |
Treatment |
FLE |
DLE |
||||||
|
GP |
SL |
RL |
SVI |
GP |
SL |
RL |
SVI |
||
|
Sava |
Control |
66.67±0.67 |
2.74±0.45 |
2.73±0.59 |
3.65 |
53.33±0.88 |
1.42±0.13 |
1.62±0.18 |
1.62 |
|
50% |
70.00±0 (-5.0) |
2.98±0.18 (-8.7) |
2.65±0.50 (2.7) |
3.95 (-8.2) |
36.67±0.88 (31.3) |
0.31±0.10 (77.8) |
0.1±0.1 (93.8) |
0.15 (90.7) |
|
|
100% |
66.67±0.67 (0.0) |
3.11±0.28 (-13.3) |
2.82±0.45 (-3.4) |
3.95 (-8.2) |
0 (100) |
0 (100) |
0 (100) |
0 (100) |
|
|
Geru |
Control |
73.33±1.45 |
1.37±0.21 |
2.08±0.47 |
2.53 |
43.33±2.33 |
0.408±0.26 |
0.75±0.66 |
0.5 |
|
50% |
66.67±1.20 (9.1) |
0.95±0.23 (30.2) |
1.57±0.39 (24.9) |
1.68 (33.6) |
3.33±0.33 (92.3) |
0.1±0.1 (75.5) |
0 (100) |
0 (100) |
|
|
100% |
36.67±0.33 (50.0) |
0.79±1.73 (42.0) |
0.82±0.27 (60.7) |
0.59 (76.7) |
0 (100.0) |
0 (100) |
0 (100) |
0 (100) |
|
|
FW=Fresh weight (g), DW=Dry weight (g), FLE= Fresh leaf extract, DLE= Dry leaf extract, FRE=Fresh root extract, DRE= Dry root extract GP= Germination percentage, SL= Shoot length, RL= Root length, SVI= Seed vigor index. The values in the parenthesis are inhibition percent. |
|||||||||
 |
Figure 1(a,b): Regression analysis of the allelopathic effect of fresh extract of Ageratum conyzoides L. |
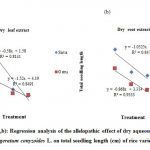 |
Figure 2(a,b): Regression analysis of the allelopathic effect of dry aqueous extracts of Ageratum conyzoides L. |
Effect of Roots on Seed Germination
FRE reduced germination percent and seed vigour index at both concentrations in Sava variety whereas in Geru variety, 100% concentration showed slight promotion in germination percent (-16.7%) and seed vigour index (-4.9%) as compared to control (Table 3). In Sava variety DRE showed slight inhibition in germination percent at both treatments. However, in Geru variety germination was slightly promoted at 50% and 100% concentration. Reduction in seed vigour index was observed for both varieties at both DRE concentrations (Table 3).
Table 3: Effect of A. conyzoides root on seed germination, growth and seed vigour index of two rice varieties
|
Variety |
Treatment |
FRE |
DRE |
||||||
|
GP |
SL |
RL |
SVI |
GP |
SL |
RL |
SVI |
||
|
Sava |
Control |
76.67±0.33 |
2.75±0.08 |
2.72±0.10 |
4.20 |
63.33±0.88 |
1.87±0.26 |
2.58±0.20 |
2.62 |
|
50% |
63.33±0.88 (17.4) |
3.28±0.25 (-19.1) |
3.56±0.12 (-30.6) |
4.33 (-3.1) |
63.33±0.88 (0.0) |
1.24±0.08 (34.3) |
1.08±0.07 (52.0) |
1.47 (43.9) |
|
|
100% |
60±0.57 (21.7) |
2.22±0.45 (19.4) |
2.49±0.58 (8.8) |
2.82 (32.8) |
60±1.53 (5.3) |
0.94±0.09 (50.1) |
0.88±0.17 (60.9) |
1.09 (58.4) |
|
|
Geru |
Control |
60±0.57 |
1.50±0.24 |
2.53±0.37 |
2.42 |
40±0.58 |
0.84±0.23 |
1.74±0.23 |
1.03 |
|
50% |
56.67±0.88 (5.56) |
1.22±0.33 (19.0) |
1.28±0.18 (49.5) |
1.42 (41.32) |
43.33±0.33 (-8.3) |
0.45±0.14 (46.4) |
0.85±0.35 (50.8) |
0.50 (51.5) |
|
|
100% |
70± 0 (-16.7) |
1.45±0.31 (3.5) |
2.17±0.40 (14.2) |
2.54 (-4.9) |
46.67±0.33 (-16.7) |
0.53±0.10 (36.5) |
0.30±0.01 (82.3) |
0.40 (61.2) |
|
|
FW=Fresh weight (g), DW=Dry weight(g), FLE= Fresh leaf extract, DLE= Dry leaf extract, FRE=Fresh root extract, DRE= Dry root extract GP= Germination percentage, SL= Shoot length (cm), RL= Root length (cm), SVI= Seed vigor index. The values in the parenthesis are inhibition percent. |
|||||||||
Effect of Roots on Seedling Growth
Both shoot (-19.1%) and root length (-30.6%) of Sava variety were promoted at 50% FRE and significant reduction in root length was observed in Geru variety as compared to control. DRE significantly reduced both shoot and root length in Sava variety (Fig. 2b) and root length in Geru variety (Table 3).
Effect of Extracts on Fresh and Dry Weight
The fresh and dry weight of Sava and Geru variety in FLE showed no significant difference with control. DLE significantly reduced fresh and dry weight at both concentrations (Table 4). FRE induced no significant change in the biomass of Sava variety, whereas in Geru variety significant increase in dry weight at 100% concentration was observed. In DRE, both varieties showed no significant changes in fresh weight at both concentrations however, dry weight of Sava variety was promoted (-39%) at 100% concentration (Table 4).
Table 4: Effect of A. conyzoides leaves and roots on fresh and dry weight of two rice varieties
|
Variety |
Treatment |
FLE |
DLE |
FRE |
DRE |
||||
|
FW (g) |
DW(g) |
FW(g) |
DW(g) |
FW(g) |
DW(g) |
FW(g) |
DW(g) |
||
|
Sava |
Control |
0.053±0.001 |
0.018±0.001 |
0.046±0.034 |
0.021±0.00 |
0.053±0.002 |
0.018±0.0007 |
0.047±0.00 |
0.018±0 |
|
50% |
0.054±0.002 (-1.9) |
0.017±0.0004 (5.6) |
0.038±0.002 (17.4) |
0.024±0.00 (56.8) |
0.059±0.003 (-11.3) |
0.019±0.0005 (-5.6) |
0.039±0.00 (17.0) |
0.019±0 (-5.6) |
|
|
100% |
0.055±0.002 (-3.8) |
0.019±0.0005 (-5.6) |
0 (100) |
0 (100) |
0.049±0.005 (7.5) |
0.019±0.0003 (-5.6) |
0.047±0.00 (0.0) |
0.025±0 (-39) |
|
|
Geru |
Control |
0.066±0.005 |
0.029±0.0083 |
0.037±0.019 |
0.022±0.01 |
0.069±0.003 |
0.028±0.0008 |
0.056±0.01 |
0.036±0 |
|
50% |
0.059±0.002 (10.6) |
0.028±0.0005 (3.5) |
0.016±0.016 (-14.3) |
0.010±0. (54.5) |
0.058±0.002 (15.9) |
0.027±0.0002 (3.6) |
0.055±0.00 (1.8) |
0.033±0 (8.3) |
|
|
100% |
0.056±0.001 (15.2) |
0.029±0.0002 (0.0) |
0 (100) |
0 (100) |
0.064±0.003 (7.2) |
0.031±0.001 (-10.7) |
0.052±0.01 (7.1) |
0.037±0 (-2.8) |
|
|
FW=Fresh weight (g), DW=Dry weight (g), FLE= Fresh leaf extract, DLE= Dry leaf extract, FRE=Fresh root extract, DRE= Dry root extract. The values in the parenthesis are inhibition percent. |
|||||||||
Discussion
Invasive plants are reported to outcompete plants growing in its vicinity by releasing inhibitory phytochemicals into the environment.23-25 In number of studies, water soluble phenolics having potential phytotoxic activity, are widely implicated in allelopathic studies.2,16,26 Batish et al.2 reported the presence of phenolics such as gallic, coaumalic, protocatechuic, catechin and p-hydroxybenzoic acid from the leaf debris and the presence of ferulic acid as main phenolic compound from root exudates and root residues.14,27 In our study, the inhibition observed could be due to presence of phenolics.
In the present study, dry leaf extract (DLE) negatively affected germination parameters of both rice varieties resembles with the previous studies.2,16 The reduction was observed concentration dependent. Phenolic compounds are responsible for affecting the cell membrane permeability of the recipient plant which affects its nutrient uptake capacity, physiology, alter enzymatic activity and cell division pattern ultimately leading to reduce growth and development.28
Fresh leaf and root extract (FLE and FRE) and dry root exert (DRE) exerted some positive effects at lower or both concentration levels in some germination parameters of rice varieties. The stimulatory effect observed could be due to growth promoting substance in tissues.29 The leaf extracts showed much inhibition than the root extracts, this could be due to high amount of phenolics reported in leaf than any other parts,16 even in the leaf debris and debris amended soil2. Dry extracts were more phytotoxic than fresh extract and showed similarity with earlier findings of.28,30 Overall the Sava variety was more tolerant to the allelopathic effect than local land race Geru variety.
Conclusion
The effects of A. conyzoides fresh aqueous extracts were positive in certain cases which might have practical use to increase agricultural yield and can be potential green manure. The dry aqueous extracts reduced germination parameters with increasing concentration. Based on the present study, the differences observed in laboratory are insufficient to establish allelopathic effect of extracts and treatments on both rice varieties needs further evaluation in the field conditions and should be further tested in a natural environment.
Acknowledgement
We are thankful to the Head, Department of Botany, DSB Campus for providing necessary lab facilities. We also thank all the three reviewers for their critical look in to the article.
Conflict of Interest
There is no known conflict of interest related to this study.
References
- Okunade AL. Ageratum conyzoides L. (Asteraceae). Fitoterapia.73 (1):1–16 (2002).
- Batish DR, Kaur S, Singh HP, Kohli RK. Nature of interference potential of leaf debris of Ageratum conyzoides. Plant Growth Regulators. 57(2):137 (2009a).
- Batish DR, Kaur S, Singh HP, Kohli RK. Role of root-mediated interactions in phytotoxic interference of Ageratum conyzoides with rice (Oryza sativa). Flora. 204 (5):388–395 (2009b).
- Kohli RK, Batish DR, Singh HP, Dogra KS. Status, invasiveness and environmental threats of three tropical American invasive weeds (Parthenium hysterophorus L., Ageratum conyzoides L., Lantana camara L.) in India. Biological Invasions. 8(7):1501–1510 (2006).
- Bargali SS, Bargali K. Diversity and biomass of the under story vegetation in an age series of Eucalyptus tereticornis plantation. International Journal of Ecology and Environmental Sciences. 26: 173-181(2000).
- Kaur S, Batish DR, Kohli RK. Ageratum conyzoides: an Alien Invasive weed in India. Invasive Alien Plants.1:57 (2012).
- Bargali, SS, Singh SP, Singh RP. Structure and function of an age series of eucalypt plantations in Central Himalaya, I. Dry matter dynamics. Annals of Botany 69: 405-411 (1992a).
- Bargali, SS, Singh RP ,Singh SP. Structure and function of an age series of eucalypt plantations in Central Himalaya, II. Nutrient dynamics. Annals of Botany 69: 413-421(1992b).
- Bargali SS, Singh RP. Pinus patula plantations in Kumaun Himalaya. I. Dry matter dynamics. Journal of Tropical Forest Science 9(4): 526-535 (1997a).
- Bargali SS, RP Singh. Pinus patula plantations in Kumaun Himalaya II.Nutrient dynamics. Journal of Tropical Forest Science 10(1): 101-104 (1997b).
- Mushtaq W, Mehdizadeh M, Siddiqui MB, Ozturk M, Jabran K, Altay V. Phytotoxicity of above – ground weed residue against some crops and weeds. Pakistan Journal of Botany. 52(3). https://doi.org/10.30848/PJB2020-3(40) (2020).
- Khatri K, Bargali K, Negi B, Bargali SS. Germination and early seedling growth of two rice varieties as affected by invasive Ageratina adenophora. Current Agriculture Research Journal (2020) (Accepted).
- Sharma PD, Sharma OP. Natural products chemistry and biological properties of the Ageratum plant. Toxicology and Environmental Chemistry 50(1-4):213–232 (1995).
- Batish DR. Tropical American Invasive Weeds in the Shiwalik Range of North Western Himalayas of India: An Assessment of Status and Impact. Available online at http//www. apafri.org/forestHealth08/DAY2_Forest%20Health°/020Workshop/Daizy_APAFRI_DAIZY_ Malaysia-1.pdf (accessed 18 October 2009) ( 2008).
- Kong C, Hu F, Xu X, Liang W, Zhang C. Allelopathic Plants. Ageratum conyzoides L. Allelopathy Journal. 14(1):1–12 (2004).
- Xuan TD, Shinkichi T, Hong NH, Khanh TD, Min CI. Assessment of phytotoxic action of Ageratum conyzoides L.(billy goat weed) on weeds. Crop Protection. 23(10):915–922 (2004).
- Bais HP, Park S-W, Weir TL, Callaway RM, Vivanco JM. How plants communicate using the underground information superhighway. Trends inPlant Sciences.9(1):26–32 (2004).
- Singh HP, Batish DR, Kaur S, Kohli RK. Phytotoxic interference of Ageratumconyzoides with wheat (Triticumaestivum). Journal of Agronomy and Crop Sciences. 189(5):341–346 (2003).
- Batish DR, Singh HP, Kaur S, Kohli RK. Phytotoxicity of Ageratum conyzoides residues towards growth and nodulation of Cicer arietinum. Agriculture, Ecosystem and Environment. 113(1-4):399–401 (2006a).
- Kumar N, Kumar K, Asma, Kumar A. Allelopathic potential of Ageratum conyzoidesL. on growth and development of Pisumsativum L.”. International Journal of Current Research. 10(07): 71659-71663 (2018).
- Vibhuti, Shahi C, Bargali K, Bargali SS. Seed germination and seedling growth parameters of rice (Oryza sativa L.) varieties as affected by salt and water stress. Indian Journal of Agricultural Sciences.85(1): 102-108(2015).
- Bargali SS, Bargali K, Singh L, Ghosh L, Lakhera ML. Acacia nilotica based traditional agroforestry system: effect on paddy crop and management. Current Science. 96(4): 581-587 (2009).
- Bargali SS, Singh RP, Joshi Mukesh. Changes in soil characteristics in eucalypt plantations replacing natural broad leaved forests. Journal of Vegetation Science. 4: 25-28(1993).
- Qasem JR, Foy CL. Weed allelopathy, its ecological impacts and future prospects: a review. Journal of Crop Production. 4(2):43–119 (2001).
- Mushtaq W, Shakeel A, Mehdizadeh M, Hakeem KR. Impact of Plant Invasions on Local Vegetation: An Indian Perspective. Biosciences, Biotechnology Research Asia. 16(4): 763-771 (2019). http://dx.doi.org/10.13005/bbra/2792
- TukeyJr HB. The leaching of substances from plants. Annual Reviewof Plant Physiology. 21(1):305–324 (1970).
- Mehdizade M, Mushtaq W. Biological Control of Weeds by Allelopathic Compounds From Different Plants: A BioHerbicide Approach. In: Egbuna C, Sawicka B. Natural Remedies for Pest, Disease and Weed Control. Academic Press. 107-117 (2019). https://doi.org/10.1016/B978-0-12-819304-4.00009-9
- Rice EL. Allelopathy 2nd Edition Academic Press. N Y. (1984).
- Li Z-H, Wang Q, Rua X, Cunde P, Jiang D-A. Phenolics and Plant Allelopathy.Mol Basel Switz. 15:8933-8952 (2010). doi:10.3390/molecules15128933
- Muhammad Z, Majeed, A. Allelopathic effects of aqueous extracts of sunflower on wheat (Triticumaestivum L.) and maize (Zea mays L.). Pakistan Journal of Botany. 46(5):1715–1718 (2014).

