Introduction
The Argane tree [Argania spinosa (L.) Skeels] is an essential species in Southwestern Morocco; it contributes to the preservation of the forest’s ecosystem by promoting the existence of a floristic and faunistic biodiversity1 and plays a considerable socio-economic role through the production of Argane oil. Significant efforts combining chemical, agronomic, and human sciences have led to the international recognition and marketing of this oil.2 A. spinosa is the only representative of the tropical family of Sapotaceae in Morocco. The climate in its natural distribution area is of arid Mediterranean type, where the rainfall is distributed unequally over the year.3 According to McGregor et al.,4 the Argane forests are part of a transitional zonation: Mediterranean-Saharan.4 The Argane tree grows naturally and abundantly in this transitional region in Southwestern Morocco, which is characterized by an arid and semi-arid climate. It displays a wide morphological diversity, which is evident even within the same locality and under similar eco-geographical conditions. This diversity provides a broad genetic basis for domestication and breeding programs.5-10
Since December 1998, the Moroccan Argane forests have been part of the worldwide network of biosphere reserves supported by UNESCO. The forests formed by Argane tree are the most extensive forests in Morocco after holm oak and red cedar. The main area of Argane forest, which create vast natural regions also known as “Arganeraie”, is estimated at 900 000 hectares.11 It originally covered a much larger area by extending over coastal, inland, and mountainous regions. The main area of A. spinosa forests distribution is limited to Southwestern Morocco, in the region of Agadir city, north of Oued Draa and south of Oued Tensift1 between 29° and 32° north. The argane forest offers multiple functions (creation of a favorable microclimate for many fauna and flora, soil erosion protection, climate change mitigation, desertification control, etc.) and uses for local populations whose socio-economic activities are strongly linked to the various products that provides (oil, soap, shampoo, cosmetic creams, livestock feed, etc.).2,12,13 The Argane tree is a “multipurpose” tree; each part or production of the tree (wood, leaves, fruits, oil) is usable and is a source of income or food for the user.14 The Argane tree adapts to all kinds of soils, presenting a wide range of soils in Western Morocco, except mobile sand. Thus, this tree can be found on poor and shallow soils due to its roots, acting as a support for its development.14
The roots are the underground parts of the plant. They generally move downward in a positive geotropism and thus responding to gravity. The main functions of the roots are the anchoring of the plant in the soil, the absorption, and then the conduction of water and mineral nutrients. They must transport water and mineral nutrients to the stems and leaves, but also import the organic molecules from stems and leaves through the phloem and xylem. Besides absorption and conduction functions, the roots produce hormones and other substances that regulate the development and structuring of the plant. Early in evolution, plants have acquired the ability to associate with soil fungi symbiotically.15 Despite the few studies on Argane root system, the results found showed important adaptive mechanisms to some abiotic stress, especially drought stress16-18 and an excellent ability to establish a mycorrhizal symbiosis.19-25 This review highlights various morphological, physiological and biochemical aspects relating to the functioning of the Argane root system in the face of different biotic and abiotic factors.
Roots of Argane Tree
A. spinosa is a long-lived tree species.1 This woody species is characterized by an original growth pattern that allows the elongation of stems and roots and the replacement of damaged or dead tissue. Like many perennial species, this tree also has a secondary growth, thus having a secondary meristem. Primary extension allows the Argane tree to explore, through its roots, the immediate surroundings to get what they need. These roots can also absorb water and mineral elements from new soil layers, deep and lateral, still rich in resources.26 While the shallow roots are generally dedicated to nutritional inputs, the deep ones allow hydration and enhance the stability of sloping soils. Like some plant species, A. spinosa possesses a dimorphic root system that seems related to flexible water uptake pattern.27,28 In adult Argane trees, the root system is gradually and slightly being substituted by the lateral root system through to trap water in the surface soil. The presence of these both roots, those that continue the proliferation in-depth (taproots) and roots that develop horizontally, allows for nutrient uptake near the surface and water uptake from deep soil layers when the surface dries.18 This species plays an essential ecological role in its territory, threatening by some biophysical processes as the advance of desertification.29,30 Also, it protects against rain erosion especially in mountainous regions. Due to its dimorphic root system, Argane tree is an effective stabilizer of soils in the mountains. In addition, the hydraulic redistribution in soil mediated by its root system (especially deep roots) contributes to lift or redistribute water from layers of wetter soil to layers of dryer soil, which allows the maintenance of soil moisture.1,26 These characteristics make it an interesting tree for developing arid zones. The soil remains relatively moist under the tree, and microbial activities are more important, especially regarding nitrogen mineralization and phosphorus availability.22,31 From the point-of-view of morphology, the Argane tree is characterized by magniloid roots, devoid of absorbing root hairs. The latter is not essential for symbiosis because of the roots of Argane tree associated with arbuscular mycorrhizae.32 Many mycelial hyphae, visible only under the microscope, emerge from the root and explore a considerable volume around this magniloid roots.24
Impact of Changing the Climate on Argane Tree
The phenological shifts in plant communities are one of the most sensitive indicators of global warming, which can have multiple impacts on ecosystem processes.33,34 The Intergovernmental Panel on Climate Change (IPCC) concluded that plant phenology is the easiest way to track the ecological effects of climate change.35 Since the roots are an important part of plant biomass and phenology roots may not respond to warming in the same way as the shoots, this constitutes a significant scientific knowledge gap on the impact of climate change on phenology and performance of plants. But root phenology, as a function of depth, can be influenced by environmental factors, such as humidity and temperature,36-38 and limiting water resources are the main risk factors responsible for tree loss and forest dieback.33 In Morocco, the scarcity of water resources is the main factor limiting productivity, particularly in the arid and semi-arid regions, which represent over two-thirds of the country.3 The main area of distribution of Argane forests is characterized by low and variable rainfall and frequent droughts. The prolonged drought has had adverse ecological consequences, including loss of vegetation cover in some areas, removal of shrubs, and lowering of groundwater. Monitoring the evolution of forest ecosystems through many parameters (growth, phenology, nutrition and health of the forest trees …), while considering the environmental variations in one hand and the other hand management of natural resources in the face of climate change require the search for indices and evaluation methods.39 According to an integrated conceptual model of degradation in the Argane woodlands, le Polain de Waroux and Lambin29 found that the Argane tree density decreased by 44.5% in the Awluz region between 1970 and 2007, as part of a long decline since the 18th century.4 The increasing aridity due to climate variability or change was the primary cause of this loss of Argane forest.29,30 Besides the effect of climate change, Zhao et al.,40 demonstrated that the human impact on Argane woodlands increases, leading to a sparse vegetation cover and increased erosion.
Effect of Drought Stress on the Roots of Argane Tree
The Argane tree is a species adapted to arid and semi-arid climatic regimes in Southwestern Morocco due to its deep root system compared to species adapted to mesic climatic conditions.26,41 Generally, tree species adapted to arid climatic conditions are characterized by a higher root/shoot ratio. In a study of 62 tropical tree species, dry forest seedlings have been shown to have underground biomass and deeper roots than seedlings from moist forests.42 Under dry conditions, adapted tree species invest more and sustainably in the root organ biomass, thus optimizing water absorption while minimizing water loss through transpiration. The Argane tree has developed effective physiological strategies to adapt to drought conditions via mechanisms related to water status and its regulation. It limits water loss through stomatal closure, increased leaf water potential and solute accumulation.43-47 But to reduce consumption and enhance water absorption, trees respond to drought stress by contributing to increase root-to-shoot ratio and rooting depth15,48,49 further. The change in dry root / shoot biomass ratio is one of mechanisms involved in drought avoidance.15 Until today the root system has been the subject of few studies (Table 1). Given the high genetic variability of the Argane tree, a study conducted on eight selected genotypes of A. spinosa has shown that under drought stress, even a severe stress, some Argane tree genotypes have significantly increased their fresh and dry root-to-shoot biomass ratio, while maintaining larger investments in the primary root and lateral roots.16 However, Chakhchar et al.,18 reported in Argane seedlings subjected to severe drought stress, by withholding the irrigation for 40 days, that root length and diameter, and root-to-shoot ratio did not change significantly in comparison with control. These findings demonstrate the capacity of Argane seedlings to maintain root elongation despite the decline in root biomass under drought stress. As a water-use strategy, Argane root system can maintain its growth in length to explore deeper soil horizons.26,41 Physiologically (Table 1), a significant decrease in root-relative water content has been recorded in Argane seedlings under drought stress conditions.17 Nonetheless, Chakhchar et al.,18 showed a considerable reduction in root hydraulic conductivity in Argane seedlings under severe drought stress. This reduction could be a biophysical response to minimize water loss by A. spinosa roots through water channels50 and to maintain leaf hydration,51 and it could also be due to cell wall suberization.52 The recourse to these strategies may allow the Argane tree to conserve and maintain the growth and functioning of its roots under drought conditions. However, this physiological response was associated with an increase of root electrolyte leakage, signaling an injury to root cell membranes.18 In addition, significant accumulation of malondialdehyde, as an indication of lipid peroxidation, was observed in the root of Argane seedlings under drought stress.17 Thus, at the biochemical level (Table 1), proteins and proline contents increased in roots of A. spinosa seedlings, as well as the peroxidase activity in response to severe drought stress.17 These changes were related to the duration of drought stress applied. These traits that occurred at the cellular level seem to be considered as heritable adaptive traits constituting the internal mechanism of tolerance to drought stress in A. spinosa.
Table 1: Summary of different root traits from studies conducted on Argania spinosa root system under different abiotic and biotic conditions.
| Root trait | Factors | Root traits | Reference |
|
Morphological and growth
|
Drought stress |
Root length |
Chakhchar et al.,18 |
|
Salt stress |
Root length |
Reda Tazi et al.,53 |
|
|
Mycorrhizal inoculation |
Root length |
Sellal et al.,24 |
|
|
Germination and in vitro techniques |
Root dry weight |
Lamaoui et al.,55 |
|
|
Physiological |
Drought stress |
Root hydraulic conductivity |
Chakhchar et al.,18 |
|
Biochemical |
Drought stress |
Root cell membrane injury |
Chakhchar et al.,18 |
|
Architectural and geophysical |
Field conditions (from early spring till midsummer) |
Electrical resistivity imaging |
Ain-Lhout et al.,26 |
|
Chemical |
Mycorrhizal inoculation |
Macroelements content (N, P, K) |
El Mrabet et al.,22 |
|
Anatomical |
Mycorrhizal inoculation |
Relative mycorrhizal dependency index |
Ouallal et al.,25 |
|
Genetic |
Chloroplast DNA phylogeography |
Polymorphisms in chloroplast DNA |
El Mousadik and Petit5 |
Effect of salt stress on the roots of Argane tree
The impact of salinity with different concentrations of NaCl on root growth in young seedlings of A. spinosa was tested by Reda Tazi et al.,53 in vitro. These authors reported decreasing of the root length with increasing concentration of NaCl in the medium. They also observed a decrease in root biomass of approximately 57.6% compared to the control, for the concentration 9 g/l NaCl.53 The Argane tree seems to be a species sensitive to salt stress.53,59 In an in vitro study, negative correlation was reported between A. spinosa callus growth and salt stress severity.55 The obtained results showed that concentration over 128 mM (NaCl) had noticeably inhibited calli growth. Nevertheless, the authors of the quoted study proved the efficiency of salt stress in boosting the Argane cell’s antioxidant status, which could be commercially applied in the field of tissue engineering and regenerative therapy.55
Mycorrhizal symbiosis in Argane tree
Most forest trees in the arid and semi-arid regions benefit from this symbiosis between their roots and specialized endophytic fungi.60 The Argane tree is characterized by a vital dependence (mutualism).24 This species is a good model of a host plant for root symbioses in arid woody plants. The Argane tree can associate with arbuscular mycorrhizae (AM) fungi belonging to the phylum Glomeromycota. Nine provenances of Argane tree from Southwestern Morocco have been the subject of a study focused on the assessment of the mycorrhizal potential of soils and determination of the community structure of the fungi in different edapho-climatic situations.25 The results of this study showed that the cytological organization of the mycorrhizae observed in Argania spinosa is arbuscular mycorrhizae in all samples analyzed with a broad dominance of the genus Glomus (approximately 80%).
When the fungus reaches the internal cortical cells of the Argane roots, the hyphae branch out and colonize the intercellular space. Once internal tissue colonization is substantial enough, the hyphae penetrate the inner cortical cells by invagination of the root cells (membrane level), forming fine branched complex structures called arbuscules (Fig. 1).24 These developed structures are a metabolic exchange interface between the root of the Argane tree and the fungus. Inoculation of young Argane seedlings with a strain of Glomus intraradices showed a beneficial and significant effect on plant size and biomass compared with non-mycorrhizal plants.19,20,21,24 Thus, Bousselmane et al.,54 reported that endomycorrhization of Argane plants by Glomus strains significantly improved height growth, dry biomass of schoot and root parts, and mineral nutrition. Sellal et al.,24 tested endomycorrhizal composite inoculum effect composed of arbuscular mycorrhizae fungi belonging to six genera: Acaulospora, Glomus, Scutellospora, Entrophospora, Pascispora and Gigaspora on Argane plants under nursery conditions. The results obtained by these authors also showed a positive and significant effect on the growth of inoculated plants (shoot and root biomass) compared with controls.
In addition, the relative mycorrhizal dependence index of inoculated Argane plants reached 80% after 6 months of growth under controlled conditions, in the presence of mineral nutrition and suitable irrigation.19 This mycorrhizal symbiosis has stimulated the absorption of macroelements, in particular nitrogen, phosphorus, potassium and calcium, and microelements such as iron, zinc, manganese and copper.22,31,58 This dependency is all the more important as the soils of Argane forests are low in phosphorus and sometimes reach the thresholds of deficiency for micronutrients. Improved mineral nutrition of Argane plants has resulted in significant biomass production.54 This mycorrhizal symbiosis is essential for the Argane tree; it allows a good recovery of plants in the natural environment, limiting the stress of transplantation and promoting initial growth through the improvement of mineral nutrition and water supply. Mycorrhization in the Argane tree has shown a long-term positive effect on inoculated and transplanted Argane plants in their natural environment.61 The beneficial effect of mycorrhization has been confirmed on several phenotypic and genotypically different Argane plants propagated by cuttings.20,54 Controlled and early inoculation of young seedlings of A. spinosa by a strain of Glomus intraradices, showed that mycorrhizal plants grow better than non-mycorrhizal controls, thus confirming the strong mycorrhizal dependence of the Argane tree.21 This also justifies the importance of inoculating the Argane tree with selected fungus strains early, at the nursery stage.
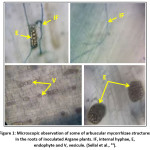 |
Figure 1: Microscopic observation of some of arbuscular mycorrhizae structures in the roots of inoculated Argane plants. IF, internal hyphae, E, endophyte and V, vesicule. (Sellal et al.,24) Click here to View Figure |
Rooting of Argane tree by different culture techniques
Assessment of the multiplication mode of the Argane tree consists in considering the state of the root system, i.e. above all the integrity and the length of the pivoting system, but also its architecture and branching, as well as the degree of mycorrhization of the secondary roots which carry the bulk of mycorrhizal symbiosis.20,21,54 Regarding the different multiplication techniques involved in the Argane tree, both rooting and growth rates of the root system are highly variable and depend primarily on the multiplied genotype.57 The genetic diversity of the Argane tree is a significant difficulty in its micropropagation, and it proves impossible to develop a single medium or combination allowing the multiplication and rooting of all genotypes. There were also problems related to Argane root formation in vitro culture, such as apical necrosis, absence of secondary roots and blocking of root elongation. However, a significant rooting rate (over 50%) have been recorded in Argane in vitro-plants.55,56,58 This technique provides the opportunity to produce genetically identical Argane plants by capturing attractive adaptive and productive traits of selected genotypes to ensure successful conservation and move from an exclusively wild plantation to an oilseed crop. Lamaoui et al.,55 pointed out that well-rooted Argane in vitro-plants were successfully acclimatized and then transferred to the field with a 100% survival rate.
The other methods of vegetative propagation of the Argane tree by cuttings, grafting, and layering (marcotting) are possible, despite the difficulty of rooting which depends on several endogenous and exogenous factors. In the natural environment of the Argane tree (Argane forest), the marcotting process is more common than suckering, especially along the Oueds, in the plains and very windy areas. In the latter case, the Argane trees are lying down by the sea winds, and their lower branches are rooted. Adventitious roots, often few, appear at the base of these branches. However, it is impossible to take advantage of these natural marcots to regenerate the Argane tree.62,63 Given the ability to develop suckers of one to two meters around the trunk in some stations, A. spinosa is therefore very apt to be propagated by cuttings of root segments.62,63 But new cultivation techniques used in the nursery have made it possible to produce seedlings with several pivoting roots and a dense network of lateral roots, which in plantations will then develop vigorously.64
Conclusion
Adaptation of A. spinosa to its environment in Western Morocco is mostly due to its root system. Despite the few studies performed on the roots of this species, these last reveal essential physiological and biochemical potentialities allowing Argane tree to overcome specific abiotic stresses and establish important mycorrhizal symbiosis. These innate and acquired features of the root system ensure the development and natural regeneration of the species in the Moroccan Argane forest. However, natural regeneration remains insufficient to maintain its existence and conservation. So, in vitro culture, after control and improvement of its rhizogenesis, seems to have many advantages for the production of selected and elite Argane genotypes with high yield oil production, and consequently, the development of an agroforestry system based on Argane tree.
Acknowledgments
We wish to thank the editor and the reviewers for their valuable critique and suggestions throughout the review and revision process.
Funding
The study was carried out as a part of a research programme supported by the Academy Hassan II des Sciences et Techniques and the Moroccan Ministry (Ministère de l’Education Nationale, de la Formation professionnelle, de l’Enseignement Supérieur et de la Recherche Scientifique).
Conflict of Interest
The authors declare that they have no competing interests.
Reference
- Msanda F., El Aboudi A., Peltier J.P. Biodiversité et biogéographie de l’arganeraie marocaine. Cah Agric, 2005; 14:357-364.
- Guillaume D., Pioch D., Charrouf Z. Argan [Argania spinosa (L.) Skeels] Oil. In., Ramadan M. (eds) Fruit Oils., Chemistry and Functionality. Springer, Cham, 2019; pp 317-352.
- Dahan R., Boughlala M., Mrabet R., Laamari A., Balaghi R., Lajouad L. A review of available knowledge on land degradation in Morocco. Allepo, ICARDA, 2012 ; 48p.
- McGregor H.V., Dupont L., Stuut J.B.W., Kuhlmann H. Vegetation change., goats., and religion., a 2000-year history of land use in southern Morocco. Quat Sci Rev, 2009; 28:1434-1448.
- El Mousadik A., Petit R.J. Chloroplast DNA phylogeography of the argan tree of Morocco. Mol Ecol, 1996; 5:547-555.
- Yatrib C., Belkadi B., Pakhrou O., Alami M., Medraoui L., El Mousadik A., Ferradous A., Msanda F., El Modafar C., Ibnsouda-Koraichi S., Filali-Maltouf A. Assessment of genetic diversity of Argania spinosa L. growing in arid and semi-arid areas of Morocco as revealed by inter-simple sequence repeats. J Agr Sci Tech B, 2015; 5:336-346.
- Mouhaddab J., Ait Aabd N., Msanda F., Filali-Maltouf A., Belkadi B., Ferradous A., El Modafar C., Ibnsouda Koraichi S., Ghazal H., El Mousadik A. Assessing genetic diversity and constructing a core collection of an endangered Moroccan endemic tree [Argania spinosa (L.) Skeels]. Moroccan J Biol, 2016; 13:1-12.
- Mouhaddab J., Msanda F., Filali-Maltouf A., Belkadi B., Ferradouss A., El Modafar C., Ibnsouda Koraichi S., El Mousadik A. Using microsatellite markers to map genetic diversity and population structure of an endangered Moroccan endemic tree (Argania spinosa L. Skeels) and development of a core collection. Plant Gene, 2017; 10:51-59.
- Pakhrou O., Medraoui L., Yatrib C., Alami M., Filali-Maltouf A., Belkadi B. Assessment of genetic diversity and population structure of an endemic Moroccan tree (Argania spinosa L.) based in IRAP and ISSR markers and implications for conservation. Physiol Mol Biol Plants, 2017; 23:651-661.
- Chakhchar A., Haworth M., El Modafar C., Lauteri M., Mattioni C., Wahbi S., Centritto M. An assessment of genetic diversity and drought tolerance in argan tree (Argania spinosa) populations., potential for the development of improved drought tolerance. Front Plant Sci, 2017a; 8:276.
- Lefhaili A. FAO forest resources assessment, Morocco country report, Rome, 2010.
- Lybbert T.J., Barrett C.B., Narjisse H. Market-based conservation and local benefits. The case of argan oil in Morocco. Ecol Econ, 2002; 41:125-144.
- Lybbert TJ., Magnan N., Aboudrare A. Household and local forest impacts of Morocco’s argan oil bonanza. Environ Dev Econ, 2010; 15:439-464.
- 14.M’Hirit O., Benzyane M., Benchekroun F., El Yousfi S.M., Bendaanoun M. L’arganier, Une espèce fruitière-forestière à usages multiples. Report for Mardaga, Sprimont, Belgium, 1998.
- Brunner I., Herzog C., Dawes MA., Arend M., Sperisen C. How tree roots respond to drought. Front Plant Sci, 2015; 6:547.
- Zahidi A., Bani-Aameur F., El Mousadik A. Growth variability in Argania spinosa seedlings subjected to different levels of drought stress. J Hortic For, 2013; 5:204-217.
- Meslem H., Djabeur A., Kharoubi O., Kaid-Harche M. Effect of water deficit on Argan tree seedlings (Argania spinosa L. Skeels)., Morphological and physiological aspect. Afr J Biotechnol, 2015; 14:1020-1028.
- Chakhchar A., Chaguer N., Ferradous A., Filali-Maltouf A., El Modafar C. Root system response in Argania spinosa plants under drought stress and recovery. Plant Signal Behav, 2018a; 13(7) e1489669.
- Nouaim R., Chaussod R. Mycorrhizal dependency of micropropagated argan tree (Argania spinosa), I. Growth and biomass production. Agroforest Syst, 1994; 27:53-65.
- Nouaim R., Chaussod R. Réponse à la mycorhization de plants d’arganier (Argania spinosa) multipliés par bouturage. Al Awamia, 2002; 105:9-22.
- Echairi A., Nouaim R., Chaussod R (2008) Intérêt de la mycorhization contrôlée pour la production de plants d’arganier (Argania spinosa) en conditions de pépinière. Sécheresse 19:277-281.
- El Mrabet S., Ouahmane L., El Mousadik A., Msanda F., Abbas Y. The effectiveness of arbuscular mycorrhizal inoculation and bio-compost addition for enhancing reforestation with Argania spinosa in Morocco. Open J For, 2014; 4:14-23.
- Elmaati Y., Msanda F., El Mousadik A., Elhamdaoui A., El Mrabet S., Ouahmane L. Contribution to the characterization of mycorrhizae in the South West of Morocco and their effect on growth parameters of Argania spinosa. Am J Innov Res Appl Sci, 2015; 1:235-243.
- Sellal Z., Ouazzani Touhami A., Mouden N., El Ouarraqi M., Selmaoui K., Dahmani J., Benkirane R., El Modafar C., Douira A. Effect of an Endomycorrhizal Inoculum on the Growth of Argan Tree. Int J Environ Agric Biotechnol, 2017; 2:928-939.
- Ouallal I., Abbas Y., Ech-cheddadi S., Ouajdi M., Ouhadach M., El Yacoubi H., Benaissa K., El Goumi Y., Rochdi A. Diversité des champignons endomycorhiziens de l’arganier et potentiel mycorhizogène des sols rhizosphériques des arganeraies du Sud-Ouest marocain. Bois For Trop, 2018; 338:73-86.
- Ain-Lhout F., Boutaleb S., Diaz-Barradas M.C., Jauregui J., Zunzunegui M. Monitoring the evolution of soil moisture in root zone system of Argania spinosa using electrical resistivity imaging. Agric. Water Manag, 2016; 164:158-166.
- Wang J., Fu B., Lu N., Zhang L. Seasonal variation in water uptake patterns of three plant species based on stable isotopes in the semi-arid Loess Plateau. Sci Total Environ, 2017; 609:27-37.
- Wang J., Fu B., Lu N., Wang S., Zhang L. Water use characteristics of native and exotic shrub species in the semi-arid Loess Plateau using an isotope technique. Agr Ecosyst Environ, 2019; 276:55-63.
- le Polain de Waroux Y., Lambin E.F. Monitoring degradation in arid and semi-arid forests and woodlands., the case of the argan woodlands (Morocco). Appl Geogr, 2012; 32:777-786.
- Alba-Sanchez F., Antonio Lopez-Saez J., Nieto-Lugilde D., Svenning J.C. Long-term climate forcings to assess vulnerability in North Africa dry argan woodlands. Appl Veg Sci, 2015; 18:283-296.
- Nouaim R., Lineres M., Esvan J.M., Chaussod R. Mycorrhizal dependency of micropropagated argan tree (Argania spinosa), II. Mineral nutrition. Agroforest Syst, 1994; 27:67-77.
- Kenny L., Galiana A., Bellefontaine R. Projet UE/MEDA/ADS. Appui à l’amélioration de la situation de l’emploi de la femme rurale et gestion durable de l’arganeraie dans le sud-ouest du Maroc- Thème 2, Multiplication végétative et symbioses racinaires de l’arganier, Optimisation des agrosystèmes à base d’arganier. Agence du développement social (Maroc) et Agropolis (France). Rapport final, 2009; 1-71.
- Allen C.D., Macalady A.K., Chenchouni H., Bachelet D., McDowell N., Vennetier M. et al. A global overview of drought and heat-induced tree mortality reveals emerging climate change risks for forests. For Ecol Manag, 2010; 259:660-684.
- Fitchett J.M., Grab S.W., Thompson D.I. Plant phenology and climate change., Progress in methodological approaches and application. Prog Phys Geog, 2015; 39:460-482.
- Solomon S., Qin D., Manning M., Chen Z., Marquis M., Averyt K., Tignor M., Miller H. eds. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change, Cambridge, UK and New York, Cambridge University Press, 2007.
- Sword M.A., Gravatt D.A., Faulkner P.L., Chambers J.L. Seasonal branch and fine root growth of juvenile loblolly pine five growing seasons after fertilization. Tree Physiol, 1996; 16:899-904.
- Hendrick R.L., Pregitzer K.S. The relationship between fine root demography and the soil environment in northern hardwood forests. Ecoscience, 1997; 4:99-105.
- Radville L., McCormack M.L., Post E., Eissenstat D.M. Root phenology in a changing climate. J Exp Bot, 2016; 67:3617-3628.
- Nicolas M., Jolivet C., Jonard M. L’apport des dispositifs de suivi vis-à-vis des enjeux de fonctionnement et de gestion des écosystèmes en relation avec les sols. Rev For Fr, 2014; LXVI: 491-500.
- Zhao X., Dupont L., Cheddadi R., Kölling M., Reddad H., Groeneveld J., Ain-Lhout FZ., Bouimetarhan I. Recent climatic and anthropogenic impacts on endemic species in southwestern Morocco. Quat Sci Rev, 2019; 221:105889.
- Zunzunegui M., Boutaleb S., Díaz Barradas M.C., Esquivias M.P., Valera J., Jáuregui J., Tagma T., Ain-Lhout F. Reliance on deep soil water in the tree species Argania spinosa. Tree Physiol, 2018; 38:678-689.
- Markesteijn L., Poorter L. Seedling root morphology and biomass allocation of 62 tropical tree species in relation to drought-and shade-tolerance. J Ecol, 2009; 97:311-325.
- Chakhchar A., Lamaoui M., Wahbi S., Ferradous A., El Mousadik A., Ibnsouda-Koraichi S., Filali-Maltouf A., El Modafar C. Leaf water status., osmoregulation and secondary metabolism as a model for depicting drought tolerance in Argania spinosa. Acta Physiol Plant, 2015a; 37:1-16.
- Chakhchar A., Wahbi S., Lamaoui M., Ferradous A., El Mousadik A., Ibnsouda-Koraichi S., Filali-Maltouf A., El Modafar C. Physiological and biochemical traits of drought tolerance in Argania spinosa. J Plant Interact, 2015b; 10:252-261.
- Chakhchar A., Lamaoui M., Aissam S., Ferradous A., Wahbi S., El Mousadik A., Ibnsouda-Koraichi S., Filali-Maltouf A., El Modafar C. Differential physiological and antioxidative responses to drought stress and recovery among four contrasting Argania spinosa ecotypes. J Plant Interact 2016; 11: 30-40.
- Chakhchar A., Lamaoui M., Aissam S., Ferradous A., Wahbi S., El Mousadik A., Ibnsouda-Koraichi S., Filali-Maltouf A., El Modafar C. Electrolyte ions and glutathione enzymes as stress markers in Argania spinosa subjected to drought stress and recovery. Afr J Biotechnol, 2017b; 16:10-21.
- Chakhchar A., Lamaoui M., Aissam S., Ferradous A., Wahbi S., El Mousadik A., Ibnsouda Koraichi S., Filali-Maltouf A., El Modafar C. Physiological and biochemical mechanisms of drought stress tolerance in the argan tree. Chapter in Plant Metabolites and Regulation under Environmental Stress. (Ahmad P., Ahanger M.A., Singh V.P., Tripathi D.K. & Alam P.). Elsevier, Academic Press, 2018b; 446p.
- Mainiero R., Kazda M. Depth-related fine root dynamics of Fagus sylvatica during exceptional drought. For Ecol Manag, 2006; 237:135-142.
- Poorter H., Niklas KJ., Reich PB., Oleksyn J., Poot P., Mommer L. Biomass allocation to leaves., stems and roots., meta-analyses of interspecific variation and environmental control. New Phytol, 2012; 193:30-50.
- Kaneko T., Horie T., Nakahara Y., Tsuji N., Shibasaka M., Katsuhara M. Dynamic regulation of the root hydraulic conductivity of barley plants in response to salinity/osmotic stress. Plant Cell Physiol, 2015; 56:875-882.
- Caldwell M.M., Dawson T.E., Richards J.H. Hydraulic lift, consequences of water efflux from the roots of plants. Oecologia, 1998; 113:151-161.
- Kreszies T., Shellakkutti N., Osthoff A., Yu P., Baldauf J.A., Zeisler‐Diehl V.V., Ranathunge K., Hochholdinger F., Schreiber L. Osmotic stress enhances suberization of apoplastic barriers in barley seminal roots., analysis of chemical., transcriptomic and physiological responses. New Phytol, 2019; 221:180-194.
- Reda Tazi M., Berrichi A., Haloui B. Germination et croissance in vitro de l’arganier (Argania spinosa L. Skeels) des Beni-Snassen (Maroc oriental) à différentes concentrations en NaCl. Actes Inst Agron Vet, 2001; 21:163-168.
- Bousselmane F., Kenny L., Achouri M. Effet des mycorhizes à vésicules et arbuscules sur la croissance et la nutrition de l’arganier (Argania spinosa L.). Actes Inst Agron Vet, 2002; 22:193-198.
- Lamaoui M., Chakhchar A., Benlaouane R., El Kharrassi Y., Farissi M., Wahbi S., El Modafar C, Uprising the antioxidant power of Argania spinosa L. callus through abiotic elicitation. C R Biol, 2019; 342:7-17.
- Justamante M.S., Ibáñez S., Villanova J., Pérez-Pérez J.M. Vegetative propagation of argan tree (Argania spinosa (L.) Skeels) using in vitro germinated seeds and stem cuttings. Sci Hortic, 2017; 225:81-87.
- Metougui M.L., Mokhtari M., Machati I., Azeroual I., Benlhabib O. Multiplication végétative de l’arganier (Argania spinosa) par bouturage et par greffage. Rev Mar Sci Agron Vét, 2017; 5:428-436.
- Nouaim R., Mangin G., Breuil M.C., Chaussod R. The argan tree (Argania spinosa) in Morocco, Propagation by seeds, cuttings and in-vitro techniques. Agroforest Syst, 2002; 54:71-81.
- Bani-Aameur F., Sipple-Michmerhuizen J. Germination and seedling survival of Argan (Argania spinosa) under experimental saline conditions. J Arid Environ, 2001; 49:533-540.
- Bitterlich M., Rouphael Y., Graefe J., Franken P. Arbuscular mycorrhizas, A promising component of plant production systems provided favorable conditions for their growth. Front Plant Sci, 2018; 9:1329.
- Nouaim R., Chaussod R. Effet de la mycorhization contrôlée sur la croissance de l’arganier (Argania spinosa) après sa transplantation en sol non désinfecté. Al Awamia, 1997; 96:65-76.
- Bellefontaine R. De la domestication à l’amélioration variétale de l’arganier (Argania spinosa L. Skeels). Sécheresse, 2010; 21:42-53.
- Bellefontaine R., Ferradous A., Alifriqui M., Monteuuis O. Multiplication végétative de l’arganier (Argania spinosa) au Maroc, le projet John Goelet. Bois For Trop, 2010; 304:47-59.
- Ferradous A., Hafidi M., Alifriqui M., Ouhammou A. Production de plants d’arganier (Argania spinosa) au Maroc, choix du conteneur et du substrat. Bois For Trop, 2017; 334:37-47.

