Introduction
Anthocyanins are pigments that belong to the flavonoid group, which are commonly found in many fruits, vegetables,and flowers. They provide colors that vary between orange, red, violet and blue, exhibiting great potential as natural colorants, due to their low toxicity.1-3 It can be used in food, cosmetic and pharmaceutical products due to their nutraceutical properties such as their ability to scavenge free radicals; reduce the risk of cardiac diseases and cancer etc.4 Black carrots are a very good source of anthocyanin pigments and contain up to 1750 mg per kg fresh weight.2,3 Moreover, black carrot anthocyanins provide an excellent bright strawberry red shade color which can be effectively used as a colorant and as a nutraceutical compound in various food products.5
Possibility of the usage of black carrot anthocyanin pigments as a natural colorant in the production of confectionery, jam, jellies,and frozen desserts was extensively reported by Birks.6 However, anthocyanin pigments are highly sensitive to factors like temperature, oxygen, light,andpH.1,3,7 Anthocyanin degradation reportedly follows first-order kinetics, i.e., anthocyanin content exponentially decreases with time. Therefore, it is imperative to protect anthocyanin pigments from temperature, oxygen, light, and pH.
Today food industries are trending towards the development of functional ingredients is under consideration with therapeutic and nutritional values.8-14 Various techniques are followed for the encapsulation process and most important for food applications include spray and freeze drying. Ersus et al.,1 used spray dryer for encapsulation black carrot anthocyanin pigments and Wilkowska et al.,14 used both spray and freeze drying for encapsulation of blueberry juice. Microencapsulation is one such technology through which any solids, liquids, or gaseous materials can be packed in miniature sealed capsules that would protect the core material from external environment.15-21Ferrari et al.,22 also reported storage stability of microencapsulated blackberry juice powders stored at 25ºC found the significant changes in the monomeric anthocyanin content with respect to a gradual increase in storage period.
In this studystorage stability of encapsulated black carrot juice powder in two different vials (transparent and amber color) under normal ambient conditions was investigated.
Materials and Methods
Ingredients and Materials
Fresh black carrots (Pusaasita) were harvested from the farm of the Department of Vegetable Science, IARI, New Delhi and washed under cold running tap water to remove the soil and foreign materials. Washed carrot wasstored under deep freezer (-20 ℃) since extraction. The coating materials such as maltodextrin (20DE), gum arabic and tapioca starch were purchased from the local market of New Delhi, India.
Extraction of Anthocyanin Rich Juice
Extraction of anthocyanin rich juice was performed following the method given by Khandare et al.,2 and stored under refrigeration since use.
Preparation of Spray Drying Feeding Materials
The feed mixture for the spray drying was prepared as suggested by Murali et al.,5 In summery encapsulating materials such as maltodextrin, gum arabic,tapioca starchwas mixed togetherwith extracted juice (6°Brix) and stirred to homogeneity with a digital Ultra-Turrax homogenizer (IKA®T25) for the stirring period of 30 min. The concentration of the feed mixture was maintained at 20° Brix.
Spray Drying of Feed Mixture
Spray drying of feed mixture was performed using the method given by Murali et al.,5 on an advanced laboratory spray dryer (LU–228, Labultima Pvt. Ltd., Mumbai, India) at the inlet&outlet temperature of 150°C&53°C, aspirator air flow rate of 50 m3/h and feed mixture flow rate of 2.5 ml/min. However, Freeze drying of feed mixturewasquick frozen by exposing at -80°C for 24 hours in sample jar followed by dried using onlaboratory scale Labconcofreeze dryer at the operating parameter of t -53°C of temperature and 0.22-0.11 mbar of vacuum.
Determination of Anthocyanin content and Color Intensity
The anthocyanin content was determined in terms of monomeric anthocyanin content (MAC)using pH differential method as given by Wrolstad et al.,23 Samples were prepared according to the method described by Kar et al.,24 and absorbance was recorded on UV-vis spectrophotometer at 520 nm and 700 nm for each sample.However, the antioxidant activity was determined using cupric reducing antioxidant capacity (CUPRAC) method as standardized by Apak et al.,25
Color intensity measurements were performed on Hunter-Lab Colorimeter (Miniscan® XE Plus 4500 L) and expressed in standard L* (whiteness or brightness/ darkness), a* (redness/greenness) and b* (yellowness/blueness) values. The same was then used to calculate the total color change using the following equation:
ΔE = [(ΔL) 2+ (Δa) 2+ (Δb)2]1/2 … (1)
Storage Stability Study
The encapsulated materials for storage were prepared using the optimized conditions obtained in the previous study using spray and freeze drying.5 The same was then packed in vials (transparent and amber) and stored at ambient temperature. Separate vials were prepared for different time periods. Vials once opened were not used for further study.
Degradation Kinetics
Degradation kinetics of encapsulated microcapsule was done at 37°C into the amber colored and transparent crew cap air tight vials, in the terms of half-life of anthocyanin (t1/2), i.e. the time needed for degradation of 50% of anthocyanin at specified storage temperature and storage period,26 calculated by the equation given below:
t1/2 = – In0.5 x k-1
Where, In (Ct/C0) = -k x t
Where Ct is the initial anthocyanin content, C0 is the anthocyanin content at the specific time and t is the time.
Statistical Analysis
A full factorial completely randomized design was conducted and SAS software (SAS, 2008) was used to analyze the results. p value of 0.05 was used to determine the level of significant difference.
Results and Discussion
Total Anthocyanin content
The anthocyanin content of spray and freeze-dried material reduced with the increase in the storage period in both the transparent and amber color vials. The loss of anthocyanin content at all storage conditions was almost similar in the first 30 days of storage. In the next 90 days, the loss varied significantly between all the experimental storage conditions. The loss in transparent vials was significantly higher than those stored in amber vials for both the spray and freeze-dried samples at all storage conditions (Fig. 1). This higher degradation of anthocyanin pigment in the transparent vial is due to direct effect of UV light, thereby more interaction of sunlight with anthocyanin pigments. Similar results have been reported by Ersus et al.,1 for encapsulated black carrot anthocyanin pigments and Tonon et al.,27 for powder of acai juice anthocyanin pigments. Moreover, the degradation was lower in the freeze-dried product stored in amber vials, and the maximum for the spray dried product stored in transparent vials. In general, powder stored in amber color vials retained more anthocyanin content due to its opaqueness to sunlight.
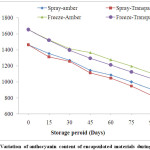 |
Figure 1: Variation of anthocyanin content of encapsulated materials during storage. Click here to View Figure |
Antioxidant Activity
Fig. 2 shows that the antioxidant activity decreases with respect to time for all the storage conditions. The decrease in the antioxidant activity followed a similar trend to that of anthocyanin content during storage.However, the degradation over the period of storage between amber and transparent vials was non-significant for both spray and freeze drying. The degradation in the first 60 days was significantly higher which then stabilizes during the last 30 days of storage under all the experimental conditions. The loss in the first 60 days is almost 80% of the total loss recorded at the end of 90 days of storage. Similar results have been reported by Ersus et al.,1 for black carrot juice powder and Pitalua et al.,28 for beetroot juice microcapsules.
Total color change
Total color change in the amber vials steadily increased with the increase in the storage period. The increase at the end of 90 days of storage was only about 5 to 7 which was non-significant (Fig. 3). This suggests that storage in amber color vials significantly retained the original characteristics of the material even after 90 days of storage for both spray and freeze-dried materials. In case of the materials stored in transparent vials, there was a significant change in the total color values in the first 15 days of storage suggesting degradation of bioactive components. Further storage period,however, does not affect the total color change significantly. Similar trends have been observed by Wrolstad et al.,23 for anthocyanin pigments of different products and Hernández‐Herrero et al.,29 for anthocyanin pigments of different juices.
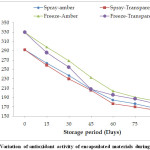 |
Figure 2: Variation of antioxidant activity of encapsulated materials during storage. Click here to View Figure |
Anthocyanin degradation kinetics and half life
Anthocyanin degradation at the end of 15th and 30th days were found to be similar for both freeze and spray dried samples stored under transparent and amber color vials (Fig. 4). Subsequent storage, however, triggers significantly higher loss of anthocyanins thereby reducing its retention in transparent vials compared to amber color vials. Similarly,freeze-dried samples at any point during the storage exhibited higher retention of anthocyanin content over spray dried samples.
Anthocyanin retention varied from 67% in the freeze-dried samples stored in amber color vials to 57% in case of spray dried powder stored in transparent vials. The half-life for freeze-dried samples stored in amber color vials was found to be the maximum (155 days) suggesting that it has the maximum storage life for 50% degradation of anthocyanin. The same for spray-dried material was found to be 130 days (Table 1).
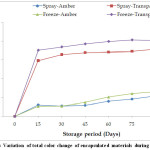 |
Figure 3: Variation of total color change of encapsulated materials during storage. Click here to View Figure |
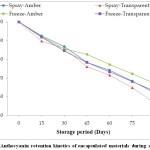 |
Figure 4: Anthocyanin retention kinetics of encapsulated materials during storage. Click here to View Figure |
Table 1: Degradation and half-life of Anthocyanins at the end of 90 days storage.
|
Drying Method |
Vial color |
K value |
Half-life(Days) |
Degradation(%) |
|
Spray |
Amber |
0.0053 |
130.2±2.04 |
38.00 |
|
Transparent |
0.0063 |
109.4±4.65 |
43.43 |
|
|
Freeze |
Amber |
0.0044 |
155.6±1.64 |
33.00 |
|
Transparent |
0.0051 |
134.8±3.58 |
37.00 |
Idham et al.,30 reported similar results for spray dried red color powder from Roselle calyces. The results indicate that the half-life followed a first-order kinetics and if encapsulation is carried out using spray drying and stored in amber colorairtight vials, anthocyanins can be effectively retained for 130 days whereas if the same is prepared using freeze-drying and stored in airtight amber containers the shelf life of the anthocyanin can be extended upto 155 days. Similar trends of anthocyanin degradation kinetic were reported by Wang et al.,31 for blackberry juice and concentrate during storage at 37ºC, respectively.
Conclusion
Storage of encapsulated material produced using spray and freeze-drying techniques can be effectively accomplished for a period of 90 days when stored under ambient conditions in both transparent and amber color vials. Retention of anthocyanin is about 67% for the freeze-dried sample and about 62% for the spray dried samples stored in airtight amber color vials at ambient conditions. The half-life of anthocyanin is about 155 days for the freeze-dried sample stored in airtight amber vials whereas the same for the spray dried ones stored in amber color vials at ambient conditions is about 130 days.
Acknowledgment
The authors acknowledge the help rendered by faculty members of the division of Food Science & Post harvest Technology, Technical officer and SRFs of Food Quality Analysis Lab, Indian Agricultural Research Institute, New Delhi for successful completion of research work.
Conflict of Interest
Authors declare no conflict of interest.
References
- Ersus S., Yurdagel U. Microencapsulation of anthocyanin pigments of black carrot (Daucus carota L.) by spray drier. J Food En. (2007); 80(3): 805-12.
CrossRef - Khandare V., Walia S., Singh M., Kaur C. Black carrot (Daucus carota ssp. sativus) juice: processing effects on antioxidant composition and color. Food Bioprod Proc. (2011); 89(4):482-6.
CrossRef - Kırca A., Özkan M., Cemeroğlu B. Effects of temperature, solid content and pH on the stability of black carrot anthocyanins. Food Chem.(2007); 101(1): 212-8.
CrossRef - Wrolstad R. E. Anthocyanin pigments – Bioactivity and coloring properties. JFood Sci.(2004); 69(5): 419-425.
CrossRef - Murali S., Kar A., Mohapatra D., Kalia P. Encapsulation of black carrot juice using spray and freeze drying. Food Sci Technol Int.(2015); 21(8): 604-12.
CrossRef - Birks S.The potential of carrots. Food Manufact.(1999); 47(4): 22-23.
- Amr A., Al‐Tamimi E. Stability of the crude extracts of Ranunculus asiaticus anthocyanins and their use as food colorants. Int JFood Sci Tech.(2007); 42(8): 985-91.
CrossRef - Patel A. S., Pandey A. K. Fortification of Limoniaacidissima Linn. fruit powder to develop the phenolic enriched herbal biscuits. J BioresEng Technol. (2014); 1: 74-85.
- Patel A. S., Pradhan R. C. Quality ranking of bottle gourd seed cake powder incorporated biscuits using fuzzy analysis of sensory attribute. BIOINFOLET-A Quart J Life Sci. (2015);12(4a): 901-908.
- Murali S., Kar A., Patel A. S., Kumar J., Mohapatra D., Dash S. K. Spray dried encapsulation of rice bran oil in soya protein isolate – tapioca starch complex using response surface methodology. Ind J Agric Sci. (2016); 86 (8): 984–91.
- Dhakane J. P., Kar A., Patel A. S., Khan I. Effect of soy proteins and emulsification-evaporation process on physical stability of lycopene emulsions. Int J Chem Studies. (2017); 5(5): 1354-1358.
- Murali S, Kar A., Patel A. S., Mohapatra D., Krishnakumar P. Optimization of process conditions of rice bran oil encapsulation using spray drying by response surface methodology. Int J Food Eng. (2017) 13(4): 25-35.
CrossRef - Patel A. S., Pradhan R. C., Kar A., Mohapatra D. Effect of Fortification of De-oiled Bottle Gourd (Lagenariasiceraria) Seed on the Functional and Chemical Characteristics of the Biscuit: A Nutritional Assessment. Curr Res Nutr Food Sci. 2018; 6(3).
CrossRef - Wilkowska A., Ambroziak W., Czyżowska A.,Adamiec J. Effect of microencapsulation by spray-drying and freeze-drying technique on the antioxidant properties of blueberry (Vaccinium myrtillus) juice polyphenolic compounds. Polish J Food Nutri Sci.(2016); 66(1): 11-6.
CrossRef - Patel A. S., Khan I., Kar A. A technique of micro capsulation by spray-drying of nutrient elements: A review. NISCAIR Public, 2016; 24(2): 164-172.
- Patel A. S., Kar A., Khan I. Process for development of β-carotene Nanocomposites with ɷ-fatty acids. 2017.
- Pradhan R. C., Patel A. S., Mishra S. Physico-chemical properties of bottle gourd kernel. J Agric Eng. (2015); 52(4): 28-34.
- Duhan S., Kar A., Nain L., Patel A. S., Dash S. K. Development of continuous flow microwave and hot water bath system for destruction of spoilage microorganisms in food. Ind J Agric Sci. (2017); 87(2): 210-214
- Ghosh P., Pradhan R. C., Patel A. S., Kar A., Mishra S. Physicochemical characteristics of Syzygiumcuminifruit. Curr Res Nutr Food Sci. (2017); 5(1), 25-35.
CrossRef - Patel, A., Balunkeswar Nayak. “Mobilization of lipophobicity of cellulose nanocrystals (CNCs): An efficient encapsulation of phycobiliproteins.” In abstracts of papers of the American Chemical Society, vol. 256. 1155 16TH ST, NW, Washington, DC 20036 USA: Amer Chemical Soc. 2018.
- Mohapatra D., Patel A. S., Kar A., Deshpande S. S., Tripathi M. K. Effect of Different Processing Conditions on Proximate Composition, Anti-oxidants, Anti-nutrients and Amino Acid Profile of Grain Sorghum. Food Chem. 2018; 271:129-135.
CrossRef - Ferrari C. C., Marconi-GermerS. P., Alvim I. D., de Aguirre J. M. Storage stability of spray-dried blackberry powder produced with maltodextrin or gum arabic. Dry Technol.(2013); 31(4): 470-8.
CrossRef - WrolstadR. E., Durst R. W., Lee J. Tracking color and pigment changes in anthocyanin products. Trends Food Sci Technol.(2005); 16(9): 423-8.
CrossRef - Kar A., Mahato D. K., Patel A. S., Bal L. M. The encapsulation efficiency and physicochemical characteristics of anthocyanin from black carrot (Daucus carota Ssp. sativus) as affected by encapsulating materials. Current Agric Res J. (2019); 7(1).
CrossRef - Apak R., Güçlü K., Özyürek M.,Karademir S. E. Novel total antioxidant capacity index for dietary polyphenols and vitamins C and E, using their cupric ion reducing capability in the presence of neocuproine: CUPRAC method. J AgricFood Chem.(2004); 52(26): 7970-81.
CrossRef - Sari, P., Setiawan, A. and Siswoyo, T. A. (2015). Stability and antioxidant activity of acylated jambolan (Syzygiumcumini) anthocyanins synthesized by lipase-catalyzed transesterification. International Food Research Journal, 22(2), 671-676.
- Tonon R. V., Brabet C.,Hubinger M. D. Anthocyanin stability and antioxidant activity of spray-dried açai (Euterpe oleracea Mart.) juice produced with different carrier agents. Food Res Inter.(2010); 43(3): 907-14.
CrossRef - Pitalua E., Jimenez M., Vernon-Carter E. J., Beristain C. I. Antioxidative activity of microcapsules with beetroot juice using gum Arabic as wall material. Food Bioprod Process.(2010); 88(2): 253-8.
CrossRef - Hernández‐Herrero J. A.,Frutos M. J. Degradation kinetics of pigment, color and stability of the antioxidant capacity in juice model systems from six anthocyanin sources. Inter J Food Sci Technol.(2011); 46(12): 2550-7.
CrossRef - Idham Z., Muhamad I. I.,Sarmidi M. R. Degradation kinetics and color stability of spray‐dried encapsulated anthocyanins from hibiscus sabdariffa l. J Food Process Eng.(2012) 35(4): 522-42.
CrossRef - Wang W. D., Xu S. Y. Degradation kinetics of anthocyanins in blackberry juice and concentrate. J Food Eng.(2007); 82(3): 271-5.
CrossRef


