Introduction
Shifting cultivation or locally known as Jhum cultivation is an age old and dominant agricultural practice in the hill states of North East India.1 Although it is considered to be well adapted to tropical climates and accessible to small farmers because of its low cost, degradation of land due to short Jhum cycle (earlier from a period of 15-20 years to now 2-3 years)2 it has become a major constraint in the soil productivity.3
As per the practice of Jhum agroforestry system, after harvesting, the site is left as such so that it rejuvenates naturally with soil nutrient components which diminish after farming. It was observed that after burning of Jhum sites of Nagaland, some of the weed species for example, Borreria hispida, Chromolaena odorata, Thysanolaena maxima and Ageratum conyzoides showed abundance growth in the starting of secondary succession of the vegetation.4 Although weed infestation was carried out for agricultural practice, when Jhum cultivation sites were left for rebuilding of ecosystem, they again grew in abundance. In later stages of succession their dominance were replaced by shrubs or other woody plant varieties. As particularly these weed species were pioneering the secondary vegetation, the soil-plant-microbe interactions associated with these ESP can be considered as most potential interaction among the other members of the plant community for rebuilding of the Jhum soil ecology.
To study these interactions, enzyme activity of root associated soils of TM were analyzed and compared with bulk soil enzyme activity. Activities of soil enzymes such as arylsulphatase, dehydrogenase, phosphatase, β-glucosidase etc. were often used as indicators of soil quality after burning and anthropogenic disturbances.5 Along with the assessment of soil enzymes, association of beneficial microbes with roots of TM was also studied, as enrichment of soil quality is further influenced by its associated microbial biomass.6
Materials and Methods
Selection of Jhum fallow site and sample collection
Considering the prevalence of the length of Jhum cycles, two fallows (secondary forest stands) of different ages viz. 5 years and 20 years (hereafter referred as F5 and F20, respectively) in Mokokchung district of Nagaland (26024.101’-26024.599’ N, 94022.409’-94022.539’ E) were selected. This study area falls under wet, warm and humid tropical climate with annual rainfall from 1800 to 2600 mm and altitude ranges from 592 to 733 m above mean sea level. The Jhum practicing tribal farmers carried out the slash and burnt operations during the last week of November, 2014 and 1st week of February, 2015, respectively in F5 and F20 fallows. On completion of burning operation, following the onset of pre-monsoon shower in the month of April, various plant species started germinating on the burnt fields. Sampling of young TM (30-35 days old) was done from 50 days old burnt field (Fig. 1). TM samples were collected from F5 and F20 hereafter referred as TMF5 and TMF20 respectively.
 |
Figure 1: The sampling of Thysanolaena maxima as early succession plant regenerating in after-burnt jhum field of Mokokchung district, Nagaland. Click here to View Figure |
To collect bulk soil and rhizospheric soil samples along with plant samples, each site was demarcated according to natural slope boundary of the hill i.e. summit, shoulder and back slope. Within a natural slope boundary, five random grids (each grid size was 25 m2) were considered.7 Soil samples from five random spots within each grid were collected and mixed them all to make one composite per grid. Thus, five composite soil samples were obtained along each natural slope boundary. Finally, each composite soil sample of summit, shoulder and backslope along the direction of slope gradient were mixed together to get overall five composite soil samples for each site. In doing so, the possible influence of slope gradient on soil attributes was confounded since, the present investigation aimed to study the effect of burning and the length of the fallow phases on soil biochemical attributes of rhizospheric soils of TM relative to bulk soils. Thus total ten composite grid samples for bulk soil (five samples for F5 hereafter referred as B1, B2, B3, B4 and B5; and five samples for F20 hereafter referred as B6, B7, B8, B9 and B10) were collected. Similarly total ten composite samples of TM for root and adhered soil were collected. Young TM (30-35 days old) were uprooted carefully using shovel. To separate rhizospheric soil, the loosely adhered soils were removed by jerking the whole root system and the tightly adhered soils along with fine roots of plant were collected in sterile zipped plastic bag. Thus from ten composite plant samples, ten composite samples for rhiospheric soil (five samples for TMF5 hereafter referred as RH1, RH2, RH3, RH4 and RH5; and five samples for TMF20 hereafter referred as RH6, RH7, RH8, RH9 and RH10) and total ten composite sample for roots (five samples for TMF5 and five samples for TMF20) were analyzed. After collection, both bulk soil samples and plant samples were stored at 40C until completion of analysis of biochemical and microbiological parameters.
Soil Enzyme Properties
Analysis of six soil enzymes was conducted on total ten composite grid samples for bulk soil and ten composite samples for rhiospheric soil keeping three replicates for each sample according to the standard procedures described by Page and his co-workers.8 In brief the procedures are described below. To determine AMY, soil supernatant was obtained after the incubation of soil with starch in citrate buffer at 37 0C for 2 hr.7 The presence of glucose was detected by Nelson-Somogyi method9 and intensity of blue colour developed was measured at 620 nm using microtiter plate reader (Multiskan, Thermo Scientific, USA). To determine ASA, soil was treated with p-nitrophenyl sulphate (PNS) in presence of toluene and acetate buffer and incubated at 370C for 1 hr. The hydrolytic release of sulphate from PNS was measured colorimetrically.10 Determination of GSA was performed by incubating soil with p-nitrophenyl- β -D-glucoside (PNG) in presence of toluene and MUB (Modified Universal Buffer, pH 6.0) solution at 370C for 1 hr. Concentration of p-nitrophenol was measured colorimetrically in yellow coloured filtrate of incubated soil.11 For determination of DHA activity, soil was incubated with 2, 3, 5-triPhenyltetrazolium chloride (TTC) along with CaCO3 at 370C for 24 hr. Filtered soil extractant was treated with methanol and formation of triphenyl formazan (TPF) was measured colorimetrically.12 For PHA determination, soil was incubated with p-NPP (p-nitrophenyl phosphate) in presence of MUB and toluene at 370C for 1 hr. After incubation suspension was treated with CaCl2 and NaOH. Released p-nitrophenol was measured colorimtrically.13 To determine PRO activity, soil was incubated with sodium caseinate along with tris buffer at 50 0C for 2 hr in shaking water bath. Stopping the reaction with trichloroacetic acid supernatant was obtained and further treated with alkaline reagent and folin-ciocalteu reagent. After 1 hr, the concentration of tyrosine in the filtrate was determined colorimetrically.14
Isolation of rhizospheric and endophytic bacteria
Isolation of rhizospheric bacteria was done from five composite rhizospheric soil samples from each fallow phase (sample RH1-RH5 from F5 and sample RH6-RH10 from F20). In similar way root samples were combined to isolate endophytic bacteria. The rhizosphere soil samples were processed as per the procedure described by Thokchom et al.,15 1 g of rhizosphere soil was serially diluted and plated separately on Reasoner’s 2 agar (R2A), Tryptic soy agar (TSA) and Potato dextrose agar (PDA). The morphologically different colonies were purified and stored in 30 % (m v-1) glycerol at – 20 0C Ultra Deep Freezer. Prior to the isolation of root endophytes, roots were surface sterilized by washing the root bits (approximate 1 cm length) with successive dip in 70 % ethanol for 1 min – 2 % hypochlorite for 5 min – 70 % ethanol for 30 sec and 4 rinses with sterile distilled water. Root suspension was prepared by dissolving the grounded root sample in 1X PBS buffer in ratio 1:5. The suspension was precipitated at 4000 rpm for 20 min and supernatant was used to isolate endophytic bacteria in above mentioned three media.
The inoculum suspension of each pure isolate, prepared in nutrient broth (18-24 hr old, OD600 2.0), was subjected to the screening for various PGP assay. For each isolate 3 replicate analyses were performed.
In-vitro assays for PGP traits of root associated bacteria
Production of Pectinase
Pure isolates were spotted in nutrient agar plates supplemented with 0.5 % pectin. After five days of incubation the plates were flooded with 2% CTAB solution for 20 min. Development of halo around the growth was considered as positive for pectinase production.16
Production of cellulase
The isolates were grown on 0.5% CMC (Carboxy Methyl Cellulose) agar plates. Two holes were made in the solid media one for sample and one for sterile distilled water17. After five days of incubation the plates were flooded with 1% congo red solution for 15 min. Destaining was done with 1 M NaCl solutions. The congo red clearing zone around the sample indicates cellulase activity of the bacteria.18
N2 fixation
100 µl of pure bacterial culture was inoculated in nitrogen-free bromothymol broth along with uninoculated control and incubated for 3-5 days at 300C. The potential N2 fixing bacteria were identified observing the blue colour development. Development of green to blue colour due to increase in pH of the medium on fixation of NH4 by the bacterial isolate was a positive test for N2 fixation.19
Production of indoleacetic acid (IAA)
Production of IAA was tested by growing the bacterium in Ashbys Mannitol broth supplemented with 1% L-Tryptophan (9:1) from NB suspension of pure culture of each bacterium.20 After 48 hr of incubation, cells were harvested at 4000 rpm for 20 min. The supernatant (2 ml) was mixed with 2 drops of orthophosphoric acid and 4 ml of Salkowski reagent (50 ml, 35% of perchloric acid; 1 ml 0.5 M FeCl3 solution). Development of pink colour indicated IAA production. Optical density was taken at 530 nm with the help of spectrophotometer (Spectrascan UV-2600, Thermo scientific, USA). Concentrations of IAA produced by cultures was measured with the help of standard graph of IAA (Hi-media) obtained in the range of 5-30 µg/ml.21
Phosphate solubilization
The isolates were tested for their ability to dissolve four different forms of insoluble iP viz. Pikovskaya broth22 amended with Ca3(PO4)2, AlPO4, FePO4 and minimal media amended with sodium phytate (Na-P). After 72 hr of incubation, cells were harvested at 4000 rpm for 20 min. In 1 ml of clear supernatant 5 ml of extractant (0.03 N NH4F, 0.025 N HCl) were added followed by addition of 5 ml of Brays reagent and 1 ml of stannous chloride to develop blue colour.23 Optical density was taken at 660 nm with the help of spectrophotometer (Spectrascan UV-2600, Thermo scientific, USA). Concentrations of inorganic P was measured with the help of standard graph of P (Hi-media) obtained in the range of 0.08-1 µg/ml.
ACCD Production
The activity of ACCD was assayed according to the modification of protocol of Honma and Shimomura,24 which measures the amount of α-ketobutyrate produced when the enzyme ACCD cleaves ACC.25 The enzyme activity was expressed as hmol α-ketobutyrate mg-1 protein hr-1 and bacterial cell protein was determined by following Bradford protein assay.26
Results
Soil enzyme properties of rhizospheric and bulk soil
Activity of AMY, ASA, GSA, DHA, PHA and PRO was different in different soil samples. In five composite grid samples for bulk soil of F5 average activity for AMY ASA, GSA, DHA, PHA and PRO was found to be 3.60 µg glucose g-1 soil hr-1, 111.09 µg pNP g-1 soil hr-1, 1.0 µg pNP g-1 soil hr-1, 3.62 µg TPF g-1 soil hr-1, 624.82 µg pNP g-1 soil hr-1 and 3.0 µg tyrosine g-1 soil hr-1, respectively (Table 1). The average activity for AMY ASA, GSA, DHA, PHA and PRO was found to be 5.46 µg glucose g-1 soil hr-1, 146.12 µg pNP g-1 soil hr-1, 1.17 µg pNP g-1 soil hr-1, 5.21 µg TPF g-1 soil hr-1, 824.91 µg pNP g-1 soil hr-1 and 4.59 µg tyrosine g-1 soil hr-1 in case of bulk soil from F20.
Table 1: The comparative activities of soil enzymes in bulk soil between 5 and 20 years Jhum fallow phases.
| Fallow phase | AMY | ASA | GSA | DHA | PHA | PRO |
| F5B1 | 3.43±0.65 | 131±4.48 | 1.15±0.12 | 5.94±0.23 | 672±64.2 | 3.72±1.81 |
| F5B2 | 3.42±0.67 | 95.0±2.05 | 0.84±0.10 | 2.53±1.11 | 628±32.3 | 4.47±1.35 |
| F5B3 | 5.36±1.23 | 123±5.84 | 0.97±0.13 | 1.95±0.23 | 583±9.10 | 0.36±0.10 |
| F5B4 | 2.39±1.08 | 91.1±6.91 | 1.01±0.19 | 1.87±0.17 | 877±78.9 | 2.91±1.58 |
| F5B5 | 3.41±1.03 | 114.±10.6 | 1.02±0.16 | 4.14±0.60 | 363±26.0 | 1.18±0.31 |
| F20B6 | 6.66±1.01 | 122±15.9 | 1.69±0.61 | 4.75±0.46 | 658±17.3 | 4.59±0.78 |
| F20B7 | 3.76±0.65 | 153±13.0 | 0.59±0.19 | 6.11±0.63 | 951±11.5 | 2.31±0.85 |
| F20B8 | 5.50±0.99 | 170±14.3 | 1.12±0.28 | 5.84±0.24 | 758±36.0 | 2.23±1.22 |
| F20B9 | 7.97±0.50 | 147±11.5 | 1.51±0.31 | 3.33±0.46 | 858±15.4 | 5.55±0.77 |
| F20B10 | 3.42±0.86 | 138±10.9 | 0.93±0.16 | 6.04±0.27 | 898±13.6 | 8.30±1.20 |
Values represent means ± SD (n = 3), F5, 5 year fallow phase; F20, 20 year fallow phase; AMY, amylase activity determined in terms of µg glucose soil g-1 soil hr-1; ASA, arylsulphatase activity determined in terms of pNP soil g-1 soil hr-1; GSA, β-glucosidase activity determined in terms of µg pNP soil g-1 soil hr-1; DHA, dehydrogenase activity determined in terms of TPF soil g-1 soil hr-1; PHA, acid-phosphomonoestarase activity determined in terms of µg pNP soil g-1 soil hr-1; PRO, protease activity determined in terms of µg tyrosine soil g-1 soil hr-1.
The rhizospheric soil from TMF5 showed average activity of 10.06 µg glucose g-1 soil hr-1, 229.64 µg pNP g-1 soil hr-1, 2.25 µg pNP g-1 soil hr-1, 8.67 TPF g-1 soil hr-1, 1296.97 µg pNP g-1 soil hr-1 and 8.39 µg tyrosine g-1 soil hr-1 for enzyme AMY, ASA, GSA, DHA, PHA and PRO, respectively (Table 2). On the other hand, in five composite grid samples for rhizospheric soil of TMF20 gave average activity for AMY ASA, GSA, DHA, PHA and PRO as 8.49 µg glucose g-1 soil hr-1, 169.02 µg pNP g-1 soil hr-1, 1.92 µg pNP g-1soil hr-1, 6.83 TPF g-1 soil hr-1, 1006.97 µg pNP g-1 soil hr-1 and 6.87 µg tyrosine g-1 soil hr-1, respectively.
Table 2: The comparative activities of soil enzymes in rhizosphere soil between 5 and 20 years Jhum fallow phases.
| Fallow phase | AMY | ASA | GSA | DHA | PHA | PRO |
| F5RH1 | 12.1±1.53 | 182±3.18 | 2.25±1.13 | 9.27±0.50 | 976±22.5 | 5.36±0.08 |
| F5RH2 | 6.82±1.48 | 324±24.6 | 1.58±0.48 | 9.64±0.60 | 1559±18.2 | 9.96±0.13 |
| F5RH3 | 12.7±2.21 | 218±33.6 | 3.75±0.64 | 6.08±0.96 | 1041±47.9 | 12.5±0.28 |
| F5RH4 | 12.6±1.73 | 267±43.6 | 1.84±0.18 | 10.6±1.07 | 1737±10.3 | 7.79±0.31 |
| F5RH5 | 6.10±1.74 | 155±7.29 | 1.85±0.52 | 6.99±0.05 | 1169±18.5 | 6.41±0.38 |
| F20RH6 | 8.28±1.10 | 139±13.5 | 1.54±0.55 | 5.10±0.39 | 1200±91.5 | 8.78±0.51 |
| F20RH7 | 12.1±1.16 | 83.1±9.49 | 2.88±0.68 | 6.92±0.89 | 975±11.0 | 4.37±0.29 |
| F20RH8 | 8.52±1.20 | 164±26.3 | 1.57±0.16 | 6.90±0.22 | 835±25.8 | 7.03±0.40 |
| F20RH9 | 6.38±1.00 | 86.7±5.69 | 1.09±0.63 | 8.39±0.46 | 1082±44.2 | 8.93±0.46 |
| F20RH10 | 7.15±0.68 | 371±22.4 | 2.54±0.96 | 6.83±0.35 | 943±14.4 | 5.24±0.22 |
Values represent means ± SD (n = 3), F5, 5 year fallow phase; F20, 20 year fallow phase; AMY, amylase activity determined in terms of µg glucose soil g-1 soil hr-1; ASA, arylsulphatase activity determined in terms of pNP soil g-1 soil hr-1; GSA, β-glucosidase activity determined in terms of µg pNP soil g-1 soil hr-1; DHA, dehydrogenase activity determined in terms of TPF soil g-1 soil hr-1; PHA, acid-phosphomonoestarase activity determined in terms of µg pNP soil g-1 soil hr-1; PRO, protease activity determined in terms of µg tyrosine soil g-1 soil hr-1.
The population incidence of root associated bacteria of TM
The population of root associated (both rhizobacteria and endophytes) bacteria (counted in media R2A, TSA and PDA) of TM showed an increasing trend in the order of F20 > F5 and the bacterial population difference between F5 and F20 were significant (Table 3).
Table 3: Root associated bacterial counts of Thysanolaena maxima from soils of different fallow phases.
| Fallow period | Population of bacteria (cfu x 107 cells g-1 soil) | |
| Rhizospheric soil | Root endophyte | |
| F5 | 0.27 ± 0.029a | 0.05 ± 0.008a |
| F20 | 0.74 ± 0.056b | 0.08 ± 0.004b |
Values are means ± SD, n = 5; Values that differed significantly are followed by different letters between F5 and F20; F5, 5 year fallow phase and F20, 20 year fallow phase.
Out of 81 roots associated bacteria of TM, 29 and 34 rhizobateria were obtained from F5 and F20 respectively; and 9 endophytes were obtained from each fallow cycle in three different media.
Incidence of plant beneficial root associated bacteria of TM
Eighty one isolates were considered for in-vitro PGP traits (Table 4 and 5). Out of 29 rhizobacteria isolated from TMF5 production of pectinase, cellulase, IAA-like substances and ACCD were obtained in 8, 9, 18 and 24 strains, respectively; and out of 34 rhizobacteria from TMF20 these activity were found in 3, 9, 17 and 19 strains, respectively. Eighteen N2 fixing rhizobacteria were isolated from TMF5 whereas 17 were isolated from TMF20. Dissolution of iP in media amended with Ca3(PO4)2, Na-phytate, AlPO4 and FePO4 were observed in 17, 28, 23 and 25 isolates from TMF5, respectively; and 16, 26, 2 and 3 strains isolated from TMF20, respectively.
Table 4: The multifaceted PGP traits of root associated bacteria (Rhizospheric and endophytic) from TM collected from 5 years fallow Jhum cycle.
| Isolate | Pect.a | Cell.b | N2-fix.c | IAAd | Dissolution/or mineralization of insoluble phosphates | ACCDi activity | |||
| Ca3(PO4)2 e | Na-phytatef | AlPO4 g | FePO4 h | ||||||
| RR1 | nd | 16 | +4 | 34.8±4.12 | 141±12.9 | 35.2±3.52 | nd | 15.9±0.32 | 43.1±2.13 |
| RR2 | 3 | nd | +3 | nd | 79.5±8.39 | 95.5±8.03 | 4.53±0.34 | 6.17±0.22 | 88.2±3.31 |
| RR3 | 3 | 15 | +5 | 64.2±9.35 | 54.2±5.93 | 85.7±9.43 | 14.3±0.52 | 18.6±1.47 | 24.4±0.64 |
| RR4 | nd | 10 | +3 | 73.2±7.47 | 98.7±14.7 | 91.0±9.58 | 20.0±0.29 | 21.5±0.67 | 36.3±0.94 |
| RR5 | nd | nd | +5 | 56.7±8.74 | 108±17.5 | 124±14.7 | nd | 19.7±1.46 | nd |
| RR6 | 4 | nd | +3 | 8.35±0.42 | 61.2±6.48 | 83.0±7.45 | 9.72±0.17 | 19.7±1.63 | nd |
| RR7 | nd | nd | +4 | 60.9±8.39 | nd | 92.0±6.93 | 4.09±0.26 | nd | 33.5±1.77 |
| RR8 | nd | nd | +3 | nd | 109±14.7 | 65.0±5.39 | 3.00±0.27 | 20.2±1.69 | 30.5±1.60 |
| TR1 | nd | nd | +1 | nd | 164±18.3 | 104±15.3 | 6.03±0.31 | 15.9±0.52 | 31.7±1.40 |
| TR2 | nd | nd | +5 | 19.6±0.73 | 106±13.7 | 147±17.0 | 25.5±0.21 | nd | 57.3±2.56 |
| TR3 | nd | nd | +1 | nd | nd | 90.5±8.33 | 3.09±0.1 | 7.00±0.58 | 38.0±2.52 |
| TR4 | 5 | 17 | +5 | 6.95±0.32 | 56±4.35 | 89.2±7.49 | 4.53±0.24 | 9.00±0.48 | nd |
| TR5 | nd | 12 | Nd | 28.9±2.03 | 51.5±3.51 | 89.5±9.43 | nd | 9.50±0.73 | 46.5±1.92 |
| TR6 | 5 | nd | +1 | nd | nd | 91.0±10.3 | 5.75±0.37 | 28.7±2.48 | 73.6±4.93 |
| TR7 | nd | nd | Nd | 17.3±1.91 | 40.2±4.52 | 87.2±8.57 | 74.5±0.33 | 10.3±1.17 | 29.4±1.63 |
| TR8 | 2 | 10 | +4 | nd | 92.2±11.5 | 89.5±9.75 | 45.4±0.42 | nd | 37.6±1.46 |
| TR9 | 13 | 17 | Nd | 87.0±13.4 | 78.2±9.07 | 83±7.09 | 48.7±0.32 | 9.25±0.74 | 22.7±1.54 |
| PR1 | nd | nd | Nd | 19.6±7.32 | nd | 104±16.0 | 60.0±0.39 | 27.9±3.89 | 41.5±2.73 |
| PR2 | nd | nd | +4 | nd | 31.5±4.32 | 95±14.07 | 27.9±0.41 | 28.2±1.07 | 29.4±0.97 |
| PR3 | nd | nd | +4 | nd | nd | 97.7±11.8 | 19.5±0.58 | 18.2±1.37 | nd |
| PR4 | nd | nd | +1 | nd | nd | 91.5±8.69 | 4.59±0.39 | 9.50±1.36 | 20.1±1.31 |
| PR5 | nd | nd | Nd | nd | nd | nd | 8.25±0.63 | 11.7±1.81 | 47.0±3.47 |
| PR6 | nd | 6 | Nd | nd | 37.5±2.63 | 121±17.0 | 4.66±0.33 | 9.00±1.32 | 13.5±0.77 |
| PR7 | 4 | 7 | +4 | 200±4.51 | 85.2±7.83 | 92.5±12.8 | nd | 9.25±0.94 | 90.5±7.60 |
| PR8 | nd | nd | Nd | 80.1±8.93 | nd | 85.7±9.37 | 14.5±0.24 | 14.2±2.13 | 53.6±3.63 |
| PR9 | nd | nd | Nd | 79.2±3.41 | nd | 81.5±8.72 | nd | 17.0±3.21 | 33.5±1.13 |
| PR10 | nd | nd | Nd | 75.0±7.05 | nd | 98.7±11.6 | 25.7±0.53 | 14.6±4.34 | 61.3±2.52 |
| PR11 | nd | nd | Nd | 42.7±4.74 | nd | 88.2±14.5 | nd | nd | 35.7±1.05 |
| PR12 | nd | nd | Nd | 50.1±4.85 | nd | 107±17.3 | 33.6±0.35 | 31.2±0.47 | nd |
| RE1 | 3 | nd | +3 | 68.2±3.01 | 167±17.1 | 153±16.5 | nd | 5.5±0.27 | 32.1±1.07 |
| RE2 | nd | nd | Nd | 83.7±7.32 | 116±14.2 | 110±10.4 | 5.56±0.23 | 45.2±4.54 | 47.2±2.67 |
| RE3 | nd | 3 | +3 | nd | 195±15.7 | 169±15.3 | nd | 14.8±2.07 | 26.2±2.94 |
| RE4 | nd | 5 | +4 | 73.1±5.06 | 269±13.5 | 208±14.9 | 5.16±0.46 | 9.92±2.35 | 13.5±0.73 |
| RE5 | 2 | nd | +2 | 9.29±0.41 | 180±12.1 | 187±15.4 | 3.91±0.17 | 27.9±5.86 | 39.3±1.05 |
| TE1 | nd | 8 | +3 | 130±12.3 | 134±12.4 | 130±12.9 | nd | 25.9±3.7 | 45.7±4.62 |
| TE2 | 2 | 2 | +3 | nd | 209±15.8 | 132±14.7 | 5.13±0.54 | 16.2±4.12 | 52.3±7.49 |
| PE1 | nd | nd | +3 | 49.8±4.91 | 246±13.2 | 138±12.4 | nd | 9.5±0.42 | 24.3±3.74 |
| PE2 | nd | nd | Nd | 56.4±4.63 | 210±14.8 | 100±11.0 | 3.61±0.33 | 11.7±1.83 | 17.4±0.63 |
Values are means (±SE) (n=3); nd, not detected; a Pectinase activity determined in terms of solubilization zone diameter (mm) ; b Cellulase activity determined in terms of solubilization zone diameter (mm); c The qualitative N2 fixation assay based on intensity of colour change in nitrogen-free bromothymol blue medium (+1 through +5, light blue to very dark blue); d Indole-acetic acid like substances production in terms of μg ml-1 hr-1; e Dissolution of Ca3(PO4)2 in terms of presence of solubilized P in μg ml-1 hr-1; f Mineralization of Na-phytate in terms of presence of solubilized P in μg ml-1 hr-1; gDissolution of AlPO4 in terms of presence of solubilized P in μg ml-1 hr-1; hDissolution of FePO4 in terms of presence of solubilized P in μg ml-1 hr-1; i1-aminocyclopropane-1-carboxylate deaminase (ACCD) production in terms of hmol mg-1 hr-1.
Table 5: The multifaceted PGP traits of root associated bacteria (Rhizospheric and endophytic) from TM collected from 20 years fallow Jhum cycle.
| Isolate | Pect.a | Cell.b | N2-fix.c | IAAd | Dissolution/or mineralization of insoluble phosphates | ACCDi activity | |||
| Ca3(PO4)2 e | Na-phytatef | AlPO4 g | FePO4 h | ||||||
| RR1 | nd | 5 | +3 | 29.4±3.84 | 101±17.3 | 125±14.3 | nd | nd | nd |
| RR2 | nd | nd | +2 | nd | 74.0±8.66 | nd | nd | nd | nd |
| RR3 | nd | nd | +3 | nd | nd | nd | nd | nd | 24.7±1.90 |
| RR4 | nd | nd | +1 | 21.0±6.23 | nd | nd | nd | nd | 30.1±2.04 |
| RR5 | nd | 7 | +2 | 28.5±6.3 | 141±16.2 | 141±13.8 | nd | nd | 24.3±3.61 |
| RR6 | nd | 11 | nd | 52.9±6.02 | 83.2±7.92 | 141±17.3 | nd | nd | 32.5±3.75 |
| RR7 | nd | 10 | nd | 43.2±3.94 | 89.5±7.01 | 101±12.4 | nd | nd | 9.2±0.54 |
| RR8 | nd | nd | +2 | nd | nd | nd | nd | nd | 14.2±0.83 |
| RR9 | nd | 8 | +4 | nd | nd | 95±13.5 | nd | nd | 24.9±2.63 |
| RR10 | nd | 8 | +3 | 28.5±5.11 | nd | nd | nd | nd | nd |
| RR11 | nd | nd | nd | nd | nd | nd | nd | nd | 39.5±3.89 |
| RR12 | nd | 14 | nd | nd | nd | 78.2±9.46 | nd | nd | nd |
| RR13 | nd | nd | +3 | 185±14.1 | nd | 83.7±8.4 | nd | nd | nd |
| RR14 | nd | 9 | nd | nd | 110±13.6 | 32.5±4.49 | nd | nd | 25.1±4.41 |
| RR15 | nd | nd | nd | 52.2±10.9 | nd | 103±14.7 | nd | nd | 19.7±4.05 |
| RR16 | 2 | nd | +1 | 39.0±4.82 | 108±14.7 | 108±16.2 | nd | nd | nd |
| RR17 | nd | nd | +1 | 51.0±8.72 | nd | nd | nd | nd | 9.40±0.83 |
| TR1 | nd | nd | nd | nd | nd | 92.0±7.35 | 14.7±0.93 | nd | nd |
| TR2 | nd | nd | nd | 39.0±4.39 | nd | 110±11.8 | nd | nd | 15.8±1.54 |
| TR3 | nd | nd | +3 | nd | nd | 33.1±5.36 | nd | nd | nd |
| TR4 | nd | nd | +2 | 41.5±7.21 | 36.2±3.72 | 89.2±9.02 | nd | nd | 7.60±2.38 |
| TR5 | nd | nd | nd | 42.6±5.83 | 35.2±6.57 | 83.7±8.4 | nd | nd | nd |
| TR6 | nd | nd | nd | nd | 60.5±5.79 | 76.0±7.92 | nd | nd | nd |
| TR7 | nd | 9 | nd | nd | 40.2±6.05 | 87.2±8.12 | nd | nd | 6.60±5.93 |
| TR8 | nd | nd | nd | nd | 47.5±4.32 | nd | nd | nd | nd |
| TR9 | nd | nd | +2 | 37.9±7.64 | nd | 79.5±12.1 | nd | nd | 33.0±4.34 |
| TR10 | nd | nd | +1 | 73.2±11.2 | 48.5±7.31 | 60.5±7.9 | nd | nd | nd |
| PR1 | nd | nd | nd | nd | nd | 73.7±5.07 | nd | nd | nd |
| PR2 | 10 | nd | nd | nd | 44.5±3.88 | 72.2±6.49 | nd | 13.5±0.73 | 11.7±2.33 |
| PR3 | nd | nd | nd | 41.4±7.3 | 36.2±3.18 | 38.7±5.65 | nd | nd | 8.70±1.03 |
| PR4 | 2 | nd | +1 | nd | nd | 31.5±5.2 | 5.22±0.43 | nd | nd |
| PR5 | nd | nd | +1 | nd | nd | 48.5±7.31 | nd | nd | 11.5±2.5 |
| PR6 | nd | nd | nd | nd | nd | 27.8±3.92 | nd | 8.75±0.29 | nd |
| PR7 | nd | nd | nd | 27.0±4.61 | 48.5±5.75 | 36.5±4.17 | nd | 3.00±0.21 | 7.30±3.1 |
| RE1 | nd | nd | nd | 16.0±4.02 | 179±15.7 | 178±16.3 | nd | nd | nd |
| RE2 | nd | 4 | +1 | 22.9±6.21 | 41.7±6.93 | 62.9±11.4 | 7.07±1.34 | 40.2±6.32 | 27.1±4.23 |
| RE3 | nd | nd | +3 | 34.9±4.03 | 189±19.2 | 90.3±10.9 | nd | nd | 23.8±5.8 |
| RE4 | nd | nd | nd | 77.2±10.2 | nd | nd | nd | nd | nd |
| RE5 | nd | nd | nd | nd | nd | 76.2±8.32 | nd | nd | nd |
| TE1 | nd | 2 | +1 | 72.3±7.9 | nd | 58.1±4.79 | nd | nd | 11.5±3.09 |
| TE2 | nd | nd | nd | 60.3±11.4 | 136±13.7 | 180±14.1 | nd | nd | nd |
| TE3 | nd | nd | nd | nd | 180±13.1 | 65.3±4.22 | nd | nd | 12.1±5.73 |
| PE1 | 1 | nd | +2 | nd | 61.5±8.33 | 61.6±6.3 | nd | 32.0±2.04 | nd |
Values are means (±SE) (n=3); nd, not detected;a Pectinase activity determined in terms of solubilization zone diameter (mm);b Cellulase activity determined in terms of solubilization zone diameter (mm);c The qualitative N2 fixation assay based on intensity of colour change in nitrogen-free bromothymol blue medium (+1 through +5, light blue to very dark blue);d Indole-acetic acid like substances production in terms of μg ml-1 h-1;e Dissolution of Ca3(PO4)2 in terms of presence of solubilized P in μg ml-1 hr-1; f Mineralization of Na-phytate in terms of presence of solubilized P in μg ml-1 hr-1; gDissolution of AlPO4 in terms of presence of solubilized P in μg ml-1 hr-1; hDissolution of FePO4 in terms of presence of solubilized P in μg ml-1 hr-1; i1-aminocyclopropane-1-carboxylate deaminase (ACCD) production in terms of hmol mg-1 hr-1.
In case of 9 endophytic strains from TMF5, 3 isolates for pectinase activity, 4 for cellulolytic activity, 7 for both N fixation and IAA-like substances production and 5 for dissolution of iPin AlPO4 were found to be positive. All isolates showed positive result while screening for iPdissolution in Ca3 (PO4)2, Na-phytate and FePO4; and production of ACCD. For sample TMF20, out of 9 endophytes production of pectinase, cellulase, IAA-like substances and ACCD; fixation of N2; and solubilization of iP in media amended with Ca3(PO4)2, Na-phytate, AlPO4 and FePO4 were observed in 1, 2, 4, 6, 6, 8, 1, 2 and 4 numbers of bacteria, respectively.
 |
Figure 2: The effect of length of fallow phase on the activities of soil enzymes. The comparison between F5 and F20 fallow phases or between rhizopheric and bulk soils within a fallow phase was determined by performing the paired t-test. The significance value (P £0.05) was indicated by paired arrows. Click here to View Figure |
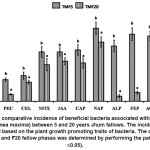 |
Figure 3: The comparative incidence of beneficial bacteria associated with broom grass (Thysolanea maxima) between 5 and 20 years Jhum fallows. The incidence was determined based on the plant growth promoting traits of bacteria. The comparison between F5 and F20 fallow phases was determined by performing the paired t-test (P £0.05). Click here to View Figure |
Discussion
The significance of TM as ESP in rejuvenating the biological activities (greater population of rhizobacteria and root endophytic bacteria and higher activities of soil enzymes) in burnt soils of shorter Jhum cycle has been demonstrated conclusively for the first time through this manuscript. In both fallow phases, activities of DHA, ASA, PHA, AMY, GSA and PRO were significantly higher (p < 0.05) in rhizosphere of TM than in bulk soils (Fig. 2). Higher activities of protease, invertase, cellulase, urease, and acid phosphatase in rhizospheric soils than bulk soils were also reported in earlier studies.27 However, the activities of these enzymes in rhizospheric soil of TM collected from F5 fallow phase were significantly (p < 0.05) higher compared to that in F20 fallow phase (Fig. 2). Comparing bulk soils of two fallow phases, activities of DHA, ASA, PHA, AMY, GSA and PRO were found to be significantly higher (p < 0.05) for F20 fallow phase as compared to those of F5 fallow phase (Fig. 2). Availability of complex types of substrates in greater amount in longer fallow phase leads to secretion of mineralizing enzymes from increased soil microbes.28 In a study conducted by Lungmuanaa et al.,29 activity of soil enzymes were found to be decreased after burning but gradually increased with time.
The root-associated bacterial population was more in TM from longer (F20) fallow phase (Table 3). Rhizobacteria and endophyte counts in rhizospheric soil and root sample of TMF20 were found to be 0.74 x107 cfu and 0.083 x104 cfu, respectively, whereas for TMF5 rhizobacteria and endophyte counts were found to be 0.27×107cfu and 0.05 x104 cfu, respectively. This is because, in longer Jhum cycle, the quantity of organic matter was high, so more number of macro- and microfaunal population exists to accomplish their decomposition.30 Hence from a greater microbial reservoir higher number of bacteria colonizes the growing roots of TM in F20 fallow phase. However the percent incidence of plant beneficial bacteria isolated from TMF5 was higher than that from TMF20 (Fig 3). In TMF5 the incidence of root associated pectinase and cellulase producers; N2 fixers; IAA like substances producers; iP solubilizers in Ca3(PO4)2,Na-phytae, AlPO4 and FePO4; and ACCD producers were found to 28.9, 34.2, 65.8, 65.8, 68.4, 97.4, 73.7, 89.5 and 86.8 %, respectively; whereas the same in TMF20 was found to be 9.3, 25.6, 48.4, 53.5, 51.2, 79.1, 7.0, 11.6 and 53.5%, respectively. In fact, higher activities of enzymes in rhizospheric soils were indication of a greater functional diversity of the microbial community.31 Plant root exudates (basically the low molecular weight compounds) acts as microbial substrate or metabolic activators to stimulate activity of these enzymes.32, 33 Again secretion of root exudates increases under the stress of nutrients and water.34 As these stresses were more in soils from F5 than F20,35 the higher activity of enzymes in rhizospheric soils could be linked with higher microbial secretion which was further influenced by release of more root exudates from TMF5.
In case of F5, soil is under more stressed condition compared to F20 soil. In F5 Jhum agroforestry, natural rebuilding process of ecosystem was destroyed due to frequent burning. Because of more nutrient availability in soil of F20 vegetation, the dependency of plant on soil microbes for their growth was minimized. In rhizosphere, the availability of nutrients was controlled by the combined effects of plant characteristics, soil properties and the interaction of roots with microorganisms.36 But in soil of F5 Jhum field, the growth of new vegetation was much more dependent on efficient and positive root-microbe interactions. Incidence of more PGPB in rhizosphere of TMF5 indicated that under stressed situation the root system of plant TM accommodated only those bacteria which would be beneficial for their growth. According to earlier reports, under stress conditions plant changes their root associated bacteria,37 which is also mainly guided by the rhizodeposition from root.38 To enhance nutrient availability in soil, more number of beneficial microbes is attracted to the rhizospheric soil.39 As plants offer a suitable environment where only some selective microorganisms can exist,40 thus filtering out the beneficial group of specific microbes only from the pool.
In conclusion, early generating plant species like TM plays important role in rejuvenating the biological activities in the burnt soils for shorter Jhum cycle and this process helps in re-establishing the linkages between above-ground and below-ground biota communities resulting gradual improvement in soil nutrient cycling processes.
Conclusion
The early generating plant species like TM plays important role in rejuvenating the biological activities in burnt soils of shorter Jhum cycle and this process facilitates in establishing the linkages between above-ground and below-ground biota communities leading to the gradual improvement in functioning of degraded Jhum lands.
Conflict of Interest
Authors declare no conflict of interest.
Acknowledgements
We thank the Department of Biotechnology, Govt. of India for financial support vide research project no. DBT-NER/Agri/14/2012 dated 31- 10-2012 and the College of Post-Graduate Studies, Central Agricultural University (Imphal), Umiam, Meghalaya for laboratory facilities.
References
- Yadav P. K., Slash-and-Burn Agriculture in North-East India. Expert Opin Environ Biol; 2013; 2: 1.
CrossRef - Ranjan R., Upadhyay V.P. Ecological problems due to shifting cultivation. Publishing web, 2001.
- Mertz O. The relationship between length of fallow and crop yields in shifting cultivation: a rethinking. Agroforest Syst; 2002; 55: 149-159.
CrossRef - Kalita H.C., Ram V. Weed Diversity in Different Fallow Cycle of Slash and burn Agriculture in Northeast India. The Bioscan; 2017; 12(4): 1987-1992.
- Yang S., Liao B., Xiao R., Li J. Effects of Amendments on Soil Microbial Diversity, Enzyme Activity and Nutrient Accumulation after Assisted Phytostabilization of an Extremely Acidic Metalliferous Mine Soil. Applied Sciences; 2019; 9: 1552.
CrossRef - Safi U. R. Q., Haroon, Arfa S. An Overview on Microorganisms Contribute in Increasing Soil Fertility. Biomed J Sci & Tech Res; 2018; 2(1): MS.ID.000641.
CrossRef - Saplalrinliana H., Thakuria D., Changkija S., Hazarika S. Impact of Shifting Cultivation on Litter Accumulation and Properties of Jhum Soils of North East India. J Indian Soc Soil Sci; 2016; 64(4): 0-0.
CrossRef - Page A. L., Miller R. L., Keeny D. R. Methods of soil analysis. In: Part-2 chemical and microbiological properties, 2nd edn. Agron Monogr; 1982; 9: 961-1010.
- Nelson N. A photometric adaptation of the Somogyi method for the determination of glucose. J Biol Chem; 1944; 153: 375-380.
- Tabatabai M. A., Bremner J. M. Arylsulfatase Activity of Soils. Soil Sci Soc Am J; 1970; 34(2): 225-229.
CrossRef - Dick R. P., Breakwell D. P., Turco R. F. Soil enzyme activities and biodiversity measurements as integrative microbiological indicators. In: Doran, J.W., Jones, A.J. (Eds.), Methods of Assessing Soil Quality. SSSA special publication 49, Soil Science Society of America, Madison, WI, USA, 1996, 247-271.
- Casida L., Klein D., Santoro T. Soil Dehydrogenase Activity. Soil Science; 1964; 98: 371-376.
CrossRef - Tabatabai M. A., Bremner J. M. Use of p–nitrophenyl phosphate for assay of soil phosphatase activity. Soil Biol Biochem; 1969; 1: 301-307.
CrossRef - Valsange A., Evarkar S. P., Tawde S. V., Kareppa B. M., Gujar R. S. Analysis of protease activity of enzyme isolated from compost soil. International Multidisciplinary Research Journal; 2012; 2(6): 01-04.
- Thokchom E., Kalita M. C., Talukdar N. C. Isolation, screening, characterization and selection of superiorrhizobacterial strains as bioinoculants for seedling emergenceand growth promotion of Mandarin orange (Citrus reticulate Blanco). Can J Microbiol; 2014; 60: 85–92.
CrossRef - Zhao L., Xu Y., Lai X. H., Shan C., Deng Z., Ji Y. Screening and characterization of endophytic Bacillus and Paenibacillus strains from medicinal plant Lonicera japonica for use as potential plant growth promoters. Braz J Microbiol; 2015; 46(4): 977-989.
CrossRef - Haripriya R., Thirumalaivasan P. Isolation of cellulolytic bacteria and production of cellulase from coir pith. Int J Res Instinct; 2017; 4(1): 13-23.
- Kasing A. Cellulase production. National Centre for Biotechnology Education, Sarawak, Malayasia, 1995.
- Okon Y. Azosiprillum as a potential inoculants for agriculture. Trends Biotechnol; 1985; 3(9): 223-228.
CrossRef - Patel M. V., Patel R. K. Indole-3-Acetic Acid (Iaa) Production by Endophytic Bacteria Isolated from Saline Dessert, the Little Runnof Kutch. Cibtech Journal of Microbiology; 2014; 3 (2) 17-28.
- Ahmad F., Ahmad I., Khan M. S. Screening of free-living rhizospheric bacteria for their multiple plant growth promoting activities. Microbiol Res; 2008; 163: 173-181.
CrossRef - Pandey D., Putatunda C. Isolation and Characterization of Phosphate Solubilizing Bacteria from the Rhizosphere of Potato Plant. Int J Curr Microbiol App Sci; 2018; 7(1): 967-975.
CrossRef - Bray R. H., Kurtz, L. T. Determination of total, organic and available forms of phosphorous in soil. Soil Science; 1945; 59: 39-45.
CrossRef - Honma M., Shimomura T. Metabolism of 1-aminocyclopropane1-carboxylic acid. Agricultu Biol Chem; 1978; 42: 1825-31.
CrossRef - Penrose D. M., Glick B. R. Methods for isolating and characterizing ACC deaminase-containing plant growth-promoting rhizobacteria. Physiol Plant; 2003; 118: 10-15.
CrossRef - Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem; 1976; 7(72): 248-54.
CrossRef - Xiao S., You H., You W., Liu J., Cai C., Wu J., Ji Z., Zhan S., Hu Z., Zhang Z., He D. Rhizosphere and bulk soil enzyme activities in a Nothotsuga longibracteata forest in the Tianbaoyan National Nature Reserve, Fujian Province, China. J For Res; 2017; 28: 521.
CrossRef - Wallenius K., Rita H., Mikkonen A., Lappi K., Lindstrom K., Hartikainen H., Raateland A., Niemi R.M. Effects of land use on the level, variation and spatial structure of soil enzyme activities and bacterial communities. Soil Biol Biochem; 2011; 43: 1464e1473.
CrossRef - Lungmuanaa, Singha S. B., Vanthawmlianaa, Sahaa S., Duttaa S. K., Rambuatsaihab, Singha A. R., Boopathia T. Impact of secondary forest fallow period on soil microbial biomass carbon and enzyme activity dynamics under shifting cultivation in North Eastern Hill region, India. Catena; 2017; 156: 10-17.
CrossRef - Reza S. K., Baruah U., Nath D.J., Sarkar D., Gogoi D. Microbial biomass and enzyme activity in relation to shifting cultivation and horticultural practices in humid subtroipcal North-Eastern India. Range Manag. Agrofor; 2014; 35: 78-84.
- Gianfreda L. Enzymes of importance to rhizosphere processes. J Soil Sci Plant Nutr; 2015; 15(2): 283-306.
CrossRef - Renella G., Landi L., Valori F., Nanniiperi P. Microbial and hydrolase activity after release of low molecular weight organic compounds by a model root surface in a clayey and a sandy soil. Appl Soil Ecol; 2007; 36: 124-129.
CrossRef - Renella G., Landi L., Garcia Mina J. M., Giagnoni L., Nanniiperi P. Microbial and hydrolase activity after release of indoleacetic acid and ethylene–polyamine precursors by a model root surface. Appl Soil Ecol; 2011; 47: 106-110.
CrossRef - Henry A., Chard J., Norton J., Petersen M., Bugbee Bruce., Hamilton M., Palmer C., Hess J. R. Water & Nutrient Stress Increase Root Exudation. Water Stress; 2002; Paper 2.
- Devi N. L., Choudhury B. U. Soil fertility status in relation to fallow cycles and landuse practices in shifting cultivated areas of Chandel district Manipur, India. IOSR J Agric Vet Sci; 2013; 4(4): 01-09.
CrossRef
- Jones D. L., Hodge A., Kuzyakov Y. Plant and mycorrhizal regulation of rhizodeposition. New Phytol; 2004; 163: 459-480.
CrossRef
- Timm C. M., Carter K. R., Carrell A. A., Jun Se-R., Jawdy S. S., Vélez J. M., Gunter L. E., Yang Z.,Nookaew I.,Engle N. L.,Lu T.Y. S.,Schadt C. W., Tschaplinski T.J.,Doktycz M. J.,Tuskan G. A.,Pelletier D. A.,Weston D.J., Abiotic Stresses Shift Belowground Populus- Associated Bacteria Toward a Core Stress Microbiome. mSystems; 2018; 3(1): e00070-17.
CrossRef - Pétriacq P., Williams A., Cotton A., McFarlane A. E., Rolfe S. A., Ton J. Metabolite profiling of non-sterile rhizosphere soil. Plant J; 2017; 1-16.
CrossRef - Rengel Z., Marschner P. Nutrient availability and management in the rhizosphere: exploiting genotypic differences. New Phytol; 2005; 168: 305-312.
CrossRef - Hallmann J., Berg G. Spectrum and population dynamics of bacterial root endophytes. In: Schulz B.J.E., Boyle C.J.C., Sieber T, N., editors. Microbial Root Endophytes. Springer; Dordercht, NL, 2006; 15-31.
CrossRef



