Introduction
Hydroponics is a method of growing plants in soil-less culture and is also known as hydro-culture or nutrient solution culture. In this method, plants are grown with their roots exposed to mineral solution throughout. The roots can be supported by a material like perlite or gravel or coco peat. The nutrient medium used in hydroponics can be by-products of vegetable waste, fish waste or liquid chemicals.1 Hydroponic system thus involves plant growth without the use of soil medium and hence is also known as soil-less culture.2 Hydroponic technology is one of the most favored, hi-tech production systems having the scope to expand to agriculture development in India. This technique if used properly can overcome problems of water availability, space for plant growth, diseases, pests and soil problems.3
The first studies on hydroponics were started at the Bengal Government experimental farm at Kalimpong in the Darjeeling District.2 About seven farmers in south Gujarat adopted this technology for growing different varieties of exotic hybrid tea roses. Localized experiments showed that other exotic crops like strawberry, green garlic and tomatoes can also be grown using this technology. Landmark Agrotech project is the second biggest hydroponics projects in Gujarat and is currently under implementation.2 Hydroponics has potential to utilize water lands having poor soil quality. Bengluru based Iot and data analysis start up Bit Mantis Innovation with its Iot solution ‘Green SAGE’ enables individuals and commercial growers to conveniently grow fresh herbs throughout the year. The ‘Green SAGE’ is a micro-edition kit that uses hydroponics methods for efficient use of water and nutrients.4,5 One of the offices of HiMedia Laboratories in Mumbai, works on basic research related to plants as an aid to promote hydroponics. Hi-Media’s new company ‘Higronics’ is developed to focus on providing plant-specific nutrient.6
Letcetra Agritech was India’s first hitech vertical hydroponics indoor farm set up in the year (2016) by its co-founder and CEO Ajay Naik. His hydroponic project focused on organically grown pesticide free vegetable plants under controlled conditions. Ajay’s transition from information technology to farming has been a novel one in recent years.
Due to the non availability of irrigated lands for fodder production, higher labour cost and small land holdings, dairy farmer of Goa were left with challenges for milk production in the state. This led to the need of hydroponic technology in the that could overcome the problems of conventional green fodder production amongst dairy farmers. This problem was tackled through fodder production by use of hydroponics. Initiative was taken by Rashtriya Krishi Vikas Yojana, in collaboration with Department of Agriculture Government of Goa and Goa State Co-operative Milk Producers Union Ltd, Curti Ponda in the year 2011-2012.
Available literature showed that hydroponically grown crop plants have increased in recent years worldwide allowing more efficient use of water and fertilizer as well as a better control of climate and pest factors. Amongst the factors affecting hydroponic production systems, nutrient solution is considered to be one of the most important one. It determines crop yield and quality. The success or failure of the soil-less culture therefore, depends primarily on the nutrient management programmes. Various researchers that work on hydroponics have shown great profit along with microbe free food produce.6
Hydroponics is a new concept and adds greenery to home gardens, offices, airports and industrial landscapes. It requires less efforts, less skills and is easy to be implemented. Although, the technology is successfully practiced, much more needs to be done in terms of understanding the physiology of plants under cultivation. In view of this, the present work was planned with an objective, to compare the physiological and biochemical parameters in plants grown in soil and in soilless culture (hydroponics or water culture).
Material and Methods
Plant material
Seeds of pisum sativum (pea), Abelmoschus esculentus (okra) and Vigna radiata (moong) were purchased from local farmer. The seeds were sterilized in 0.1% mercuric chloride for 1 minute and cleaned repeatedly using distilled water. The seeds were later soaked in water for 24 hours and allowed to germinate. After 48 hours of germination 3 sets of each seed type were grown in soil and 3 sets were grown in hydroponics culture. Four week old seedlings were used for morphological, physiological and biochemical studies.
Experimental set of Hydroponic culture
Our hydroponic setup was constructed using thermocol board with holes dug in it to fit the pots filled with sterile coco-peat. The thermocol sheet was then placed in a tray in such a way that it stood above the tray surface holding the pots (Figure 1). The tray was filled with nutrient solution and were placed in the laboratory at 26⁰C ± 20C temperature and 800 µmol of light intensity.
 |
Figure 1: Pea seedlings grown in soil (left) and in hydroponics. (right) Click here to View Figure |
 |
Figure 2: Okra seedlings grown in soil (left) and in hydroponics. (right) Click here to View Figure |
 |
Figure 3: Moong sedlings grown in soil (right) and in hydroponics. (left) Click here to View Figure |
 |
Figure 4: Extensive rooting in pea seedlings grown in hydroponics. Click here to View Figure |
We also designed another setup involving plywood and plumbing pipes (Figure 5). Appropriate holes were dug on the pipe to hold the pots filled with coco-peat. These pipes were connected to a reservoir water bucket enclosed with a submerged motor that provided continuous water flow into the system. The setup was placed in a laboratory conditions maintained at similar temperature and light conditions. This setup can also be used as an alternative method of growing plants hydroponically (data not shown in this paper).
 |
Figure 5: Hydroponic experimental setup 2. Click here to View Figure |
Parameters studied (Morphological, physiological and biochemical)
Morphological and physiological parameter studied were root shoot ratio and relative water content
Root shoot ratio7
Four week old seedlings from each setup were uprooted without damaging the root system and washed under running tap water. They were placed on a plane sheet of paper and the length of root and shoot in centimeter (cm) were noted to calculate root: shoot ration. Average of five plants from each setup was taken.
Relative water content (RWC)8
Leaves of similar sizes from the plants under study were taken and weighed for fresh weight (FW). They were then kept in water for 30 minutes. After 30 minutes, excess water was removed from the surface using blotting paper. Leaves were re-weighed to get turgid weight (TW). These leaves were placed in hot air oven for complete dryness and were weighed to get dry weight (DW).
Relative water content (RWC) was calculated using the following formula:
RWC = (FW – DW)/(TW – DW) X 100
Biochemical studies
Chlorophyll content9
Leaf tissue (0.1gm) was weighed and homogenized using mortar and pestel with 10 ml of 80% acetone. The extract was centrifuged at 5,000 rpm for five minutes. Supernatant was transferred in another test tube and absorbance of the solution was read at 645 and 663 nm. Acetone (80%) was used as blank.
Total sugar content10
Leaf tissue (200 mg) was weighed and homogenized in 10 ml of 80% hot alcohol. It was centrifuged at 5000 rpm for 15 min. The supernatant was evaporated on evaporating dish using a water bath. The residue was then dissolved in distilled water making the total volume to 10 ml. Series of different concentration (0.2, 0.4 and 0.5 ml) from the above solution were taken in separate test tube. 1 ml of 5% phenol was added to each tube followed by mixing the contents. 5ml of concentrated sulphuric acid was then added to each test tube and mixed. Contents of the tube were cooled at room temperature and absorbance was read at 490nm. Total sugars (mg/g F.w.) were calculated using glucose as standard.
Total protein content11
Leaf tissue (0.5 grams) was weighed and homogenized in 5 ml of phosphate buffer (pH 7). It was centrifuged at 5000 rpm for 30 min. The supernatant was diluted with equal amount of water. Series of different concentration (0.2, 0.4, 0.6, 0.8 and 1.0 ml) were taken in separate test tubes using the above solution and volume was made to 1Ml using distilled water. Blank consisted of water alone. Reagent C (5 ml) was added to each test tube. They were allowed to stand for 10-15 min in a test tube stand. Folin reagent (0.5 ml) was then added to each of the test tubes followed by incubation at 37oC for 40 min. Appearance of violet color was read at 660 nm on a spectrophotometer. Protein content (mg/ml) was calculated using Bovine Serum Albumin (BSA) as standard.
Statistical Analysis
Statistical analysis was performed by using 1 way analysis of variance (ANOVA). All the experiments were repeated thrice and data presented were mean of three experiments. Statistical analysis was performed by using Microsoft Excel version -7
Results
Our results on root shoot ratio and RWC of moong, okra and pea seedlings grown in hydroponics and soil are shown in table 1. Results showed that hydroponically grown pea and okra seedlings had higher root shoot ratio in comparison to those grown in the soil. However, in moong seedlings the root shoot ratio was seen to be higher in soil grown plants than in hydroponics. Our results on relative water content (RWC) showed that the seedlings grown in soil had higher relative water content than the ones grown in hydroponics.
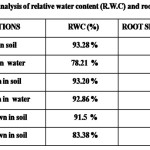 |
Table 1: Click here to View Table |
Total chlorophyll content
Our results on total chlorophyll content of seedlings (moong, pea and okra) grown in hydroponics and soil are shown in figure 6. The result showed higher chlorophyll content in the okra and moong seedlings grown in hydroponics as compared to the ones grown in the soil. However total chlorophyll was found to be more in soil grown pea plants in comparison to those grown hydroponically.
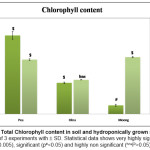 |
Figure 6: Total Chlorophyll content in soil and hydroponically grown seedlings. Click here to View Figure |
Total sugar and total protein content
Our experimental results of total sugars and total protein content in pea, okra and moong, are shown in figure 7. There was slight variation in total sugars and protein content in pea and moong seedlings grown in soil and in hydroponics. Sugar and protein content in soil grown okara and moong seedlings were slightly higher in comparison to those grown hydroponically. We also observed more protein in hydroponically grown pea seedlings than the ones grown in soil.
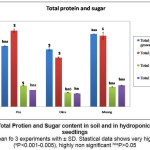 |
Figure 7: Total Protien and Sugar content in soil and in hydroponically grown seedlings. Click here to View Figure |
Discussion
Hydroponics has emerged as a agricultural branch for plant growth with no usage of soil. All necessary nutrients that a plant normally gets through soil can be supplied in a dissolved state in water or nutrient medium in a hydroponic setup.
Hydroponics success depends on health of the seedlings, instruments used for hydroponics, the culture media and the seeds. Contamination risks of seeds can be taken care by proper sterilization of the seeds before transplanting to hydroponics setup. Other factors that need to be considered in hydroponics system are proper light intensity, oxygen and temperature at the setup.11 Freshness of the nutrient medium determines the success of the soilless culture and directly influences the plant heath. Replacement of hydroponic solution at least twice a week is recommended for better plant growth.12 Large containers if used should be well aerated or be equipped with air pump system. In such cases frequent replacement of nutrient solution throughout the experiments is not necessary.12
Phenotypic studies of plants and their response to different nutrient conditions can be studied by hydroponic experiments.13 Although it is difficult to completely eradicate plant pathogens in the root zone in hydroponic culture, it is necessary to maintain the sterile root-zone environment for good plant vigour.14 In comparison to soil grown plants, the risk of soil-borne insect, pest attacks, weed growth etc. can be minimized by growing plants in a homogenous nutrient medium. Soil-less cultures thus appear to be an efficient nutrient regulating system with dense planting, improved quality and yield of the produce. In our study the root shoot ratio of the seedlings grown in hydroponics was found to be higher as compared to the seedlings grown in soil. This could be because the roots are in direct contact with water through-out and hence it grew longer and fibrous. Hydroponically grown roots although longer were fragile in comparison to sturdy roots of soil grown seedlings. Research done on Gledistsia tricanthos L. var. inermis15 wild (honey locust) found that the root growth was similar to that of our study. They also reported longer roots with less secondary growth of plants grown hydroponically in comparison to those grown in soil.
In another study on plant nutrition, where plants were grown on various zinc concentrations gradients showed that plants grown in lower Zn concentration had more roots in comparison to those grown with higher Zn concentrations. This may be because microelement like Zn is directly related to growth and metabolism and can be taken by plant roots directly through their roots suspended in water.16
All hydroponic cultures rely on a nutrient solution to deliver essential elements to the plants. In addition to the nutrients roots also need a steady supply of oxygen. Under anoxic conditions roots are unable to transport metabolites to rest of the plant. In hydroponics, nutrient solution can either be saturated with air prior to its use and changed frequently, or air can be continuously supplied in solution throughout plant life cycle.13
Our biochemical analysis included total chlorophyll content, total sugars and total proteins.
Possible reason for increased chlorophyll content in moong and pea could be because the roots being in direct nutrient solution, uptake of magnesium is favoured accounting for more chlorophyll content in them. Okra plant having larger leaf expansion than pea and moong, may have developed less chlorophyll content in soil grown conditions.
The results have shown that soil grown seedlings have more protein content than the one’s grown hydroponically except that in pea plant. Sugar content did not reveal much changes. However, hydroponically grown okra and moong seedlings showed higher total chlorophyll content. Amount of chlorophyll in leaf is related to plant nutrient status. A similar study was done in spinach that showed increased sugar and protein content in aquaponics and in hydroponics than the ones grown in soil conditions.17
Conclusions
From our result, we conclude that root shoot ratio of seedlings grown in hydroponics was seen to be higher than the seedlings grown in soil. Hydroponic cultures of vegetable crops with more leaf biomass is more beneficial. No significant change was observed in the total sugar content in plants grown hydroponically and in soil. Total protein content was higher in hydroponically grown seedlings than the ones grown in soil. Overall soil appears to be better environment for efficient growth and metabolism of shrubby and higher plants. Soil can also increase shelf life of a plant providing a natural environment. However, hydroponics is an alternative method suitable for leafy vegetable seedlings. Hydroponic technology does not require conventional approach of farming requires less space and can be practiced as a hobby. Hydroponic culture can best be suitable for herbaceous seedlings only.
Acknowledgment
The authors would like to thank Plant Physiology laboratory funded by DST (SERB) grant, Department of Botany, Parvatibai Chowgule College for necessary laboratory facilities to undertake the work.
References
- Santos, J.D., A.L. Lopes da Silva, J.L. Costa, G.N. Scheidt, A.C. Novak, E.B. Sydney and C.R. Soccol. 2013. Development of a vinasse nutritive solution for hydroponics. J Environ Manage., 114: 8-12.
CrossRef - Sanchar Pal. Growing soil-less with Hydroponics: An introduction to innovative farming at home. The better India. 2016 https://www.thebetterindia.com
- Shalini K., Pratibha P., Ranjeet Y., Santosh K. Hydroponic technique: a soilless cultivation in agriculture. Journal of pharmacognosy and phytochemistry, 2018; (7) 1-6.
- Pandey R., V. Jain and K. P. Singh, “Hydroponics Agriculture: Its Status, Scope and Limitations,” in Division of Plant Physiology, Indian Agricultural Research Institute, New Delhi, 2009; 20-29.
- Nidhi Singh. Hydroponics in India. correspondent, The Entrepreneur India Asia-Pacific.2017; https://www.entrepreneur.com/article/295531
- Karayamparambil, J., Bhaskar, R. Hydroponic: the future of agriculture. The free press journal. 2018. https://www.freepressjournal.in/business/hydroponics-the-future-of-agriculture.
- Mane V., Saratale G.D., Karadge B.A., Samant J.S. (2011). Studies on the effects of salinity on growth, polyphenol content and photosynthetic response in Vetiveria zizanioides (L) Nash. Emir. J. Food Agric; 23(1):59-70.
CrossRef - Turner, N. C. (1988). A pressure chamber for measurement of plant water potential. Irrigation Sci. 1988; (9)289-308.
CrossRef - Arnon, D. I. Copper enzymes in isolated chloroplasts polyphenol-oxidase in Beta vulgaris. Plant Physiol.1949; (24)1-15.
CrossRef - Michel Dubois, Gilles K. A., Hamilton J.K., Rebers P.A., Fred Smith. Colorimetric Method for Determination of Sugars and Related Substances. Analytical Chemistry, 1956;28( 3) 350-356.
CrossRef - Lowry O.H., Rosebrough, N.J., Farr A.L.,Randall R.J. Protein measurement with the Folin phenol reagent. J.Biol.Chem. 1951; 193-265.
- Kopittke P.M., McKenna B.A., Blamey F.P.C., Wehr J. B, Menzies N.W. Metal induced cell rupture in elongating roots is associated with metal ion binding strengths. Plant and Soil.2009; 322: 303–315.
CrossRef - Nguyen, N.T., McInturf, S.A., Mendoza-Cózatl, D.G. Hydroponics: A Versatile System to Study Nutrient Allocation and Plant Responses to Nutrient Availability and Exposure to Toxic Elements. 2016. J. Vis. Exp. (113), e54317, doi:10.3791/54317. http://www.jove.com/video/54371
CrossRef
- Pii, Y., Cesco, S., & Mimmo, T. Shoot ionome to predict the synergism and antagonism between nutrients as affected by substrate and physiological status. Plant Physiol. Biochem. 2015. (94), 48-56.
CrossRef - Sardare, M., Admane S. A Review on Plant without Soil –Hydroponics, International Journal of Research in Engineering and Technology. 2013; 2(3), 299-304.
CrossRef - Graves, W. R. Influence of hydroponic culture method on morphology and hydraulic conductivity of roots of honey locust. Tree Physiology. 1992; 11(2) 205-211.
CrossRef - Ranawade P.S., Tidke S.D., and Kate A.K. Comparative cultivation and biochemical analysis of Spinacia oleraceae grown in aquaponics, hydroponics and field conditions. Original research article. 2017; 6(4) 1007-1013.
CrossRef


