Introduction
Neolamarckia cadamba Miq., (Family-Rubiaceae) commonly called as Kadam is one of the best sources of raw material for plywood industry, besides pulp and paper production. Its leaves and barks have medical applications while dried barks can be used to relieve fever and as a tonic. The leaf extract can serve as a mouthwash. Other than medical applications, its leaves have also used as fodder to cattle.11 The leaf area, pruning frequency, moisture content and nutritional characters of this species outreached it as an excellent fodder tree, which used to meet the green, dry and concentrate feed utility. Such a multiple utility tree reached only little research attention. Hence, Forest College and Research Institute, Mettupalayam has undertaken the improvement program through progeny and clonal tests.
DUS testing is useful for identification of varieties, registration of varieties and plant variety protection (PVP) Act, for varietal information system and classification of varieties into different groups, and for genetic resources.2 The exhibited variation in the established genetic resources needs to preserve for protection of IPR (Intellectual Property Rights) genetics in the species.
Materials and Methods
Clonal evaluation trial of Neolamarckia cadamba was established at Forest College and Research Institute, Mettupalayam from the best performing trees of Neolamarckia cadamba. The clonal evaluation trial comprises twenty-five clones and these clones were subjected to DUS characterization for the characters viz., leaf shape, base shape, apex shape, leaf margin, leaf venation, base symmetry, leaf length in cm, leaf breath in cm, leaf petiole length in cm, Waxiness in upper side of Leaf, bark colour and bark texture and observations were recorded as per UPOV.12
Descriptors
Descriptors are developed based on the morphological assessment of Twenty-five Neolamarckia cadamba genetic resources for DUS characterization. The selection made on phenotypic assessment of character with distinctiveness for bark and leaf. Descriptors developed for qualitative (QL) and quantitative characteristics (QN) as per the procedure followed by Sivakumar et al.,10
Results and Discussion
Twenty-five Neolamarckia cadamba clones characterized for DUS traits in order to protect the genetic resources through possible IPR mechanism. Accordingly, the clones characterized for bark and leaf attributes, are presented in table 1 & 2. Considerable variation was recorded among the kadam genetic resources for the following characters viz., leaf shape, leaf base shape, apex shape, leaf length, leaf petiole length, Waxiness in upper side of leaf, bark colour and bark texture.
Table 1: Leaf DUS characterization of Neolamarckia cadamba
| Sl. No. | Characteristics | State | Distribution classes of descriptor | Source | Type of assessment |
| 1 | Leaf shape | Elliptic | 18 (72%) | MTPAC 01, MTPAC 02, MTPAC 03, MTPAC 04, MTPAC 05, MTPAC 09, MTPAC 10, MTPAC 11, MTPAC 13, MTPAC 14, MTPAC 15, MTPAC 17, MTPAC 18, MTPAC 21, MTPAC 22, MTPAC 23 MTPAC 24, MTPAC 25 | VG |
| Ovate | 2 (8%) | MTPAC 07, MTPAC 08 | |||
| Broadly ovate | 3 (12%) | MTPAC 06, MTPAC 12, MTPAC 19 | |||
| Nearly round | 2 (8%) | MTPAC 16, MTPAC 20 | |||
| 2 | Leaf base shape | Cordate | 19 (76%) | MTPAC 01, MTPAC 02, MTPAC 03, MTPAC 04, MTPAC 05, MTPAC 10, MTPAC 11, MTPAC 13, MTPAC 14, MTPAC 15, MTPAC 16, MTPAC 17, MTPAC 18, MTPAC 20, MTPAC 21, MTPAC 22, MTPAC 23, MTPAC 24, MTPAC 25 | VG |
| Obtuse | 5 (20%) | MTPAC 06, MTPAC 07, MTPAC 08, MTPAC 12, MTPAC 19 | |||
| Acute | 1 (4%) | MTPAC 09 | |||
| 3 | Leaf apex shape | Cuspidate | 6 (24%) | MTPAC 09, MTPAC 10, MTPAC 16, MTPAC 18, MTPAC 21, MTPAC 24 | VG |
| Acuminate | 9 (36%) | MTPAC 03, MTPAC 04, MTPAC 05, MTPAC 06, MTPAC 08, MTPAC 11, MTPAC 13, MTPAC 14, MTPAC 22 | |||
| Apiculate | 5 (20%) | MTPAC 02, MTPAC 17, MTPAC 19, MTPAC 22, MTPAC 25, | |||
| Acute | 3 (12%) | MTPAC 01, MTPAC 07, MTPAC 12 | |||
| Obtuse | 2 (8%) | MTPAC 15, MTPAC 20 | |||
| 4 | Leaf length | Short (< 12 cm) | 3 (12%) | MTPAC 08, MTPAC 09, MTPAC 12 | MG |
| Medium (12 – 21 cm) | 4 (16%) | MTPAC 05, MTPAC 10, MTPAC 16, MTPAC 19 | |||
| Long (> 21 cm) | 18 (72%) | MTPAC 01, MTPAC 02, MTPAC 03, MTPAC 04, MTPAC 06, MTPAC 07, MTPAC 11, MTPAC 13, MTPAC 14, MTPAC 15, MTPAC 17, MTPAC 18, MTPAC 20, MTPAC 21, MTPAC 22, MTPAC 23, MTPAC 24, MTPAC 25, | |||
| 5 | Leaf petiole length | Short (< 2cm) | 2 (8%) | MTPAC 05, MTPAC 20 | MG |
| Intermediate (2-3 cm) | 5 (20%) | MTPAC 04, MTPAC 09, MTPAC 10, MTPAC 19, MTPAC 24 | |||
| Wide (> 3 cm) | 18 (72%) | MTPAC 01, MTPAC 02, MTPAC 03, MTPAC 06, MTPAC 07, MTPAC 08, MTPAC 11, MTPAC 12, MTPAC 13, MTPAC 14, MTPAC 15, MTPAC 16, MTPAC 17, MTPAC 18, MTPAC 21, MTPAC 22, MTPAC 23, MTPAC 25, | |||
| 6 | Leaf waxiness in upper side | Absent or weak | 10 (40%) | MTPAC 03, MTPAC 04, MTPAC 05, MTPAC 11, MTPAC 12, MTPAC 13, MTPAC 18, MTPAC 21, MTPAC 23, MTPAC 24 | VG |
| Medium | 3 (12%) | MTPAC 06, MTPAC 07, MTPAC 08 | |||
| Strong | 12 (48%) | MTPAC 01, MTPAC 02, MTPAC 09, MTPAC 10, MTPAC 14, MTPAC 15, MTPAC 16, MTPAC 17, MTPAC 19, MTPAC 20, MTPAC 22, MTPAC 25 |
Table 2: Bark DUS characterization of Neolamarckia cadamba
| Sl. No. | Characteristics | State | Distribution of classes of descriptor | Example source | Type of assessment |
| 1 | Bark colour | Brown | 4 (16%) | MTPAC 16, MTPAC 19, MTPAC 22, MTPAC 23, | VG |
| Light Brown | 11 (40%) | MTPAC 01, MTPAC 02, MTPAC 03, MTPAC 04, MTPAC 05, MTPAC 06, MTPAC 07, MTPAC 11, MTPAC 14, MTPAC 17, MTPAC 24 | |||
| Black | 10 (44%) | MTPAC 08, MTPAC 09, MTPAC 10, MTPAC 12, MTPAC 13, MTPAC 15, MTPAC 18, MTPAC 20, MTPAC 21, MTPAC 25 | |||
| 2 | Bark texture | Smooth | 14 (56%) | MTPAC 01, MTPAC 02, MTPAC 03, MTPAC 06, MTPAC 07, MTPAC 11, MTPAC 12, MTPAC 13, MTPAC 14, MTPAC 15, MTPAC 16, MTPAC 17, MTPAC 19, MTPAC 24 | VG |
| Moderate | 8 (32%) | MTPAC 04, MTPAC 05, MTPAC 08, MTPAC 09, MTPAC 10, MTPAC 20, MTPAC 21, MTPAC 22, | |||
| Rough | 3 (12%) | MTPAC 18, MTPAC 23, MTPAC 25 |
Leaf Characterization
Leaf Shape
The leaves of kadam exhibited four different shapes viz., elliptic, ovate, broadly ovate and nearly round (Fig. 1). Elliptic shape was predominantly reported and showed predominance of 72% occurrence in the Neolamarckia cadamba genetic resources. Broadly ovate shape of leaves was found in three clones viz. MTPAC 06, MTPAC 12, MTPAC 19, which contributed to 12% of occurrence. Nearly round shape of leaves found in MTPAC 16, MTPAC 20. The elliptic character showed predominance with 72% occurrence in the Neolamarckia cadamba genetic resources followed by broadly ovate (12%), ovate (8%) and nearly ovate (8%). Similar variation observed by Hare Krishna et al.,5 in Ziziphus mauritiana. It showed three types of leaf shape viz., ovate, cordate and oval in Ber. The study showed similar variation in leaf shape as recorded in Eucalyptus10 and Neem.9
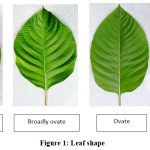 |
Figure 1: Leaf Shape Click here to View Figure |
Leaf Base Shape
The Base shape of Kadam leaf sample recorded three categories viz., cordate, obtuse and acute (Fig. 2). MTPAC 09 registered acute leaf base. Five clones viz., MTPAC 06, MTPAC 07, MTPAC 08, MTPAC 12 and MTPAC 19 recorded obtuse leaf base and the remaining clones recorded cordate leaf base. In Base shape, cordate was predominantly found (76%) in kadam genetic resources followed by obtuse (20%) and acute (4%). These variations authenticated by the study on Ziziphus mauritiana5 and Eucalyptus.10
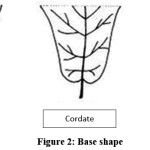 |
Figure 2: Base Shape Click here to View Figure |
Leaf Apex
Apex shape of leaf in Neolamarckia cadamba clones represent five different shapes viz., Acute, Acuminate, Apiculate, cuspidate and Obtuse (Fig. 3). Among the five different shapes, occurrence of acuminate (36%) and cuspidate (24%) was given major contribution while considering kadam genetic resources. Similar results recorded in Ziziphus mauritiana5 and Eucalyptus.10 Leaf margin, leaf venation and base symmetry are same across the clones having undulate, pinnate and bilateral symmetry, respectively (Fig. 4,5,6).
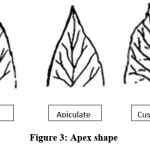 |
Figure 3: Apex Shape Click here to View Figure |
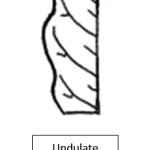 |
Figure 4: Leaf Margin Click here to View Figure |
 |
Figure 5: Leaf Venation Click here to View Figure |
 |
Figure 6: Base Symmetry Click here to View Figure |
Leaf Length and Width
Leaf length was categorized into three viz., Short (< 12 cm), Medium (12-21cm) and Long (> 21 cm). Three clones viz., MTPAC 08, MTPAC 09 and MTPAC 12 recorded short leaf length, four clones viz., MTPAC 05, MTPAC 10, MTPAC 16 and MTPAC 19 recorded medium leaf length, and the remaining clones registered long leaf length. Among the three distinctive characters of leaf length, maximum kadam genetic resources recorded long leaf length (72%) followed by medium (16%) and short (12%). Similar variation reported in Neem.9 Leaf breadth was same across the clones, which comes under the category of wide (> 4 cm).
The study conducted by George et al.,4 showed that leaf length varies from 12.5 cm (HC 27) to 7.6 cm (HC 19) among the 27 back cross clones of Jatropha curcas. These results are at par with the current study.
Leaf Petiole Length
Leaf petiole length of kadam is categorized into three viz. short (< 2 cm), intermediate (2-3 cm) and wide (> 3 cm). MTPAC 05 and MTPAC 20 recorded the short leaf petiole length. Five clones viz., MTPAC 04, MTPAC 09, MTPAC 10, MTPAC 19 and MTPAC 24 recorded intermediate leaf petiole length and the remaining clones showed wide leaf petiole length. Wide petiole length (72%) was recorded in maximum number of Neolamarckia cadamba genetic resources showed followed by intermediate petiole length (20%) and Short petiole length (2%). George et.al. [4] showed variation in leaf petiole length ranged from 12.1cm (HC 12) to 4.5cm (HC 16) among the 27 back cross clones of Jatropha curcas, which is at par with current results. Similar variations were reported in Neem.9.
Waxiness in upper side of Leaf
Waxiness in upper side of the leaves was categorized into weak (or) absent, medium and strong. Three clones viz., MTPAC 06, MTPAC 07 and MTPAC 08 recorded Medium waxiness in the upper side of the leaf. Among the Neolamarckia cadamba genetic resources, 48% genetic resources showed strong leaf waxiness in upper side of the leaves followed by 40 % (Weak or absent) and 12 % (Medium).
Study conducted by Gnanasekar and Balasubramanian9 shows significant variations in leaf length and width, rachis length, petiole length within the type in Neem. Similar findings were reported in Teak,6 Eucalyptus,3 Sandal1 and Jamun.8
Bark Characterization
Bark Color
Bark colour of the Neolamarckia cadamba categorized into brown, Light brown and black (Fig. 7). Four clones viz., MTPAC 16, MTPAC 19, MTPAC 22 and MTPAC 23 exhibited brown bark colour and Ten clones viz., MTPAC 8, MTPAC 9, MTPAC 10, MTPAC 12, MTPAC 13, MTPAC 15, MTPAC 18, MTPAC 20, MTPAC 21 and MTPAC 25 recorded black bark colour. The remaining clones showed light brown bark colour. Among the kadam genetic resources, 44% of genetic resources with black bark colour followed by light brown (40%) and brown (16%).
 |
Figure 7: Bark Colour Click here to View Figure |
Bark Texture
Bark texture grouped in to smooth, moderate and rough (Fig. 8). Three clones viz., MTPAC 18, MTPAC 23 and MTPAC 25 recorded rough bark texture and eight clones viz., MTPAC 4, MTPAC 5, MTPAC 8, MTPAC 9, MTPAC 10, MTPAC 20, MTPAC 21 and MTPAC 22 exhibited moderate bark texture. The remaining clones recorded smooth bark texture. Among the kadam genetic resources, 56% of clones registered smooth bark followed by 32% (medium) and 12% (rough).
Similar descriptor study showing significant genotypic variations in bark texture, annual peeling type, colour of fresh bark, colour of dried bark and colour of rhytidome bark characters were studied in casuarina7 and Eucalyptus.10
 |
Figure 8: Bark Texture Click here to View Figure |
Conclusion
Neolamarckia cadamba genetic resources were characterised for DUS traits based on UPOV11 guidelines. Twenty-five clones of kadam were characterised based on the morphological characters of leaf and bark. In leaf, four distinct leaf shapes were recorded viz., elliptic, ovate, broadly ovate and nearly round. Two clones viz., MTPAC 16 and MTPAC 20 showed nearly round shape of leaf. In leaf base shape, three base shapes were recorded and higher variation was recorded for the leaf apex shape. Obtuse leaf apex shape registered lower frequency (8%) among the kadam genetic resources. Leaf margin, leaf venation and base symmetry are same across the clones. Leaf length has showed considerable variation among the clone, which grouped into three categories viz., short, medium and long. Leaf width has not revealed any variation among clones. Leaf petiole length is categorized into three viz., short, Intermediate and wide. MTPAC 05 and MTPAC 20 were showed short petiole length. Higher variation was registered due to waxiness on the upper side of the leaf. Bark colour is categorised into brown, light brown and black. In bark texture, three characters were recorded viz., smooth, moderate and rough. Three clones (MTPAC 18, MTPAC 23 and MTPAC 25) are showed rough bark texture. The clones depicted wide variability for various morphological traits, which is used for conservation of germplasm through IPR mechanism.
Acknowledgements
The authors profusely thank the Indian Council of Agricultural Research (ICAR), New Delhi for having funded the research project entitled “Development of High Yielding Short Rotation varieties for Industrial Agroforestry” through extramural research project wherein the current study formed a part of the objectives.
Reference
- Bagachi S.K., Veerendra H.G.S. Study on intra tree and inter tree variation in leaves of Santalum album Linn. My Forest. 1985;21(1): 33-39.
- Bhim Jyoti, Usha, Padam Singh. Role of DUS testing in registration of plant varieties under PPV & FR Act, 2001. Rashtriya Krishi. 2015;10(2):5-6
- Bramwells H.W., Wiffin T. Patterns of variation in Eucalyptus sideroxylon A.Cunn. ex Woolls. I. Variation in leaf morphology. Australian journal of Botany. 1984;40: 657-676.
CrossRef - George A.K., Parthiban K.T., Vikas Kumar. Development and documentation of descriptors for Jatropha (Jatropha curcas) and their hybrid derivatives. Indian Journal of Tropical Biodiversity. 2016;24(1).
- Hare Krishna R., Bhargava, Nitesh Chauhan, Sharma S. K. Morphological descriptor for DUS testing of Indian jujube (Ziziphus mauritiana). Indian Journal of Agricultural Sciences. 2016;86 (6): 809–14
- Gunaga R.P., Surendran T. Leaf morphological variation in Teak (Tectona grandis Linn.F.) clones. Evergreen. 2002; 48:8-9.
- Nicodemus A., Warrier R.R., Sivakumar V., Anandalakshmi R. Guidelines for the conduct of test for Distinctiveness, Uniformity and Stability on Casuarinas (Casuarina equisetifolia L. and Casuarina junghuhniana Miq.). Protection of Plant Varieties and Farmers’ Rights Authority, Government of India. 2012.
- Prabhuraj S., Swamy G.S.K., Athani S.I., Patil B.R., Hulamani N.C., Patil P.B. Variability in morphological characters of Jamun (Syzygium cumini skeels) trees. My forest. 2002; 38(2): 187- 190.
- Gnanasekar S., Balasubramanian A. DUS Descriptors for Registration of Neem (Azadirachta indica A.Juss.) for Varietal Protection. Trends in Biosciences. 2014; 7(16): 2303-2305.
- Sivakumar V., Anandalakshmi R., Nicodemus A., Warrier, R.R. Guidelines for the conduct of test for Distinctiveness, Uniformity and Stability on Eucalypts (Eucalyptus camaldulensis Dehnh. and Eucalyptus tereticornis). Protection of Plant Varieties and Farmers’ Rights Authority, Government of India. 2012.
- Soerianegara I., Lemmens RHMJ. (eds.). Plant Resources of South-East Asia. No. 5(1): Timber trees: major commercial timbers. Backhuys Publishers, Leiden. 1993.
- UPOV. Guideline for the conduct of the test for distinctness, homogeneity and stability Banana (Musa acuminate) TG/123/3. International Union for the Protection of new Varieties and plants (UPOV), Geneva. 1989;26.


