Introduction
Medicinal and aromatic plants (MAPs) form the basis for plant based therapies including the Indian/ Chinese/ Tibetan Systems of Medicines, which are being popularized in many parts of the world. Quality of these plants largely depends on active ingredients present in them. Though most of the raw drugs are sold on fresh/dry weight basis in the domestic market,1 during the extraction process it is observed that superior the chemotype, better is the quality of the extract, and maximum the returns obtained per unit of charge. From time immemorial, crude drugs have been collected from wild and thus, population of most of the important MAP species is dwindling in nature.2 A number of species are on the verge of extinction due to problems such as destructive harvesting, poor seed set, low seed viability, pest and disease incidence etc.3-4 Though the existing populations of these species are being regenerated in the nature by means of sexual reproduction, the rate of multiplication is slow and thus insufficient to meet the requirements in most of the cases.5 The ever-increasing demand for these products indomestic and international markets also calls for a faster multiplication strategy.6 Good number of improved varieties of MAPs is now available7 that are being popularized amongst farmers due to their higher net returns and ease of cultivation, thereby reducing extra burden on the forests. Propagation by vegetative means is not only relevant in case of improved varieties/hybrids of commercially cultivated species but also for conservation of those, which are endemic, threatened and are on the verge of extinction.3 Multiplication of these species by clonal means such as cuttings can make cultivation much economic, providing more uniform population and active ingredient yield per unit area.8 In some cases such as Centellaasiatica, plants raised from cuttings have been proven to perform better for biomass production than those raised from seeds.9
Understanding the skills involved in vegetative propagation techniques ensures faster multiplication tomeet the growing demand from consumers, and thereby keeping collectors away from forests for collecting such resources. Adventitious root formation has been regarded as a complex process as it is highly influenced by different internal and external factors.10 Poor adventitious root development is considered as a major hindrance in propagation through cuttings.11 Some species may produce roots even without any treatments as observed in case of Cinnamomumasomicum and C. impressinervium,12 whereas in other species propagation by cutting might not be feasible as in Aconitum heterophyllum.13 For example, hard wood cuttings of a threatened species Saracaasoca exhibited rooting in mere 16.67% cuttings, when treated with 500mg/lIndole-3 butyric acid (IBA) and hence, propagation via air layering was advocated for propagation with 90% success.14 Nevertheless, considering the practicality of the technique, ease of operation, cost involved etc., propagation by cuttings has remained a technique of choice in the past and will continue to be in future as well. Even though propagation through cuttings appears to be the simplest among all the methods of vegetative propagation, some aspects need to be understood to obtain better results. Considering these, factors governing the success of vegetative propagation through cuttings in MAPs (Figure 1) have been reviewed hereunder.
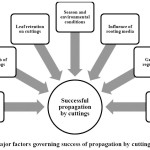 |
Figure 1: Major factors governing success of propagation by cuttings in MAPs Click here to View Figure |
Factors governing success of propagation through cuttings in MAPs
Physiological maturity of cuttings
Stem cuttings are classified as hardwood, semi-hardwood, softwood and herbaceous cuttings based on physiological age of the wood from which they are excised.15-16 Hardwood cuttings are taken from dormant, mature stems of more than one year and are commonly adopted in MAPs such as Indian Myrrh, Rosa spp., Henna etc. Semi-hardwood cuttings are usually prepared from partially mature wood of the current season’s growth, which is practiced in species such as Embelia spp., Salaciaspp.etc. Softwoodandherbaceous cuttings are prepared from soft, succulent new growth of plants16 and are commonly employed for the propagation of species of mints (Mentha spp.), brahmi (Bacopa monnieri) and other herbs.
Dick and Dewar17 opined that variation in carbohydrate pools could be the main factor determining rooting ability. Classically, internal factors such as auxins, rooting co- factors, C:N ratio etc. have shown to influence root initiation process.18 Hardwood stem cuttings of Gongronemalatifolia, an important African medicinal species,produced significantly higher number of roots compared to when propagated by semi-hardwood and softwood cuttings.19 Similarly, in case of J. grandiflorum, hardwood cuttings exhibited highest regeneration capacity compared to semi-hardwood and softwood cuttings.20 In case of Pogostemonheyneanus, two nodal hardwood cuttings performed better in rooting parameters when compared to semi-hardwood and softwood cuttings.21 Positive relationship between diameter of cutting and rooting capacity in Azadirachtaindica has been previously reported,22 which could be explained by higher carbohydrate reserves in thicker cuttings.
On the contrary, in Salacia fruticosa, Salacia reticulata and Embeliaribes, use of semi-hardwood cuttings gave superior rooting response than that by hardwood cuttings.4,23-24 Based on diameter, thin (0.43 cm) and medium (0.52 cm) cuttings were found to be superior for increasing sprouting and survival in Embeliatsjeriam-cottam and Caesalpiniabonduc than thicker cuttings.3 Secondly, portion of the stem used for excising cutting has been found to contribute in root induction. Rooting ability of cuttings taken from different parts of the stem of Ficus species indicated that higher rooting rates (>52%) were obtained with pole and nodal cuttings as against 5% in case of terminal cuttings.25 Low rooting ability of apical cuttings could be attributed to their herbaceous character, which made them sensitive to moisture stress, resulting in desiccation.26 Middle or apical cuttings of about 25 cm length were adjudged as the best planting material for regeneration of Commiphorawightii, the Indian myrrh.27 Similarly, in Damask rose (Rosa damascena), basal woody cuttings were found to give highest rooting percentage with maximum number of roots.28 Nevertheless, the response is species dependent as Khosla and Pushpangadan29 observed that the current year, young lateral shoots were the best planting material for rooting of clocimum (Ocimumgratissimum). Most suitable physiological maturity of the cuttings in some important MAPs have been presented in Table 1.
Table 1: Some examples of different types of cuttings based on their physiological maturity in MAPs.
| Type of cutting | Species | Reference |
|
Herbaceous cutting |
Bacopa monnieri, Centellaasiatica,Pogostemon patchouli |
7, 9, 90 |
|
Softwood cutting |
Nothapodytesnimmoniana, Pelargonium graveolens, Tylophoraindica |
83, 91, 92 |
|
Semihard wood cutting |
Adhatodavasica,Bixaorellana,Boswellia serrata, Clerodendrumindicum, Crataegusoxyacantha, Embeliaribes, Ginkgo biloba; Jasminum sambac, Leptadenia reticulata, Nothapodytesnimmoniana, Piper longum, Plumbago rosea, Plumabgozeylanica, Rutagraveolens; Salacia fruticosa |
4, 7, 23, 33, 82, 83, 84, 94, 95, 96, 97 |
|
Hard wood cutting |
Celastruspaniculata, Jasminumgrandiflorum, Lawsoniainermis¸ Nothapodytesnimmoniana,Premnaintegrifolia, Rauwolfia serpentina, Streblus asper, Rosa damascena, Taxusbaccata, Vitex negundo, Wrightiatinctoria |
20, 83,97, 93, 99, 100, 101, 102 |
|
Terminal cutting |
Baliospermummontanum, Bursera delpichiana, Coleus forskohlii, Lippiajavanica
|
32, 94, 95, 103 |
|
Root cuttings |
Hemidesmus indicus, Chlorophytumboriviliamum, Rauwolfia serpentina, Rubiacordifolia |
94 |
Length of cuttings
Went30 observed that functional buds present on the cutting produced root promotingchemicals, which signifies the importance of length of cutting in inducing rooting. The length that could produce sufficient root and shoot system for the plant to grow however varies with the species. For example, about 15 cm long cuttings were rated superior in Plumbago zeylanicaunder mist,31 while longer cuttings of 25 cm were required in case of Bursera delpichiana32 and Rutagraveolens to obtain maximum rooting percentage, fresh root weight and higher field survival.33 Superior rooting percentage with better survival has been reported using 12-15 cm long cuttings in Rosa damascena34 and Drymis brasiliensis35 under polyhouse conditions.
Availability of mother stock is a limiting factor in a number of species and efforts are beingmade to increase the multiplication ratio to make it cost efficient as well. For instance, in noni (Morindacitrifolia), earlier reports36 recommended use of longer (20 to 40 cm) hardwood cuttings; however, subsequent reports suggested that 4 node37 or even 3 node38 cuttings could suffice development of healthy plantlets. Similarly, in case of black pepper (Piper nigrum), Singh and Singh39obtained maximum rooting and subsequent plant development using two node cuttings than that from three and four node cuttings. However, recently, single node cuttings have been found to increase the multiplication ratio by manifolds.40 In a related species, long pepper (Piper longum), Kempe Gowda et al.41 reported that triple node cuttings treated with IBA 1000 mg/l produced better shoots and roots. Efforts could be made to propagate long pepper using single node cuttings as in black pepper. However, reduction in length of cuttings is not always useful42 as significantly higher sprouting percentage with better shoot and root growth were observed, when 15 cm cuttings were used over 7.5 cm in stevia (Stevia rebaudiana). Similarly, four nodal cuttings werefound to be superior to two nodal cuttings for increasing sprouting and survival in Embeliatsjeriam- cottam and Caesalpinia bonduc.3 In Piper sarmentosum, use of double node cuttings was rated superior to single and triple node cuttings.43
Leaf retention on cuttings
Retention of leaves on the stem cutting is known to have an impact on the rooting success in some species, mainly because of the presence of auxins in leaves, which are translocated to the base of cuttings for promotion of rhizogenesis.44 Leaf is also known to provide energy to the cuttings especially those with limited food reserves.45 However, if excessive leaves are present on the cuttings, moisture may get lost from cuttings due to evaporation resulting into dehydration of cuttings.46 The requirement for retention of leaves on a cutting is variable, as success varies greatly amongst the species. In some species, cuttings with lesser leaves root better than more leafy ones,47 while rooting cannot occur in other species in the absence of leaves.48 According to Okoro and Grace,49 the initial carbohydrate reserve in leafless hardwood cuttings may not act as a limiting factor for rootinduction but may govern the subsequent growth and development of leaves.
In case of Pipersarmentosum, retention of leaves had no positive influence on rooting percentage butimproved plant growth.43 Limited efforts have been made in MAPs to study the effect ofthis factor on success of propagation (Table 2).A comparison was made between half-leaf and full-leaf stem cuttings in Enantiachloranthausing different auxins. Results revealed that full-leaf cuttings were superior to half-leaf cuttings as 100% rooting was noticed in those cuttings even without applicationof auxins.50 In another study on Lavandula dentata, cuttings were planted with 1/3, 1/2 or2/3 of their leaves. It was noticed that maintaining higher number of leaves was beneficialand 2/3 leaf retention was found to be most suitable for obtaining better rooting percentage.51 In Salacia oblonga, highest regeneration percentage (72.3%) was observed, when leafless cuttings were used as against cuttings with leaves (55.5%) at 300 ppm IBA. 6
Table 2: Influence of leaf retention on success of propagation through stem cuttings.
| Species | Remarks | Reference |
|
Barleriaprionitis
|
Better survival of 81.48% was noticed in leaved cuttings than leafless cuttings (70.37%) |
104 |
|
Commiphorawightii |
Leafless stem cuttings are preferred for early and better rooting |
94 |
|
Dilleniasuffructicosa |
Juvenile cuttings with leaves gave 100% survival as against 100% mortality in leafless cuttings. |
45 |
|
Enantiachlorantha |
Full-leaf cuttings were superior (100% rooting) to half-leaf cuttings even without application of auxins |
50 |
|
Lavandula dentata |
Maintaining higher number of leaves (2/3 leaf retention) was beneficial for obtaining better rooting percentage |
51 |
|
Marsdeniatenacissima |
Fresh and healthy leafy cutting having two or three nodes of axillary buds gives better rooting |
94 |
|
Pogostemoncablin |
Removal of all the leaves was not recommended due to higher mortality percentage |
54 |
|
Piper sarmentosum
|
Retention of leaves on the cuttings did not enhance rooting percentage but improved plant growth. |
43 |
|
Plectranthus barbatus |
Removal of all leaves except apical bud gives better rooting |
94 |
|
Salacia oblonga |
Highest regeneration percentage (72.3%) was observed, when leafless cuttings were used as against cuttings with leaves (55.5%) using 300 ppm IBA |
6 |
In one case, rooting and successful establishment in patchouli (Pogostemoncablin) was found to be superior with 6 leaves + IBA (2000 mg/l),52 while other report suggested useof 2-4 leaves + IBA (150 mg/l) combination.53 These variations could be attributed to thedifferences in the growing environments and the varieties used in these studies. Removal ofall the leaves in patchouli cuttings was not recommended due to higher mortality percentage.54
Season and environmental conditions
Season plays an important role onsuccess of different methods of vegetativepropagation including cuttings (Table 3). Day temperature, cloud cover and relative humidity have great influence on the success of rooting, sprouting and growthof propagules.25,55 These variations largely govern the physiological activities in a plantsystem, including the sugar levels (due to retention of leaves on the cuttings, which iscommon in evergreen species) and temperature of the substrate.55 It has been reported thatwet season with high relative humidity is congenial for rapid callus production and earlyrooting.56 In Salacia fruticosa and Embeliaribes, plant propagation by semi-hardwoodcuttings was most successful when cuttings were collected during January-April.4,23
Table 3: Influence of season and growing environment on success of propagation in MAPs.
| Species | Remarks | Reference |
|
Caralluma edulis, Leptadenia reticulata and Tylophoraindica |
Aeroponics system was found to be superior in terms of rooting percentage, plant growth and survival than the conventional soil planting |
68 |
|
Commiphorawightii |
Four to five days seasoning of cuttings in the month of August supported better sprouting and field survival (50%) |
105 |
|
Clerodendrumserratum |
Stem cuttings treated with IBA 400 ppm for early sprouting |
106 |
|
Embeliaribes |
Semi-hardwood cuttings collected during January-April were most suitable |
23 |
|
Ginkgo biloba |
Cuttings maintained in polyhouse (25 °C and 70% RH) gave 56.7% rooting after 6 months as against two years under open condition |
84 |
|
Ginkgo biloba |
Cuttings taken during July rooted better (90%) than those taken during April (40%) with the same dose of IAA |
107 |
|
Jasminum sambac, J. auriculatumand J. grandiflorum |
Better rooting under mist than in open condition |
59, 60 |
|
Magnolia fuscata |
Propagation under mist reported highest rooting (%) and survival of rooted cuttings |
58 |
|
Operculinaturpethum |
Stem cuttings with two nodes may either be planted directly in the field during monsoon (July) or may be rooted in mist chamber during March-June |
93 |
|
Pelargonium graveolens |
Shade of Putranjeeva roxburghii was equally suitable as shadenet house for rooting |
108 |
|
Piper longum |
Growth and field establishment was improved in greenhouse than those in natural shade |
66 |
|
Plumbago zeylanica |
15 cm long cuttings were rated superior under mist conditionsBasal stem cuttings up to seventh to ninth nodes under mist chamber gives 80-100% rooting success |
3193 |
|
Salacia fruticosa |
Semi-hardwood cuttings collected during January-April were most suitable |
4 |
|
Stevia rebaudiana |
Growth and field establishment was improved in greenhouse than those in natural shade |
65 |
|
Stevia rebaudiana |
Better rooting in autumn season with 50 ppm NAA + 500 ppm IBA, while 2000 ppm NAA + 2000 ppm IBA is required during kharif season |
109, 110 |
|
Tinosporacordifolia |
Obtaining stem cuttings from mother plant during June-July gives better rooting and field survival |
94 |
|
Vanilla planifolia |
Growing cuttings under 50% shade was found to be cost effective |
61 |
|
Vanilla planifolia |
Greenhouse was most suitable for producing vigorous plants, early rooting with highest rooting percentage |
64 |
|
Zanthoxylumaramatum |
Stem cuttings planted in nursery during monsoon (July – August) gives good rooting |
94 |
Under open conditions, ideal conditions for propagation cannot be maintained throughout the year; however, manipulation of environment is possible under protected conditions. Kindof structuresusedforraising nursery also governs the success as light penetration, temperature, relative humidity and gaseous composition inside the structure varies with thematerial used. Several workers have reported good results with cuttings under mist. Raines57was first to report the use of mist chamber for induction of rooting in stem cuttings.Balakrishna and Bhattacharjee58 reported in Magnolia fuscata that shoot tip cuttings withtwo leaves treated with IBA (6000 ppm) gave the highest rooting percentage and survival ofrooted cuttings under mist. Stem cuttings of Jasminumsambac, J.auriculatum and J.grandiflorum were rooted better under mist than in open condition.59-60 Under costeffective growing conditions, Konedenedo61 obtained best results with one meter long vanilla (Vanilla planifolia) cuttings with 50% shade.Under Indian conditions, use of low cost polyhouse with ambient condition wasrecommended for propagation of MAPs through cuttings as against shade net conditions.62 Medium cost greenhouse was also found to be ideal due to maintenance of relatively high temperature and humidity,63 wherein the extent of rooting of MAPs was better (76.3%)than that in shade net (25.0%).
Propagating structuresviz. greenhouse, shade net and natural shade of Muntingia calabura were evaluated for propagation of vanilla (Vanilla planifolia), which revealed that greenhouse was most suitable for producing vigorous plants, early rooting and maximumrooting percentage.64 Similarly, rooting, shoot growth and field establishment of stevia (Stevia rebaudiana) and long pepper cuttings were improved when experiments wereconducted in greenhouse than those in natural shade.65,66 Higher temperature and relativehumidity prevailing in such structures compared to outside condition could result in enhancedphotosynthetic efficiency due to early sprouting and leaf production, better root growth andestablishment of cuttings.67Recently, aeroponics system has become popular in the multiplication of plantingmaterial. In three medicinal species viz. Carallumaedulis, Leptadenia reticulata and Tylophoraindicathe aeroponics system was found to be superior in terms of rootingpercentage, plant growth and survival than the conventional soil planting.68 Majoradvantage of this system is production of completely disease free planting material, andhence could be exploited on commercial scale.
Influence of rooting media/ substrate
Various characteristics of rooting mediumviz. structure, texture, porosity, chemicalcomposition, water holding ability of the media and pH have pronounced effect on rootingability as well as the quality of root system formed.18,69-70 Hence, adequate attentionshould be paid, while selecting a medium for the concerned species. In a number of medicinalplant species, such studies are lacking, however spice species having numerous medicinalproperties such as black pepper are adequately studied (Table 4). In black pepper variety Panniyur-1, highest rooting (30.9%) and plant growth wereobtained in the medium containing sand + farmyard manure (FYM), followed by sand alone.71 Bogantes-Arias72 tested six substrates as rooting media for black pepper cuttings, wherein lowestmortality with better root growth was observed in soil + Bio-rigi + sawdust (1:1:1) and soil +sand (1:1) combinations, while soil was not recommended. Thankamani et al.,73 reported that use of vermicompost as a substrate supported better plant growth over standard pottingmixture comprising of soil, sand and farmyard manure (3:1:1). Mixture containing topsoil,sand and vermicompost (1:1:1) was found to be the best for root induction and recoverypercentage in black pepper var. Panniyur-1 under rapid multiplication technique that couldproduce 23.5 rooted cuttings per vine within three and a half months.74
Table 4: Effect of substrate on rooting, survival and plant growth.
| Species | Substrate | Remarks | Reference |
|
Barleriaprionitis |
Sand+ soil+ vermicompost (1:1:1) |
Better (97%) survival and plant growth |
104 |
|
Cinnamomumverum var. Navashree and Nityashree |
Sand and coir dust (1:1) |
Superior rooting parameters |
78 |
|
Leptadenia reticulata |
Sand : FYM : Red soil |
Good rooting and field establishment |
94 |
|
Morindacitrifolia |
Forest soil + sand, volcanic cinder + compost |
Superior rooting parameters |
36 |
|
Piper longum |
Soil + sand + vermicompost (1:1:1) |
Better shoot and root growth parameters |
66 |
|
Piper nigrum |
Soil + Bio-rigi + sawdust (1:1:1) and soil + sand (1:1); while soil was not recommended |
Lowest mortality with better root growth |
72 |
|
Piper nigrum |
Vermicompost |
Better plant growth |
73 |
|
Piper nigrum var. Panniyur-1 |
Topsoil, sand and vermicompost (1:1:1) |
Better multiplication ratio (23.5 cuttings per vine) under rapid multiplication technique |
74 |
|
Pogostemonheyneanus |
Top soil: sand: compost (1:1:1) |
Better root growth |
21 |
|
Premnaintegrifolia |
Sand : soil : FYM (1:1:1) |
Better rooting |
94 |
|
Salacia reticulata |
Top soil and compost (1:1) |
Highly congenial for establishment of cuttings |
24 |
|
Stevia rebaudiana |
Sand: perlite (1:3) |
Better rooting and growth |
75 |
|
Stevia rebaudiana |
Soil + sand + vermicompost (1:1:1) |
Better shoot and root growth parameters |
65 |
|
Vanilla planifolia |
Vermicompost and coir pith compost |
Most efficient and cost effective |
76 |
Better rooting and growth response have been reported in stevia (Stevia rebaudiana),when stem cuttings were planted in sand: perlite (1:3) media under polythene film.75 Inanother study on stevia, use of soil + sand + vermicompost media (1:1:1) was found toproduce longer and thicker sprouts, better root girth, dry weight of shoot and roots.65 Samemedium combination was also found to be promising in long pepper (Piper longum) also.66 In Pogostemonheyneanus, a combination of top soil, sand and compost (1:1:1) wasfound to be the most suitable potting medium.21 In Salacia reticulata, use of top soil andcompost (1:1) was found to be highly congenial for establishment of cuttings.24Siddagangaiah et al., 76 evaluated various rooting substrates and found that vermicompostand decomposed coir pith were most efficient, cost effective and hence ideal for theproduction of vanilla stem cuttings. Vermicompost is commonly incorporated in the rootingmedium due to various properties such as its richness in nutrients and growth stimulants,higher water holding capacity and improved soil texture that facilitate the root growth.27,77 Krishnamoorthy et al.,78 reported that sand and coir dust (1:1) was the most appropriaterooting medium for two cinnamon (Cinnamomumverum) varieties viz. Navashreeand Nityashree as highest rooting percentage, length of primary roots and secondary roots percutting were obtained in this combination. Nelson36 recommended weed and nematodefree forest soil mixed with sand, volcanic cinder and composted organic matter forpropagation in noni (Morindacitrifolia).
Growth Regulators
Inherent capability of a cutting to induce rooting could largely be altered by chemicaltreatments. Many synthetic growth substances have been used to aid the rooting of cuttingssince long time.79 Of those, auxins are the most commonly used as they are known to help in accumulation of metabolites, synthesis of new proteins, cell enlargement and increasenitrogen in roots.80 They also regulate different aspects of plant growth and developmentby affectingphysiological processes including cell division, cellelongationanddifferentiation.81Indole-3-butyric acid (IBA), naphthaleneacetic acid (NAA) and indole-3-acetic acid (IAA)are the most commonly employed auxins due to their ability to initiate roots, stability and lowmobility in plants. Although the induction of rooting by auxin application has been reportedin different plant species, reports on the mechanisms of this response are contradictory.81
In Bixaorellana, use of 4000 mg/l concentration of IAA supported root induction in 22%cuttings, whereas none of the IBA concentrations could induce rooting in these cuttings.82It is noticed that the concentration of growth regulators required for rooting varies accordingto nature of the plant and as a thumb rule, woody plants require higher concentration than theherbaceous ones. The optimum kind and concentration of auxin are species-specific e.g. adose of 2000 mg/l of IBA is recommended for Jasminum grandiflorum, whereas double doseof 4000 mg/l was required in case of Jasminum auriculatum.60 Hormonal requirement also differs with the type of cutting used for propagation e.g. in Nothapodytesnimmoniana, IBA concentrations of 2000 mg/l, 3000 mg/l and 4000 mg/l were optimum for soft wood, semihardwood and hardwood cuttings, respectively.83 Hence, a number of other factors need tobe studied together with growth regulators to get comprehensive results.
Unlike other aspects discussed in this article, role of plant growth regulators have beenstudied in multiplication of a number of MAPs (Table 5). In a number of instances, rootingcould not be obtained in the absence of external application of growth hormones, therebyindicating their pivotal role in the propagation of these species. Apart from auxins,compounds such as catechin 5 mg/L and gallic acid 10 mg/L have been reported to inducerooting in semi hardwood cuttings of Ginkgo biloba with 53 and 57% success.84
Table 5: Interactions between type of cuttings and different growth regulators in propagation of MAPs.
| Species | Type of cutting | Growth regulators | Remarks | Reference | |
|
Adhatodavasica |
SHWC |
1500 ppm IBA |
Higher (67.5%) rooting than control (30.8%) |
96 |
|
|
Azadirachtaindica |
SC |
1000 ppm IBA |
Superior (51%) rooting than control (35%) |
112 |
|
|
Baliospermummontanum |
SHWC |
IBA, NAA, Boric acid |
Improved sprouting percentage to nearly 90 to 100 % |
94 |
|
|
Berberis aristata |
TC |
5000 ppm IBA |
Significantly higher (50%) rooting as against no rooting in control |
98 |
|
|
Bursera delpichiana |
HWC |
2000 ppm IBA |
Better rooting (67%) than control (15.3%) |
32 |
|
|
Caesalpiniabonduc |
SC |
1000 ppm IBA |
Superior sprouting and field survival |
3 |
|
|
Celastruspaniculata |
HWC |
2000 ppm IBA |
Improved (72.6%) rooting percentage as against 54% in control |
102 |
|
|
Caralluma edulis |
SC |
2000 ppm IBA |
100 % root induction in hydroponics system |
68 |
|
|
Coleus forskohlii |
TC |
500 ppm IAA |
Superior rooting response |
103 |
|
|
Commiphorawightii |
SC |
100 ppm IBA |
Hasten rooting and better survival |
94 |
|
|
Embeliaribes |
SHWC |
3000 ppm IBA |
100 percent rooting |
23 |
|
|
Embeliatsjerium-kottam |
SC |
1000 ppm NAA |
Superior sprouting and Survival |
3 |
|
|
Glycyrrhiza glabra |
SC |
500 ppm IBA |
90% rooting as compared to 67% in control |
113 |
|
|
Gymnemasylvestre |
BC |
500 ppm IAA |
Superior rooting, root length and survival |
94, 95 |
|
|
Jasminum auriculatum |
SC |
4000 ppm IBA |
Better rooting percentage (13.3%) than control (6.7%) |
60 |
|
|
Jasminum grandiflorum |
SC |
2000 ppm IBA |
Better rooting percentage (50%) |
60 |
|
|
Jasminum sambac |
SC |
2000 ppm IBA |
Better rooting and survival |
114 |
|
|
Lavendula angustifolia |
BC |
2000 ppm IBA |
Superior rooting percentage |
111 |
|
|
Leptadenia reticulata |
SC |
3000 ppm IBA |
Better root induction (97.7 %) in hydroponics system |
|
|
|
Lippiajavanica |
BC and TC |
3000 ppm IBA |
Better rooting percentage |
103 |
|
|
Magnolia fuscata |
SC |
6000 ppm IBA |
Superior rooting and survival under mist |
58 |
|
|
Marsdeniatenacissima |
SC |
100 ppm IBA |
Better rooting |
94 |
|
|
Nothapodytesnimmoniana |
1. SWC |
1. 2000 ppm IBA |
Superior rooting, survival and growth parameters |
83 |
|
|
Pelargonium graveolens |
SC |
2000 ppm IBA |
Better rooting percentage and field survival |
92 |
|
|
Picrorhizakurroa |
Runners |
50 µM IBA for 12 h |
Superior rooting (87%) than control (53%) |
115 |
|
|
Piper longum |
BC |
500 ppm IAA |
Superior rooting percentage, root length and survival |
95 |
|
|
Piper longum |
SC |
500 ppm IBA |
Better rooting and field survival |
116 |
|
|
Piper nigrum |
NC |
2000 ppm IBA |
Superior root growth parameters |
117 |
|
|
Piper sarmentosum |
SWC |
1000 mg/l |
Superior rooting (92.5%) |
43 |
|
|
Plumbago zeylanica |
SC |
500 ppm NAA |
Promote quick rooting |
94 |
|
|
Pogostemon patchouli |
LHC |
1500 ppm IBA |
Superior rooting percentage and survival |
90 |
|
|
Pogostemon patchouli |
SC |
IBA and NAA |
4-6 folds increased rooting over control |
118 |
|
|
Premnaintegrifolia |
HWC |
1000 ppm IBA |
Superior rooting percentage, growth and field survival |
101 |
|
|
Rauwolfiaserpentina |
HWC |
30 ppm IAA |
75-95% success with better rooting |
119 |
|
|
Rosa damascena |
SC |
50 ppm NAA |
Maximum rooting and shoot growth |
99 |
|
|
Rutagraveolens |
SHWC |
300 ppm IBA |
Maximum rooting and field survival |
33 |
|
|
Salacia fruticosa |
SHWC |
3000 ppm IBA |
Superior rooting percentage and root length |
4 |
|
|
Taxusbaccata, |
HWC |
500 ppm IBA |
Higher callusing percentage, rooting percentage, number of roots |
93 |
|
|
Terminalia arjuna |
SC |
2000 ppm IBA |
Superior rooting parameters, shoot proliferation, maximum shoot and root biomass |
120 |
|
|
Terminalia chebula |
Juvenile SC |
4000 ppm IBA |
Better (55%) rooting than control (25%) |
121 |
|
|
Tinosporacordifolia |
SC |
100 ppm IBA |
Better rooting and plant growth |
122 |
|
|
Thymus vulgaris |
SC |
150 ppm IBA |
Early and better rooting |
123 |
|
|
Tylophoraindica |
SWC |
1000 ppm IBA |
Maximum rooting and survival |
124 |
|
|
Tylophoraindica |
SC |
3000 ppm IBA |
Better RP (93.3 %) in hydroponics |
68 |
|
|
Vitex negundo |
SC |
3000 ppm IBA |
100% sprouting |
125 |
|
|
Wrightiatinctoria |
HWC |
4000 ppm IBA |
Higher root induction |
100 |
|
(BC: basal cuttings; HWC: hard wood cuttings; LHC: leafy herbaceous cuttings; NC: nodal cuttings; SC: stem cuttings; SHWC: semi hard wood cuttings; SWC: soft wood cuttings; TC: terminal cuttings)
Role of Microbial inoculants
Microbial inoculation could help in getting healthy and vigorous transplants with well-developed root system. This in turn, helps in reducing the transplant injury and improving field establishment. Further, a number of microbial species are known to offer protection against soil borne nursery diseases. Microorganisms such as Trichoderma, Azotobacter, Azospirillum, Bacillus, Rhizobacteria, Pseudomonas, Phosphate Solubilising Bacteria (PSB),Vesicular Arbuscular Mycorrhiza (VAM) etc. have been used to induce rooting in severalspecies.
The mechanism of action of these species is diverse. Trichoderma strains are known to assist the process of decomposition of plant residues in the soil85 and also act as bio-control agent.86 Root colonization by Trichoderma spp. enhances root growth and development, crop productivity and the uptake and use of nutrients.87 Incorporation of VAM fungus, Glomus fasciculatus into the rooting medium enhanced rooting and increased the plant biomass mainly by increasing concentration of endogenous hormone level in blackpepper cuttings.88 Similarly, increased feeder root production and absorptive surface areadue to colonization of Pseudomonas fluorescens has been reported.89 Table 2 represents the applications of different microbial strains in nursery propagation of MAPs. It is evidentthat a good number of reports are available on black pepper; however, studies on otherspecies are limited (Table 6).
Table 6: Examples of use of microbial inoculants in propagation of MAPs.
| Species | Microbial inoculant | Remarks | Reference |
|
Coleus aromaticus |
G. fasciculatum, T. harzianum |
Maximum growth, total biomass and nutrients in plants |
126 |
|
Piper longum |
T. viride |
Improved plant growth and yield |
66 |
|
Piper nigrum |
Trichoderma viride (1 g/kg) |
Superior growth, survival percentage and lower disease incidence |
127 |
|
Piper nigrum |
Azospirillumsp. and Phosphobacteria |
Superior plant growth and biomass |
128 |
|
Piper nigrum |
Trichoderma spp. and VAM |
Robust, disease free cuttings |
129 |
|
Piper nigrum |
T. harzianum and Pseudomonas fluorescens |
Improved growth, reduced incidence of soil borne diseases |
130 |
|
Piper nigrum |
P. fluorescence or T. harzianum (1g/kg) |
Superior plant growth and biomass |
131 |
|
Stevia rebaudiana |
T. viride |
Superior growth and field establishment compared to growth regulator treatments |
65 |
|
Vanilla planifolia |
T. harzianum (2 g/polybag) |
Better rooting and plant growth than in growth regulators treated cuttings |
67 |
Conclusion
In recent years, there has been an ever increasing demand for MAPs from national and international markets leading to over-exploitation of their wild sources, resulting in theirdwindling population in the wild. Hence, there is an urgent need to bring these plants undercultivation for which standardization of propagation techniques is of prime importance.Though sexual propagation through seeds helps in maintaining the diversity in nature,commercial scale exploitation of these species demands uniformity, thereby necessitating thestandardization of an alternative vegetative propagation method. Considering the work donein various species as described in the article, there is tremendous scope for studying variousfactors influencing rooting of stem cuttings in MAPs. Once standardized, it could increase theefficiency of this method for mass multiplication in the MAPs for their cultivation andconservation.
Acknowledgements
Authors are thankful to the staff and officials of Department of Horticulture, University of Agricultural Sciences, Bengaluru for the support provided during the conduct of various studies compiled herein.
Conflict of Interest
Authors declare that there is no conflict of interest.
References
- Waman A.A., Bohra P. Sustainable development of medicinal and aromatic plantssector in India: an overview. Sci Cult, 2016; 82:245-250.
- Waman A.A., Bohra P. Choice of explant- a determining factor in tissue culture ofashoka (Saracaindica L.). Int J ForUsufMngt, 2013; 14:10-17.
- Tiwari R.K.S., Das K. Effect of stem cuttings and hormonal pre-treatment onpropagation of Embeliatsjeriam- cottam and Caesalpiniabonduc, two importantmedicinal plant species. J Med Plants Res, 2010; 4:1577-1583.
- Saumya M.T., Jijeesh C.M., Hrideek T.K., Surendran T. Standardization ofPropagation through cuttings in Salacia fruticosaHeyne ex Lawson: a medicinal plantendemic to Western Ghats. Intl J Agric Environ Biotechnol, 2014a;7:565-570.
CrossRef - Nalawade M.S., Tsay, Hsin-Sheng.In vitro propagation of some important Chinesemedicinal plants and their sustainable usage. In Vitro Cell Dev Biol- Plant, 2004; 40:143-154.
CrossRef - Deepak K.G.K., Suneetha G., Surekha C. A simple and effective method forvegetative propagation of an endangered medicinal plant Salacia oblonga Wall. J NatMed, 2016; 70:115–119.
CrossRef - Smitha G.R., Waman A.A. A Handy Guide to Medicinal and Aromatic Plants. DayaPublishing House, New Delhi, 2015; pp. 241.
- Gehlot A., Arya S., Arya I.D. Vegetative propagation of Azadirachtaindica A. Juss(Neem) through cuttings: A review. NativaSinop, 2014; 2:239-246.
CrossRef - Wankhar B., Tripathi R.S. Competitive fitness of Centellaasiatica populationsraised from stem cuttings and seedlings. Proc: Plant Sci, 1990; 100:239-245.
- Ahkami A.H., Melzer M., Ghaffari M.R., Pollmann S., Javid M.G., Shahinnia F., HajirezaeiM.R., Druege U. Distribution of indole-3-acetic acid in Petunia hybrid shoot tipcuttings and relationship between auxin transport, carbohydrate metabolism andadventitious root formation. Planta, 2013; 238:499–517.
CrossRef - De Klerk G.J. Rooting of microcuttings: Theory and practice. In Vitro Cell DevBiol- Plant, 2002; 38:415–422.
CrossRef - Baruah A. Vegetative propagation of two species of Cinnamomum Schaeffer. MedPlants, 2009; 1(2):121-123.
CrossRef - Beigh S.Y., Nawcho A.I., Iqbal M. Cultivation and conservation of Aconitumheterophyllum: a critically endangered medicinal herb of the Northwest Himalayas. JHerbs Spices Med Plants, 2005; 11:47-56.
CrossRef - Smitha G.R. Vegetative propagation of Ashoka [Saracaasoca (Roxb.) de Wilde]-an endangered medicinal plant. ResCrops, 2013; 14:274-283.
- Rice R.P., Rice L.W., Tindall H.D. Fruit and vegetable production in warm climates.Macmillan Education Limited, London and Basingstoke, 1990; pp.486.
- Evans E. Propagation by stem cutting: instructions for a home gardener. NewCarolina Cooperative Extension Service, New Carolina, 1999; pp. 6
- Dick J.M., Dewar R.C. A mechanistic model of carbohydrate dynamics duringadventitious root development in leafy cuttings. Ann Bot, 1992; 70:371–377.
CrossRef - Hartmann H.T., Kester D.E. Plant propagation principles and practices. Prentice Hallof India Pvt. Ltd., New Delhi, India, 1978.
- Agbo C.U., Obi I.U. Macropropagation technique for different physiological ages of Gongronemalatifolia Benth. cuttings. Afr J Biotech, 2006; 5:1254-1258.
- Nagaraja G.S., Muthappa Rai B.B., Guruprasad T.R. Effect of intermittent mist andgrowth regulators and propagation of Jasminum grandiflorum by different types ofcuttings. Haryana J Hortic Sci, 1991; 20:183-188.
- Rathnayake R.M.D.H., Dharmadasa R.M., Abeysinghe D.C. Suitable maturity stage,type of cuttings and potting media for vegetative propagation of PogostemonheyneanusBenth. World J Agric Res, 2015; 3:203-207.
- Palanisamy K., Kumar P. Effect of position, size of cuttings and environmentalfactors on adventitious rooting in neem (Azadirachtaindica A. Juss). For Ecol Mgt, 1997;98:277–288.
CrossRef - Saumya M.T., Surendran T., Hrideek T.K. Vegetative propagation for differentphysiological ages of Embeliaribes cuttings in different seasons. Res J Agric For Sci, 2014b;2:8-12.
- Nayana E.K.E., Subasinghe S., Amarasinghe M.K.T.K., Arunakumara K.K.I.U., KumarasingheH.K.M.S. Effect of maturity and potting media on vegetative propagation of Salaciareticulata through stem cuttings. Intl J Minor Fruits Med Arom Plants, 2015;1:47-58.
- Danthu P., Soloviev P., Gaye A., Sarr A., Seck M., Thomas I. Vegetative propagationof some West African Ficus species by cuttings. Agrofor Sys, 2002; 55:57–63.
CrossRef - Gangoo S.A., Qaisar K.N., Mughal A.H., Makaya A.S. Propagation of FicuspalmataForsk by cuttings. Indian For, 1997; 123:87–88.
- Dinesh Kumar,Ram Chandra, Aishwath O.P. Rooting potential of Indian bdellium (Commiphorawightii)cuttings taken from different shoot positions.Indian J Hort, 2006;63:319-323.
- Farooqi A.A., Shenoy R., Sreeramu B.S. Influence of planting material and growthregulators on the rooting of cutting of Rosa damascena Mill. Ind Perf, 1994; 38:133-143.
- Khosla M.K., Pushpangadan P.A faster method for vegetative propagation ofClocimum (Ocimumgratissimum L.). Indian J For, 1995; 18:56-60.
- Went F.W. On a substance causing root formation. In: Proc Kon Ned Akad Wet, 1929;32:547-555.
- Dhar A.K. Propagation of Plumbago zeylanica.J Med Arom Plant Sci, 1999; 21(2):304-307.
- Shwetha H. Propagation of Indian lavender (Bursera delpichiana) through cuttingsunder mist. M.Sc. (Agri.) thesis, 2005; University of Agricultural Sciences, Dharwad, India.
- Niranjan Kumar. Influence of planting material and growth regulators on rooting ofstem cuttings in Garden Rue (Rutagraveolens L.). M.Sc.Thesis, 1993; University ofAgricultural Sciences, Bengaluru, India.
- Shenoy R. Influence of planting material and growth regulatorson rooting of stemcuttings in Rosa damascena. M.Sc. (Hort.) thesis, 1992; University of Agricultural Sciences,Bangalore, India.
- Zem L.M., Zuffellato-Ribas K.C., Radomski M.I., Koehler H.S. Rooting of semihardwood stem cuttings from current year shoots of Drymisbrasiliensis.Ciencia Rural, 2016;46:2129-2134.
CrossRef - Nelson S.C. Noni cultivation in Hawaii. Res. Note, 2001; University of Hawaii, Honolulu.
- Vezhavendran S., Ponnaiyan P. An approach to obtain true noni through cuttings.Intl J Noni Res, 2005; 1:27-30.
- Srikantaprasad D. Standardization of vegetative propagation of MorindacitrifoliaL. var. citrifolia by cuttings, M.Sc. (Hort.) thesis, 2009; University of Agricultural Sciences,Bangalore, India.
- Singh S.S., Singh S. Effect of nodal cuttings and rooting media on the propagationof black pepper under South Andaman condition. Indian Cocoa, Arecanut Spices J, 1989;12:122-123.
- Devasahayam S., John Z.T., Jayashree E., Kandiannan K., PrasathD.,Santhosh J.E.,Sasikumar B., Srinivasan V., Suseela B.R. Black pepper, extension pamphlet, 2015; ICAR-Indian Institute of Spices Research, Kozhikode, pp. 24.
- Kempegowda K., Ganesh, Mahendrakar P.Efficacy of growth regulators for rootingand sprouting of long pepper cuttings (Piper longum L.). J Plantation Crops, 2006; 34:650-651.
- Chalapathi M.V., Thimmegowda S., Devakumar N., Rao G.G.E., Mallikarjuna K. Influence of length of cuttings and growth regulators on vegetative propagation of stevia(Stevia rebaudiana Bert.). CropRes, 2001; 21:53-56.
- Waman A.A., Bohra P., Chakraborty G. Vegetative propagation of Piper sarmentosumRoxb.- a medicinally important species. Curr Agric Res J, 2019; 7:46-52.
CrossRef - Bordin I., Hidalgo P.C., Bürkle R., Roberto S.R.Efeitodapresença da folha noenraizamento de estacassemilenhosasdeporta-enxertos de videira. CiênciaRural, 2005; 35:215-218.
CrossRef - Abidin N., Metali F. Effects of different types and concentrations of auxins onjuvenile stem cuttings for propagation of potential medicinal Dilleniasuffruticosa (Griff.Ex Hook. F. and Thomson) Martelli Shrub. Res J Bot, 2015; 10:73-87.
CrossRef - Lima R.L.S., Siqueira D.L., Weber O.B., Cazetta J.O.Comprimentodeestacas e partedo ramonaformação de mudasdeaceroleira. Rev Bras Fruticultura, 2006; 28:83-86.
CrossRef - Santos M.Q.C., Lemos E.E.P., Salvador T.L., Rezende L.P., Salvador T.L., Selva J.W., Barros P.G.,Campos R.S. Rooting soft cuttings of soursop (Annona muricata) ‘Giant ofAlagoas’. ActaHort, 2011; 923:241-246.
CrossRef - Correa C.F., Biasi L.A.Área foliar e tipo de substratonapropagaçãoporestaquia decipó-mil-homens (Aristolochia triangularis). Rev Bras Agrociência, 2003; 9:233-235.
- Okoro O.O., Grace J. The physiology of rooting Populus cuttings. I. Carbohydratesand photosynthesis. PhysiolPlanta, 1976; 36:133–138.
CrossRef - Gbadamosi A.E., Oni O. Macropropagation of an endangered medicinal plant,EnantiachloranthaOliv. J Arboricult, 2005; 31:78-82.
- Bona C.M., Biasi L.A. Influence of leaf retention on cutting propagation ofLavendula dentata L. Revista Ceres, 2010; 57:526-529.
CrossRef - Bhattacharjee S.K., Thimmappa D.K. Studies on the growth hormone, length ofcuttings and number of leaves on root formation of Pogostemon patchouliBenth. Ind Perf, 1991;35:71-76.
- Jadhav S.G., Jadhav B.B., Apte U.B. Influence of growth regulators on growth and oilcontent of Patchouli (PogostemoncablinBenth.). Ind Perf, 2003; 47:287-289.
- Bettoni M.M., Storck R.C., Penuela L.F., de Moraes C.P. Vegetative propagation ofpatchouli by cutting. Sci Agric, 2010; 11:417–420.
CrossRef - Danthu P., RamarosonN., Rambeloarisoa G. Seasonal dependence of rootingsuccess in cuttings from natural forest trees in Madagascar. Agrofor Sys, 2008; 73:47–53.
CrossRef - Arce P., Balbo. Seasonality in rooting of Prosopis chilensis cuttings and in vitromicropropagation. For Ecol Mgt, 1991; 40:163–173.
CrossRef - Raines M.A. Use of spray chamber in experiments in plants. Am J Bot, 1940; 27:18-26.
- Balakrishna M., Bhattacharjee S.K. Studies on propagation of ornamental treesthrough stem cuttings. Indian J Hortic, 1991; 48:84-87.
- Singh S.P., Singh. Effect of rooting media and Indo-3-Butric acid on root formationin Jasminum sambac cv. ‘Motia’ Semi hardwood cuttings under intermittent mist. ProgHortic, 1979; 11:49-51.
- Sreelatha U., Gopikumar K., Aravindakshna M. Vegetative propagation of jasminethrough cuttings and layers. Agric Res J Kerala, 1991; 29:67-70.
- Konedenedo J.R., Propagation of vanilla by cuttings. Philippines Agric, 1955; 39: 393-401.
- Vasundhara M., Farooqi A.A. Propagation of some of medicinal and aromatic plantsunder cover, In: Proceedings of the International Seminar on Protected Cultivation in India, 1997; University of Agricultural Sciences, Bengaluru, India.
- Farooqi A.A., Vasundhara M., Khan M.M., Krishna M.R., Kariyanna, Shyamalamma S. Studies on the propagation of difficult to root species of medicinal and aromatic crops in medium cost greenhouse. A decade of R and D underPlasticulture Development Center, 1998; University of AgriculturalSciences, Bengaluru, India.
- Murthy G. Studies on the influence of environmental conditions, type of cuttings,growth regulators and bioinoculants on rooting and growth of vanilla (VanillaplanifoliaAndr.) cuttings, M.Sc. (Hort.) Thesis,2005; University of Agricultural Sciences,Bengaluru, India.
- Smitha G.R., Umesha K. Vegetative propagation of stevia [Steviarebaudiana(Bertoni) Hemsl.] through stem cuttings. J Trop Agric, 2012; 50(1-2):72-75.
- Smitha G.R. Standardization of organic cultivation and propagation through stemcuttings in long pepper (Piper longum Linn.) and stevia (Stevia rebaudiana (Bertoni)Hemsl. Ph.D.(Hort.) Thesis, 2010; University of Agricultural Sciences, Bengaluru, India.
- Murthy G., Umesha K., Smitha G.R., Krishnamanohar R. Effect of growth regulatorsand bio-inoculants on rooting and growth of vanilla stem cuttings.Indian JHortic, 2010;67(1):90-93.
- Mahendru P., Shekhawat N.S.,Rai M.K., KatariaV., Gehlot H.S. Evaluation ofaeroponics for clonal propagation of Caralluma edulis, Leptadenia reticulata andTylophoraindica–three threatened medicinal Asclepiads. Physiol Mol Biol Plants, 2014;20(3):365–373.
CrossRef - Long J.C. The influence of rooting media in the character of roots produced bycuttings. Proc Am Soc Hortic Sci, 1932; 29:352-355.
- Bruckel D.W., Johnson E.P. Effect of pH on rootability of Thujaorientalis. PlantPropagation, 1969; 15(4):10-12.
- Shridhar, Singh S. Effect of nodal cutting and rooting media on thepropagation of black pepper. J Andaman Sci Assoc, 1989; 5(2):149-150.
- Bogantes-Arias A. Rooting of pepper (Piper nigrum L.) cuttings in differentsubstrates. Investigation Agricola, 1989; 3(1):18-20.
- ThankamaniC.K., Sivaraman K., KandiannanK. Response of clove (Syzygiumaromaticum) seedlings and black pepper (Piper nigrum L.) cuttings to propagating mediaunder nursery conditions. J Spices Arom Crops, 1996; 5(2):99-104.
- Sit A.K., Chenchaiah K.C., Acharya G.C. Effect of rooting media on production ofquality black pepper cuttings by rapid multiplication. The Hortic J, 2005; 18(1):39-41.
- Zubenko V.F., Rogovskii S.V., Chudnoski B.D. Stimulation of rooting of steviacutting and root of transplants by phyto-hormones.V. T. Lenina, 1991; 2:16-18.
- Siddagangaiah, Vadiraj B.A., Sudharshan M.R., Kumar K. Standardization of rootingmedia for propagation of vanilla (VanillaplanifoliaAndr.). J SpicesArom Crops, 1996;5(2):131-133.
- Srinivasan V., Hamza S. Use of coir pith compost as a component of nurserymixture for spices. Contributory papers, Centennial Conference on Spices and AromaticPlants, 2005; Indian Society of Spices, Calicut, pp. 91-96.
- Krishnamoorthy B., Rema J., Mathew P.A., Abraham J. Standardization of rootingmedia for propagation of cuttings of Cinnamon varieties, Navyashree and Nityashree.Spice India, 2000; 8:10-11.
- Garner R.J. Technical communication No. 14 on propagation of cuttings and layers, 1944;Imperial Bureau of Horticulture and Plantation Crops East Malling Kent, England.
- Strydem D.K., Hartman H.T. Effect of indolebutyric acid on respiration and nitrogenmetabolism in Marianna 2624 plum soft wood cuttings. Proc Am Soc Hortic Sci, 1960; 16:125-133.
- Woodward A.W., Bartel B. Auxin: regulation and interaction. Ann Bot,2005;95:707-735.
CrossRef - Navamaniraj K.N., Srimathi P., ParamathmaM.,ParthibanK.VegetativePropagation of Annatto (Bixaorellana) (Linn.). Madras Agric J, 2008; 95 (7-12):493-495.
CrossRef - Paneerselvam K., Bhavanisankar K., Jayapragasam K. Effect of growthregulators and planting media on rooting of cuttings of NothapodytesnimmonianaMabberly. Ind J Plant Physiol, 2004; 9:308-312.
- Gopichand, Nehria R., Meena R.L., Singh R.D., Ahuja P.S. Effect of different growthregulators on vegetative propagation of Ginkgo biloba. BharatiyaVaigyanikevamAudyogikAnusandhanPatrika, 2006; 14(1):21-24.
- Herper S.H.T., Lynch J.M.Colonization and decomposition of straw by fungi.Transact Brazilian Mycol Soc, 1995;85:655-661.
CrossRef - Harman S., Schichler H., Chef I. Molecular mechanism of lytic enzymes involved inthe biocontrol activity of Trichoderma harzianum. Microbiol, 1996; 142:2321-2331.
CrossRef - Harman G.V., HomellC.R.,Viterbo A., Chet I.,LoritoM. Trichodermaspp.opportunistic, avirulent plant symbionts. Nature Rev Microbiol, 2004; 2:43-56.
CrossRef - Anandaraj M., Sarma Y.R. Effect of vesicular arbuscular mycorrhiza on rooting ofblack pepper (Piper nigrum L.). J Spices Arom Crops, 1994; 3:39-42.
- Anandaraj M., Sarma Y.R. The potential of IISR-6 in disease management of spicecrops. In: VI International workshop on plant growth promoting Rhizobacteria, 2003; IndianInstitute of Spices Research, Calicut.
- Selvarajan M., Madhav Rao V.N. Propagation of patchouli through leaf, single node and split cuttings. Ind Perf, 1981; 25(1): 40-45.
- Nayagam J.R., Varghese M.K.I. Sprouting value index: a mathematical approach for evaluation of rooting media efficiency. Am J Environ Protect, 2015; 4(6): 271-274.
CrossRef - Siddique M.A.A., Anjum A., Khan F.U., Khan F.A. Clonal propagation of scented geranium (Pelargonium graveolens (L.) Herit). Prog Agric, 2012; 12(1): 19-25.
- Anjum Q., Sharma L.K., Ganie S.A., Rather M.M., Rather H.A. Effect of auxins on macropropagation of Taxus baccata Linn. through stem cuttings. Indian For, 2011; 137(12): 1382-1385.
- Somashekhar B.S., Sharma M. Propagation Techniques of Commercially Important Medicinal Plants, 2002; Foundation for Revitalisation of Local Health Traditions, Bangalore
- Sundharaiya K., Ponnuswami V., Jayajasmine A. Effect of growth regulators in the propagation of Gymnemasylvestre, Coleus forskohlii and Piper longum. South Indian Hortic, 2000; 48: 172-174.
- Suseela T., Reddy G.S. Influence of IBA and NAA on rooting of Adathodavasica. Prog Hortic, 2015; 47(2): 354-356.
CrossRef - Agro-Techniques of selected Medicinal Plants, Volume III, 2016. National Medicinal Plants Board, Ministry of Ayush, New Delhi.
- Ali M., Malik A.R., Sharma K.R. Vegetative propagation of Berberis aristata DC an endangered Himalayan shrub. J Med Plants Res, 2008; 2(12): 374-377.
- Khan M.S., Khan R.U., Waseem K. Effect of some auxins on growth of damask rose cuttings in different growing media. J Agri Soc Sci, 2006; 2(1): 13-16.
- Rao P.S., Rao M., Venkaiah K.A., Satyanarayana W.V. Rooting of stem cuttings of Wrightiatinctoria(Roxb.) R.Br.–an important medicinal plant. Indian For, 1999;125: 427-428.
- Sharma Y., Venugopal C.K., Hegde R.V. Propagation of a rare medicinal plant species Premnaintegrifolia by hardwood cuttings. Indian J Hortic, 2011; 68(1): 108-112.
- Sharma Y., Venugopal C.K., Vasudeva R., Manjunath A.V., Yashwant Kumar K.H. Propagation of an endangered species, Celastruspaniculata by hardwood cuttings. Asian J Hort, 2010; 5(2): 383-387.
- Soundy P., Mpati K.W., duToit E.S., Mudau F.N., Araya H.T. Influence of cutting position, medium, hormone and season on rooting of fever tea (Lippiajavanica L.) stem cuttings. Med Arom Plant Sci Biotech, 2008; 2(2): 114-116.
- Lone S.A., Yadav A.S., Bajaj A., Sharma A.K., Badkhane Y., Raghuwanshi D.K. Conservation strategies for threatened medicinal plant–Barleriaprionitis L.–using in vitro and ex vitro propagation techniques. Arch Phytopathol Plant Protect, 2012; 45(11): 1327-1340.
CrossRef - Kulloli R.N., Kumar S., Jindal S.K., Singh M. Effect of seasoning on sprouting of stem cutting and their survival in threatened medicinal important Plant, CommiphoraWightii(Arn.) Bhan. Intl J For Crop Improve, 2015; 6(2): 116-122.
CrossRef - Agro-Techniques of selected Medicinal Plants, Volume II, 2014. National Medicinal Plants Board, Ministry of Ayush, New Delhi
- Bhattarai N., Joshi S.D. Seasonal variation in rooting behavior of Gingko biloba L. cuttings. J Nat Hist Mus, 2012; 26:175-180.
CrossRef - Upadhyay R.K., Verma R.S., Singh V.R., Bahl J.R., Sharma S.K., Tewari S.K. New agrotechnology for quality planting material production of rose-scented geranium (Pelargonium graveolens L. Herit.). J Appl Res Med Arom Plants, 2016; 3: 128–130.
CrossRef - Rajashekara. Standardization of vegetative propagation of stevia (Stevia rebaudianaBertoni) through stem cuttings. M.Sc. (Hort.) thesis, 2004; University of Agricultural Sciences, Bengaluru, India.
- Rekha K.M. Standardization of vegetative propagation of stevia (Stevia rebaudianaBert) through stem cuttings by using growth regulators. M.Sc. (Hort.) Thesis, 2008; University of Agricultural Sciences, Bengaluru, India
- Kumar N., Sreeja K.V. Effect of growth regulators on the rooting ability of Lavender (Lavendula angustifolia Mill.). Ind Perf, 1996; 40(3): 93-94.
- Devarnavadagi S.B., Sajjan A.S., Wali S.Y. Effect of growth regulator on induction of adventitious rooting in stem cutting of neem. Karnataka J Agric Sci, 2005; 18(1): 210-211.
- Massodi N.A., Srivastava L.L., Mir N.A. Studies on the effect of growth regulators on initiation of rooting in Glycyrrhiza glabra L. cutting. Indian J Plant Physiol, 1994; 37(1-4): 28-29.
- Singh A.K. Effect of auxins on rooting and survival of jasmine (Jasminum sambac) stem cuttings. Prog Hortic, 2001; 33(2): 174-177.
- Chandra B., Palni L.M.S., Nandi S.K. Propagation and conservation of PicrorhizakurrooaRoyle ex Benth.: an endangered Himalayan medicinal herb of high commercial value. BiodiverConser, 2006; 15: 2325–2338.
CrossRef - Singh A.K., Rajesh S., Mittal A.K., Singh Y.P., Shiva J. Effect of plant growth regulators on survival, rooting and growth characters in long pepper. Prog Hort, 2003;35(2): 208-211.
- Zeubini R. Effects of IBA and planting method on the growth of pepper. PemberitaanPenelitianTanamanIndustai, 1984; 49: 55-59.
- De Guzman C.C., Reglos R.A., Tuico A.V., Flavier M.E.E. Rooting performance of cuttings of Patchouli, Pogostemoncablin (BICO) Benth. Philippine Agric, 1994; 77(4): 431-436.
- http://www.svlele.com/herbal/sarpagandha.htm.
- Agro-Techniques of selected Medicinal Plants, Volume I, 2009; National Medicinal Plants Board, Ministry of Ayush, New Delhi.
- Madhwal K., Kumar P., Nautiyal S., Rayal S.P., Nautiyal D.P. Rooting response of juvenile shoot cuttings of Terminalia chebula Retz. under different hormonal treatments. Indian For, 2008; 134(2): 270-274.
- Mishra Y., Usmani G., Chawhaan P.H., Mandal A.K. Propagation of Tinosporacordifolia(Willd.)Miers ex Hook. f. &Thoms. through mature vine cuttings and their field performance. Indian For, 2010; 136(3): 88-94.
- Chandregowda M., Shiva P.B.L. Effect of growth regulators and methods of application on rooting of thyme (Thymus vulgaris) cuttings. Mysore J Agric Sci, 2008; 42(1): 9-14.
- Pal M., Goel C.L., Bhandary H.C.S. Effect of auxins on rooting branch cutting of anatmul (Tylophoraindica). Indian J For, 1993; 16(2): 183-186.
- Bhagya H.P., Sreeramu B.S. Effect of growth regulators on vegetative propagation of Vitex negundo L. Asian J. Hort, 2013; 8(1): 209-212.
- Eranna N., Mallikarjumaiah R.R., Bagyaraj D.J., Suresh C.K. Response of Coleus aromaticusto Glomus fasciculatusand other beneficial soil microflora. J Spices Arom Crops, 2001;10(2):141-143.
- Satapathy S.K., Ray A.K., Chakrabarthy K., Subramanian P., Maheshwarappa H.P., Acharya G.C. Performance of rooting media for production of quality rooted cuttings of black pepper under Assam conditions. Spice India, 2006; 19(2): 22-26.
- Thankamani C.K., Srinivasan V., Hamza S., Kandianna K., Mathew P.A. Evaluation of nursery mixture for planting material production in black pepper (Piper nigrum L). J Spices Arom Crops, 2007; 16(2): 111-114.
- Sarma Y.R. 2000. Diseases of black pepper and their management. Training manual. IISR, Calicut, India.
- Jisha P.J., Paul D., Kumar A., Anandaraj M., Sarma Y.R. Biocontrol consortium for a cropping system involving black pepper, ginger and cardamom. Indian Phytopathol, 2002; 55: 374.
- Thankamani C.K., Sreekala K., Anandaraj M. Effect of Pseudomonas fluorescence (IISR-6) and Trichoderma harzianum(P-26) on growth of black pepper (Piper nigrum L.) in the nursery. J Spices Arom Crops, 2005; 14(2): 112-116.
Abbreviations
Indole-3-acetic acid (IAA); Indole-3 butyric acid (IBA); Farmyard manure (FYM); Medicinal and aromatic plants (MAPs); Naphthaleneacetic acid (NAA); Vesicular Arbuscular Mycorrhiza (VAM)
Views: 4,579

