Introduction
Polycyclic aromatic hydrocarbons (PAHs) are organic pollutants which are ubiquitously found in all environmental compartments.1 High concentration PAHs may be present in petroleum-related industries, coal-tar, gas production as well as timber-based industries.2 Marine and coastal ecosystems are one of the major sediments for PAHs3 which become available to organisms dwelling in sediments like shellfish and able to bioaccumulate PAHs actively. Consumption of those contaminated dwelling organisms will lead to concentrated of PAHs in human body. PAHs have been included in the priority pollutant lists of European Union (EU)4 and Environmental Protection Agency (EPA)5 owing to the fact that they are carcinogenic. Diet is the main contributor to human exposure to PAHs that is through the consumption of contaminated foods (88-98%).6
Monitoring of harmful PAHs becomes a crucial analytical issue due to their very low concentration plus the complexity of environmental matrices. Sample pre-treatment is therefore required to achieve the required sensitivity and selectivity.7 Sample pre-treatment is an essential part, and often the time-determining step, of an analytical scheme. It significantly affects the quality of the results obtained, throughput and cost of analyses. Thus, selecting an appropriate pre-concentration technique and optimizing it are key steps in the development of an accurate, reliable analytical procedure. Several extraction methods such as Soxhlet and liquid-liquid extraction methods have been investigated for sample preparation of soil and most of these involved an evaporation step. However, evaporation leads to the loss or low recoveries of the volatile PAHs.8 Solid phase extraction (SPE) is a versatile pre-concentration technique in which analytes in solution can be isolated from their sample matrix through interactions with a solid phase. SPE first gained attraction as an analytical technique in the 1970’s, leading to the development of commercial SPE cartridges containing bonded silica absorbents, which are still the most commonly used today.9 For aqueous sample, silica-based is the most commonly employed SPE absorbents to extract analytes.10
Recent efforts focused on the production of silica using renewable precursors with potential for satisfying both economic and environmental targets. Silica produced from agro-wastes has been noted to give high significant advantages. In Malaysia, after every harvesting season, rice husk is produced with large amounts of more than 400,000 metric tons annually.11 At harvesting time, the rice husk was burnt to get rid of its large quantities. The combustion of rice husk has produced rice husk ash (RHA) with its main content is silica as well as released carbon dioxide, a well-known greenhouse gas. By using RHA as alternative silica sources not only reducing the greenhouse effect but also cost of purchasing expensive commonly used silica precursor such as tetra ethyl orthosilicate. The Si surface consists of pairs of atoms that are formally linked together with a very weak Si-Si double bond.12 In another study reported that the bonding occurs between acenaphthylene with silicon (Si) through Si-C linkage, which represent a potential for coupling extended π-electron systems with silica surfaces.13
The enthusiasm of researchers in synthesizing new nano-materials without considering their toxic effects on the plant growth has become a newly emerging topic nowadays. The impacts of nanoparticles (NPs) and carbon nanotubes (CNTs) on plant germination have been reported in previous studies with different results.14-18 For instance, silica NPs have been found to reduce the germination of cotton14 and Arabidopsis thaliana,15 whereas the germination of tomato is not affected by CNTs.16 In view of the acclaimed reports on the use of nanotechnology as an emerging discipline that appears in virtually all areas of technology, it is important to understand the germination process associated with the nano-materials. The increase of current advancement of nanotechnology and its use in waste utilization has caused the understanding of the role of nano-materials derived from the wastes in seeds germination to be vital.
In the present work, silica nano-powder has been extracted from rice husk for use as SPE absorbent to extract three selected PAHs, namely acenaphthene, acenaphthylene and naphthalene from wastewater samples. A number of extraction parameters including amount of absorbent, sample volume and, the type and volume of elution solvent were optimized. To the best of our knowledge, no report has been published on the use of silica derived from rice husk as the SPE absorbent combined with gas-chromatography equipped with mass spectrometry (GC-MS) method to determine PAHs content in industrial wastewater samples. In addition, the toxicity study of the silica nano-powder derived from agro-waste was investigated because of limited information available on its toxicity.
Materials and Methods
Reagents and Chemicals
Rice husk (RH) was obtained from local company in Kedah, Malaysia. RH is used as main material to produce silica nano-powder. Hydrochloric acid (HCl) and sodium hydroxide (NaOH) were purchased from Friendemann Schmidt Supplies (Selangor, Malaysia) and Merck Mellipore (United State), respectively. Both ammonium hydroxide (NH4OH) and sulphuric acid (H2SO4) were purchased from J. T. Baker Chemical Co. (San Francisco, California). Acenaphthene, acenaphthylene and naphthalene was purchased from Sigma Aldrich (Darmstadt, Germany)). Acetonitrile (HPLC grade) was obtained from J.T. Baker Chemicals (China)) and dichloromethane (HPLC grade), n-hexane (HPLC grade) and ethyl acetate (HPLC grade) were supplied by Merk Mellipore (Darmstadt, Germany). All chemicals were analytical reagent grade and were used without further purification. Ultrapure deionized water (resistivity, 18.2 MΩ /cm) was generated by a model Elga Purelab Option-Q DV25 system (United Kingdom). Stock solution of PAHs (100 mg/L) was prepared by dissolving an appropriate amount of PAHs in acetonitrile and stored at 4 °C when not use. Working standard solutions were freshly prepared by diluting the stock solution with water. Wastewater samples were collected from wastewater drainage near the petroleum industrial area in Gebeng, Pahang, Malaysia. Tomato seed was purchased from a local market in Kuantan, Pahang, Malaysia.
Apparatus
SPE polypropylene tube with frits was purchased from Sigma Aldrich (Darmstadt, Germany) while 12-port SPE vacuum manifold from Supelco (Bellefonte, PA, USA). Analysis of PAHs was performed on a Varian Saturn 2000 GC-MS (Agilent, USA) equipped with fused-silica capillary column (30×0.25mm ID, 0.25 μm film thickness). The injector was set at 280°C in split less mode. Injection volume was 1.0 μl. The temperature programmed was: 40°C, held for 5 min, rate 10°C/min to 179°C, held for 2 min, rate 9°C/min to a final temperature of 300°C, held for 10 min. The mass spectrometer was operated in the electron ionization (EI) mode. Samples were analyzed in the selected ion monitoring (SIM) mode.
Procedure
Preparation of silica nano-powder as SPE absorbent
Initially, rice husk was washed thoroughly with distilled water to separate any other compounds or impurities before dried at 110°C for 24 hr. Further treatment of the dried rice husk (RH) was conducted according to the literature19 with some modifications. The RH was refluxed with acidic solution (1.0 M HCl) for 90 min by constant stirring. After that, it was cooled at room temperature and kept in desiccator for 20 hr before decanted. Next RH was washed with warm distilled water until the acid is free from the rinse. Again, the treated RH was dried at 110°C for 24 hr. The dried, treated RH was burned inside furnace at 700°C by 10°C/min to get rice husk ash (RHA). 20 g of RHA was stirred in 160 ml of 2.5 M NaOH and heated for 3 hr in a covered beaker by constant stirring. Next, it was filtered and washed by using 40 ml of boiling distilled water. Then, the transparent, viscous, and colorless solution was cooled down at the room temperature. About 10 M of H2SO4 was added with constant stirring until pH 2. Then, add NH4OH until pH is 8.5 by allowing them to stand for 3 hr under room temperature.
The obtained silica is refluxed for 4 hr in 6 M HCl. Next, it was washed several times with deionized water to make it free from acid. The next step, it was dissolved in 2.5 M of NaOH solution and stirred before adding 1.0 M H2SO4. The warm deionized water was used repeatedly to make it free from alkali. Lastly, it was dried at temperature of 80°C for 24 hr. The silica nano-powder (200 mg) was ground using a pestle and mortar and packed manually into an empty 3 ml SPE polypropylene tube with frits.
Extraction Procedure
The filled SPE cartridge was then placed in a 12-port SPE vacuum manifold and conditioned by passing 5.0 ml methanol. 10.0 ml deionized water and 1.0 ml of 2.0 ppm spike wastewater sample were loaded in each cartridge at a flow rate of 0.5 ml/min and passing air for 10 min. The analytes were eluted by 2 × 2.0 ml methanol at a flow rate of 0.5 ml/min. The volume was then reduced to 1.0 ml under nitrogen flow prior to GC-MS.
Optimization of variables affecting the extraction procedure
In the present study, several important SPE parameters including absorbent amount, sample volume, type of elution solvent and elution solvent volume were thoroughly optimized. During the optimization absorbent amount (100, 150, 200, 250, 300 and 350 mg), other parameters are kept constant as follows: sample volume of 1.5 ml and 2.0 ml of acetonitrile as type of elution solvent. While for optimization of sample volume (1.0, 1.5, 2.0 and 2.5 ml), other parameters are kept constant as follows: absorbent amount of 200 mg and 2.0 ml of acetonitrile as type of elution solvent. For optimization of type of elution solvent (acetonitrile, dichloromethane, n-hexane and ethyl acetate), other parameters are kept constant as follows: absorbent amount of 200 mg, sample volume of 2.0 ml and 2.0 ml of elution solvent. Lastly, to optimize the volume (2.0, 4.0, 6.0 and 8.0 ml) of elution solvent (n-Hexane), other parameters are kept constant as follows: absorbent amount of 200 mg and sample volume of 2.0 ml. All experiments were repeated thrice. Once the optimum conditions were identified, wastewater samples were analyzed using the SPE packed with silica nano-powder and GC-MS method.
Method Validation
The linearity, limit of detection (LOD), limit of quantification (LOQ), repeatability, reproducibility and recovery of the developed method was validated under the optimum conditions. A calibration curve was plotted for PAHs using water spiked with known concentration of PAHs. The repeatability (intra-day) and reproducibility (inter-day) were obtained from three replicates and expressed as relative standard deviations at 2.0 ppm of PAHs. The recoveries of all samples were calculated by comparing the final amounts of PAHs after extraction from water samples with the corresponding spiking amounts on the calibration curve.
Toxicity Study
The effect of silica nano-powder on seed germination was tested according to Siddiqui & Al-Whaibi.20 Tomato seed was bought from a local market in Kuantan, Pahang, Malaysia. Ten tomato seeds in each petri dish was watered with S1 (50 ppm), S2 (125 ppm), S3 (250 ppm), S4 (375 ppm), respectively and compared with S0 (control). The silica nano-powder was diluted in water. The percent seed germination was reported in terms of mean germination time21 and vigour index.22 All the treatments were conducted in two replicates.
Results and Discussion
Optimization of extraction parameters
Four important factors that affect the extraction efficiency of silica nano-powder as SPE absorbent were studied, namely absorbent amount, sample volume, type of elution solvent and elution solvent volume. To achieve high adsoprtion performance for the PAHs, different amounts of silica-nano powder were experimentally investigated. The peak area of naphthalene was notably increased with the increase of absorbent amount from 100 to 150 mg and showed a decreasing trend when the absorbent amount increased to 200 until 350 mg (Figure 1). However, the highest amount of both acenaphthene and acenaphthylene extracted were observed with 200 mg of absorbent, but no significance differences when using 150 mg absorbent. Thus 150 mg of silica absorbent was chosen as the optimum absorbent amount for use in subsequent experiments. Silica nano-powder improves extraction efficiency because their nano-size provide large surface area-to-volume ratio.23,24 Additionally, poor recovery of analytes when using absorbent amount more than 150 mg might be due to the incomplete elution of the analytes from the absorbent,25 which is also depending on the elution solvent volume.
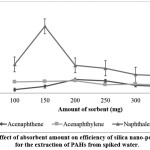 |
Figure 1: Effect of absorbent amount on efficiency of silica nano-powder SPE for the extraction of PAHs from spiked water. Click here to View Figure |
The percentage recovery of the analytes is highly depended on the sample volume.10 The sample volume was varied between 1.0 to 2.5 ml. Figure 2 shows that the sample volume profile for the acenaphthene extracted increase as the sample volume was increased from 1.0 to 1.5 ml, but decreased when the sample volume was further increased to 2.0 and 2.5 ml. As for both acenaphthylene and naphthalene, the highest peak areas were obtained when using 2.0 ml of sample. However when compared to 1.5 ml sample volume, there were no significant differences in acenaphthylene and naphthalene peak areas, therefore, sample volume of 1.5 ml was selected for further essays to decrease analysis time.
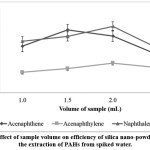 |
Figure 2: Effect of sample volume on efficiency of silica nano-powder SPE for the extraction of PAHs from spiked water. Click here to View Figure |
The analytes from the SPE absorbent can be eluted by using elution solvent. The most important characteristic that plays a role in the eluting of analyte is the polarity of elution solvent used.26 Acenaphthene and acenaphthylene are moderately polar PAHs while naphthalene is a non-polar PAH. Four solvents were selected in this study including acetonitrile, dichloromethane, n-hexane and ethyl acetate. Figure 3 shows the types of elution solvent profiles for the silica nano-powder SPE and the use of n-hexane gave the best extraction efficiency for acenaphthene and naphthalene while dichloromethane gave the highest peak area of acenaphthylene. There were no significant differences in the amount of extracted acenaphthylene when using n-hexane, hence it was chosen as a compromise elution solvent.
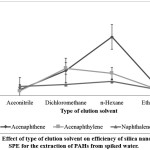 |
Figure 3: Effect of type of elution solvent on efficiency of silica nano-powder SPE for the extraction of PAHs from spiked water. Click here to View Figure |
All analytes is able to be completely eluted from the SPE absorbent by optimizing the elution solvent volume.27 Different n-hexane volumes (2.0, 4.0, 6.0 and 8.0 ml) were evaluated. As shown in figure 4, the highest signals were obtained when using 2.0 ml of n-hexane, hence it was selected as the optimum elution solvent volume for the extraction of PAHs.
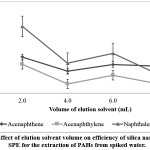 |
Figure 4: Effect of elution solvent volume on efficiency of silica nano-powder SPE for the extraction of PAHs from spiked water. Click here to View Figure |
Method validation and analytical performances
The final optimum SPE conditions using silica nano-powder as its absorbent were: 150 mg absorbent amount, 1.5 ml sample volume and 2.0 ml volume of n-hexane as the elution solvent. The obtained coefficient of determination (R2), LOD and LOQ are listed in table 1. The method exhibited good linearity (R2 ≥ 0.9956) within the concentration range of 0.1 to 10.0 mg/L. The LOD obtained was in the range of 1.5×10-6 – 1.0 mg/L and the LOQ obtained was in the range of 5.0×10-6 – 3.2 mg/L. Repeatability (intra-day) and reproducibility (inter-day) were expressed as relative standard deviations (RSD %). The results showed good RSDs ranged from 0.32 to 2.78% (n=3) for spiked PAHs levels at 2.0 mg/L (Table 2).
Table 1: Method validation of PAHs in wastewater samples.
| Acenaphthene | Acenaphthylene | Naphthalene | |
| Correlation coefficient, R2 | 0.9996 | 0.9956 | 0.9994 |
| LOD (mg/L) | 1.5×10-6 | 1.0 | 0.4 |
| LOQ (mg/L) | 5.0×10-6 | 3.2 | 1.2 |
Table 2: Repeatability (intra-day) and reproducibility. (inter-day)
| Acenaphthene | Acenaphthylene | Naphthalene | |
| Repeatability (%) | 2.78 | 1.36 | 2.77 |
| Reproducibility (%) | 2.20 | 0.32 | 0.56 |
Analysis of Real Samples
The proposed method was applied for determination of the three PAHs in wastewater samples. The samples showed negative results for PAHs. Thus, to assess the applicability of the synthesized silica nano-powder to real samples, wastewater samples were spiked at two concentration levels of PAHs (0.5 and 10.0 mg/L). Three replicate sample extractions were conducted for each concentration. The results showed satisfactory average recoveries ranging from 94.7 to 99.9% (Table 3).
Table 3: Determination of PAHs in wastewater samples.
| Spiked concentration (mg/L) | Recovery (%) | ||
| Acenaphthene | Acenaphthylene | Naphthalene | |
| 0.5 | 94.7 | 97.6 | 98.1 |
| 10.0 | 99.8 | 99.9 | 99.7 |
Toxicity Study
Application of silica nano-powder derived from rice husk was founded to increase the characteristics of tomato seed germination (Figure 5a, 5b and 5c). Mean germination time and vigour index were increased with the increase of silica solution levels up to 125 mg/L. The use of 125 mg/L of silica solution proved best by giving the highest values of seed germination parameters. Application of 125 mg/L of silica solution increased percent seed germination by 11.11%, mean germination time by 29.17% and seedling vigour index by 210.53% over the respective controls. The results from the current study showed that the seeds may absorp and utilize silica nano-powder28 and enhanced seed germination potential, thus proved to be non-toxic when apply to wastewater treatment in future.
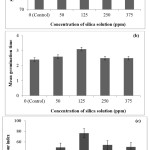 |
Figure 5: Effect of silica nano-powder on the (a) percentage of seed germination, (b) mean germination time and (c) vigour index of tomato. Click here to View Figure |
Conclusions
High purity silica nano-powder has been successfully prepared from rice husk and applied as absorbent for SPE of three selected PAHs from industrial wastewater samples. High recovery of the PAHs was achieved by using the synthesized silica nano-powder. The porous surface of the absorbent helped to increase surface area and obtained high recoveries of targeted analytes. The toxicity study showed that the plants have grown which are not at risk of toxicity. With the good recoveries obtained, rapid pre-treatment step and non-toxic to the plants, the use of silica nano-powder absorbent for SPE method has high potential for other applications.
Acknowledgements
The authors gratefully acknowledged the grant provided by Universiti Malaysia Pahang (UMP) under the RDU Grant (Vote number: RDU 170301).
References
- A. O. Adeniji, O. O. Okoh, and A. I. Okoh, “Levels of Polycyclic Aromatic Hydrocarbons in the Water and Sediment of Buffalo River Estuary, South Africa and Their Health Risk Assessment”, Arch. Environ. Contam. Toxicol., 2019, 76, 657.
CrossRef - H. I. Abdel-Shafy, and M. S. M. Mansour, “A review on polycyclic aromatic hydrocarbons: Source, environmental impact, effect on human health and remediation”, Egypt. J. Petrol., 2016, 25, 107.
CrossRef - C. Guigue, M. Tedetti, D. C. Dang, J. U. Mullot, C. Garnier, and M. Goutx, “Remobilization of polycyclic aromatic hydrocarbons and organic matter in seawater during sediment resuspension experiments from a polluted coastal environment: insights from Toulon Bay (France)”, Environ. Pollut., 2017, 229, 627.
CrossRef - Priority Substances Under the Water Framework Directive. http://ec.europa.eu/environment/water/water-dangersub/pri_substances.htm
- Toxic and Priority Pollutants Under the Clean Water Act. https://www.epa.gov/eg/toxic-and-priority-pollutants-under-clean-water-act#priority
- B. Strandberg, A. Julander, M. Sjöström, M. Lewné, H. K. Akdeva, C. Bigert, “An improved method for determining dermal exposure to polycyclic aromatic hydrocarbons”, Chemosphere., 2018, 198, 274.
CrossRef - H. Wang, X. Liu, K. Nan, B. Chen, M. He, and B. Hu, “Sample pre-treatment techniques for use with ICP-MS hyphenated techniques for elemental speciation in biological samples”, J. Anal. At. Spectrom., 2017, 32, 58.
CrossRef - T. T. Tran-Lam, Y. H. Dao, L. K. T. Nguyen, H. K. Ma, H. N. Tran, and G. T. Le, “Simultaneous Determination of 18 Polycyclic Aromatic Hydrocarbons in Daily Foods (Hanoi Metropolitan Area) by Gas Chromatography⁻Tandem Mass Spectrometry”, Foods, 2018, 7, 201.
CrossRef - K. Mejía-Carmona, M. Jordan-Sinisterra, and F. M. Lanças, “Current Trends in Fully Automated On-Line Analytical Techniques for Beverage Analysis”, Beverages, 2019, 5, 13.
CrossRef - C. Bylda, R. Thiele, U. Kobold, and D. A. Volmer, “Recent advances in sample preparation techniques to overcome difficulties encountered during quantitative analysis of small molecules from biofluids using LC-MS/MS”, Analyst, 2014, 139, 2265.
CrossRef - S. Noor Syuhadah and H. Rohasliney, “Rice Husk as Biosorbent: A Review”, Health Environ. J., 2012, 3, 89.
- T. Matsuo, and N. Hayakawa, “π-Electron systems containing Si=Si double bonds”, Sci. Technol. Adv. Mater., 2018, 19, 108.
CrossRef - K. Ozaki, K. Kawasumi, M. Shibata, H. Ito, and K. Itami, “One-shot K-region-selective annulative π-extension for nanographene synthesis and functionalization”, Nat. Commun., 2015, 6, 6251.
CrossRef - V. N. Le, Y. Rui, X. Gui, X. Li, S. Liu, and Y. Han, “Uptake, transport, distribution and Bio-effects of SiO2 nanoparticles in Bt-transgenic cotton”, J. Nanobiotechnology., 2014, 12, 50.
CrossRef - D. L. Slomberg, and M. H. Schoenfisch, “Silica nanoparticle phytotoxicity to Arabidopsis thaliana”, Environ. Sci. Technol., 2012, 46, 10247.
CrossRef - M. V. Khodakovskaya, B. S. Kim, J. N. Kim, M. Alimohammadi, E. Dervishi, T. Mustafa, and C. E. Cernigla, “Carbon nanotubes as plant growth regulators: effects on tomato growth, reproductive system, and soil microbial community”, Small, 2013, 9, 115.
CrossRef - M. K. Kanwar, S. Sun, X. Chu, and J. Zhou, “Impacts of Metal and Metal Oxide Nanoparticles on Plant Growth and Productivity”, 2019, Springer Nature, Switzerland, 379.
CrossRef - A. Rastogi, D. K. Tripathi, S. Yadav, D. K. Chauhan, M. Živčák, M. Ghorbanpour, N. I. El-Sheery, and M. Brestic, “Application of silicon nanoparticles in agriculture”, 3 Biotech., 2019, 9, 90.
CrossRef - E. Rafiee, M. Khodayari, M. Kahrizi, and R. Tayebee, “H5CoW12O40 supported on nano silica from rice husk ash: A green bifunctional catalyst for the reaction of alcohols with cyclic and acyclic 1,3-dicarbonyl compounds”, J. Mol. Catal. A-Chem, 2012, 358, 121.
CrossRef - M. H. Siddiqui, and M. H. Al-Whaibi, “Role of nano-SiO2 in germination of tomato (Lycopersicum esculentum seeds Mill.)”, Saudi J. Biol. Sci., 2014, 21, 13.
CrossRef - S. Matthews, and M. Khajeh-Hosseini, “Length of the lag period of germination and metabolic repair explain vigour differences in seed lots of maize (Zea mays)”, Seed Sci. Technol., 2007, 35, 200.
CrossRef - A. Vashisth, and S. Nagarajan, “Effect on germination and early growth characteristics in sunflower (Helianthus annuus) seeds exposed to static magnetic field”, J.Plant Physiol., 2010, 167, 149.
CrossRef - W. N. Wan Ismail, “Application of Sol-Gel Hybrid Extraction Sorbent for Gas Chromatographic Analysis of Organophosphorus Pesticides”, J. Sol-Gel Sci. Technol., 2016, 80, 50.
CrossRef - L. Wang, C. Hu, and L. Shao, “The antimicrobial activity of nanoparticles: present situation and prospects for the future”, Int. J. Nanomedicine, 2017, 12, 1227.
CrossRef - J. K. S. Nigel, “Solid Phase Extraction – Principles, Techniques and Applications”, 2000, Marcel Dekker, New York, 213.
- K. Ramandeep, K. Ripneel, S. Rani, A. K. Malik, A. Kabir, K. G. Furton, and V. F. Samanidou, “Rapid Monitoring of Organochlorine Pesticide Residues in Various Fruit Juices and Water Samples Using Fabric Phase Sorptive Extraction and Gas Chromatography-Mass Spectrometry”, Molecules, 2019, 24, 1013.
CrossRef - J. A. de Oliveira, L. J. P. Izeppi, R. F. Loose, D. K. Muenchen, O. D. Prestes, and R. Zanella, “A multiclass method for the determination of pharmaceuticals in drinking water by solid phase extraction and ultra-high performance liquid chromatography-tandem mass spectrometry”, Anal. Methods, 2019, 17, 2333.
CrossRef - S. Shinde, P. Paralikar, A. P. Ingle, and M. Rai, “Promotion of seed germination and seedling growth of Zea mays by magnesium hydroxide nanoparticles synthesized by the filtrate from Aspergillus niger”, Arab. J. Chem., 2018, in press.
CrossRef


