Introduction
Anthocyanins (Greek anthos, flower and Greek kyanose, blue) are pigments of many flowers, fruits, and vegetables which are responsible for red, blue and purple colors.1 Nowadays used as a natural colorant, only small doses of anthocyanins are required to display the color desired in several food matrixes (e.g. 30 to 40 ppm for soft drinks and 20–60 ppm for fruit preserves). Besides their use as a natural colorant, they play a major role in reducing the risk of coronary heart disease, cancer, and stroke,2 improve visual acuity, anticancer and antioxidant activities.3 Anthocyanins are glycosides of anthocyanidins (also called aglycones). Till date, 539 anthocyanins have been reported from plants4 but mostly six anthocyanidins (cyanidin, delphinidin, malvidin, pelargonidin, peonidin, and petunidin) are found widespread in fruits and vegetables.5,6
Black carrot (Daucus carota ssp. sativus) anthocyanin attributes higher stability to hydration, light, pH and temperature changes due to the presence of acylated groups like p-coumaric, ferulic, p-hydroxybenzoic and sinapic acids.7 In addition to their high anthocyanin stability, the black carrot is a rich source of anthocyanin having 1750 mg of anthocyanin per kilogram fresh weight.8 Being a rich source of natural colorant, black carrot in India is underutilized and could not gain much consumer acceptance as a vegetable. Therefore, some processing techniques implied to renovate the raw food materials by means of either chemical or physical changes into stable, consumable, marketable and valorized form.3,6,9-11
Increase awareness about health and healthy food development of functional ingredients food products with nutritional and medicinal values are under consideration.12-18 Microencapsulation is a process technique of incorporating natural ingredients, polyphenols, volatile additives, colors, enzymes, and bacteria in small capsules in order to stabilize and protect them against nutritional as well as health losses. It does package materials in the form of micro- and nanoparticles.19 The active agent inside the microcapsule is referred to as the core, encapsulants, internal phase, payload phase or fill, whereas the wall is called the shell, coating, wall material, encapsulating agents or membrane shell, carrier material, external phase, or matrix.20 Microencapsulation by spray dryer is an economical method for preservation of natural colorants by entrapping the active ingredient in a wall material.21 The most significant parameters of spray dryer, which affects the physicochemical properties of core materials as well as wall materials, are inlet temperature, outlet temperature and feed speed.22-25 Microencapsulation of black carrot extract using spray dryer at inlet and outlet temperature of 150°C and 76°C with 2 mL/min feed rate were studied the best for efficient quality anthocyanin powder.26 Several studies have been conducted for the protection of core material from adverse environmental conditions viz., high acidity, oxygen stress, and temperature many researchers using different carrier materials.27-30 The choice of carrier material should be low solubility so that it is protected while passing through in vitro and in vivo environmental conditions.31,32 Jackfruit seed starch (JSS) has been chosen as a carrier material because jackfruit seed is a source of protein (13.50%), carbohydrates (79.34%), crude fiber (3.19%) and total mineral matter (3.1%)33,34 and it has excellent gelling, thickening capacity, stability to mechanical and thermal shear, binding capacity, acid resistivity and non-toxicity.35 Whey protein isolate (WPI) with polysaccharides has been also studied by several researchers in microencapsulation of different bioactive compounds using spray drying.30,36,37 Similarly gum arabic also plays a key role in the encapsulation of different bioactive compounds.38,39 However, availability and caste of production have been limited the utilization of gum arabic in food industries.39-43 Hence, NBRE-15 has similar chemistry to gum arabic which acts as an emulsifying agent was tried to optimize the effective concentration in microencapsulation of anthocyanin.
In this study the feed mixture containing Jackfruit Seed Starch, Whey protein and NBRE-15 as carrier materials for the encapsulation of anthocyanin pigment of black carrot juice was evaluated statistically by the use of RSM; the process was optimized in terms of the modeling equations for the anthocyanin content, antioxidant activity, and the encapsulation efficiency and the particle size and its structure have been characterized using Particle Size Analyser and Scanning Electron Microscope (SEM) respectively.
Materials and Methods
Materials
Black carrot (Pusa asita – black carrot variety) sample was procured from the archer of Indian Agricultural Research Institute, New Delhi, India. The carrot was properly cleaned, washed and kept at (4-10) °C in cold storage before juice extraction. WPI (>99.0%) was purchased from the local market of New Delhi, India. NBRE-15 (an emulsifier) was obtained National Botanical Research Institute (NBRI), Lucknow, India. Jackfruit seed starch was isolated from jackfruit seeds purchased from the local market. Other chemicals were purchased from Sigma Aldrich, India and Central Drug House (CDH) Private Ltd. Pvt, New Delhi, India.
Juice Extraction
Juice extraction was done as per the method optimized by Kirca et al.,10 with slight modification. The carrot was manually peeled and crushed by a hydraulic press to extract the juice. Solid fibers were separated from the juice by filtering through Whatman No.1 filter followed by addition of 1% citric acid as a preservative. It was given a pectinase enzyme treatment at 50°C for 2 hours. The total solids were measured to be 6°Brix of the extracted juice and stored in cold storage at (4-10) °C for further use.
Carrier Agents for Spray Drying
Dry Jackfruit seeds were procured from the local market, New Delhi, India. The seeds were decorticated and powdered using hammer mill and further used for starch extraction. The starch was extracted using the distilled water method as described by Murali et al.,22,24 with slight modification. Jackfruit seed powder was sieved using 150-170µ pore-size and slurry made with distilled water. The slurry was centrifuged for 2 min at 9000 rpm and the pellet collected was washed with 50% ethanol followed by vacuum drying for 48 hours. The fat was removed by adding petroleum ether which was evaporated at room temperature. The starch obtained was fineally ground with a mortar and pestle and packed in plastic bag and kept at room temperature for further use. Whey protein isolate (WPI) was obtained from the local market. NBRE-15 a type of emulsifier was excessed from National Botanical Research Institute (NBRI), Lucknow, India.
Preparation of Feed Mixtures
Jackfruit seed starch (JSS), whey protein extract and NBRE-15 were combined with the black carrot juice (6% solid content) in different proportions as per the BBD arrangements of design-expert 8.0.7.1 (Table 1) and stirred to homogeneity with IKA®T25 digital Ultra-Turrax (USA) homogenizer for 30 min.
Spray Drying Conditions
The feed mixtures were spray dried using SonoDry 1000 (Sono-Tek Corporation, USA) with the inlet temperature of (150 ± 2) °C and the outlet temperature of (76 ± 2)°C being optimized for the encapsulation of black carrot juice using spray drying.26 The aspirator speed was set at 79 rpm with a constant feed flow of 2 ml/min with D-block on time of 1 second.
Determination of Anthocyanin Content
The total monomeric anthocyanin pigment was determined using pH differential method.26 The samples were diluted in two buffer systems- pH 1.0 buffer (potassium chloride, 0.025M) and pH 4.5 buffer (sodium acetate, 0.4M) and the absorbance measured at 520 nm and 700 nm for each using UV-Visible spectrophotometer (GENESYS 10 VIS, USA). The results were expressed as cyaniding-3-glucoside equivalents as:
![]()
Where
A = Absorbance = = (A520nm – A700nm) pH 1.0 – (A520nm – A700nm) pH 4.5
MW = Molecular weight = 449.2; DF = Dilution factor; 1 = path length in cm and L.mol–1.cm–1.
Determination of Antioxidant Activity
The Antioxidant activity was done by Cupric Reducing Antioxidant Capacity (CUPRAC) method developed by Apak et al.,41 100 µl of sample solution was added to 1 ml of distilled water which was further added to 1mL each of copper (II) chloride solution (10−2M), neocuproine solution (7.5 × 10−3M), and ammonium acetate buffer solution (pH 7) solution in a test-tube to make the final volume of 4.1 ml. The tubes were capped properly and left for 1 hour. The absorbance was taken at 450 nm against a reagent blank using UV-Visible spectrophotometer (GENESYS 10 VIS, USA). The results were expressed in µmol TE/g.
Encapsulation Efficiency
The encapsulation efficiency is the content of anthocyanin being encapsulated with respect to the content in the juice. It was calculated using the following method.26
![]()
Experimental Design for Optimization using Box-Behnken Design (BBD) by Response Surface Methodology (RSM)
Box-Benhken Design (BBD) was used for the optimization of the encapsulation process. Three independent were taken as TSS, the ratio of whey and JSS and NBRE-15 each at 3 levels (Table 1) with anthocyanin content (mg/100g of dry matter), antioxidant activity (µmolTrolox/100g dry matter) and encapsulation efficiency (%) taken as the dependent variables. The BBD consisted of
17 experiments with 1 block containing 5 center points per block. Experimental data obtained were fitted into the second order polynomial equation.
![]()
Where is predicted response; is fitted response at the centre point; a1, a2 and a3 are linear terms; a12, a13 and a23 are interaction effects; a11, a22 and a33 are squared effects. and C are the independent variables.
Table 1: The Arrangement of the BBD for the Three Independent Variables Along with the Experimental Responses.
| Experimental order | Actual and coded value for design variable | Responses | ||||
| TSS (°Brix, X1) | WPI:JSS (X2) | NBRE-15 (%,X3) | Anthocyanin content (mg/100g of dry matter) | Antioxidant activity (µmol Trolox/g dry matter) | Encapsulation efficiency (%) | |
|
1 |
20 (-1) |
1:1 (-1) |
0.2 (0) |
2409.24 |
204.86 |
67.12 |
|
2 |
30 (1) |
1:1 (-1) |
0.2 (0) |
2524.84 |
253.86 |
70.34 |
|
3 |
20 (-1) |
1:5 (1) |
0.2 (0) |
2445.10 |
232.52 |
68.72 |
|
4 |
30 (1) |
1:5 (1) |
0.2 (0) |
2651.78 |
283.54 |
73.88 |
|
5 |
20 (-1) |
1:3 (0) |
0.1 (-1) |
2253.24 |
160.28 |
62.76 |
|
6 |
30 (1) |
1:3 (0) |
0.1 (-1) |
2522.54 |
206.72 |
70.28 |
|
7 |
20 (-1) |
1:3 (0) |
0.3 (1) |
2486.23 |
179.03 |
66.48 |
|
8 |
30 (1) |
1:3 (0) |
0.3 (1) |
2551.42 |
220.54 |
71.14 |
|
9 |
25 (0) |
1:1 (-1) |
0.1 (-1) |
2560.36 |
242.62 |
71.33 |
|
10 |
25 (0) |
1:5 (1) |
0.1 (-1) |
2734.25 |
260.56 |
76.18 |
|
11 |
25 (0) |
1:1 (-1) |
0.3 (1) |
2680.54 |
253.53 |
74.67 |
|
12 |
25 (0) |
1:5 (1) |
0.3 (1) |
2807.59 |
289.32 |
78.22 |
|
13 |
25 (0) |
1:3 (0) |
0.2 (0) |
2630.57 |
245.59 |
74.93 |
|
14 |
25 (0) |
1:3 (0) |
0.2 (0) |
2622.77 |
246.45 |
74.72 |
|
15 |
25 (0) |
1:3 (0) |
0.2 (0) |
2696.06 |
234.34 |
73.97 |
|
16 |
25 (0) |
1:3 (0) |
0.2 (0) |
2684.89 |
245.24 |
76.45 |
|
17 |
25 (0) |
1:3 (0) |
0.2 (0) |
2664.54 |
248.68 |
73.09 |
Note: The mean values of the experimental responses are taken for optimization
Color Measurement
The color of the optimized powder was measured using a Hunter-Lab Colorimeter (Miniscan® XE Plus 4500L). The color was expressed in hunter lab units, L* (varying from 0 black to 100 white), a* (varying + value, red to – value, green), b* (varying from +value, yellow to – value, blue). The test was performed in triplicates and average data was recorded.
Moisture Content
5g of powder was weighed in a clean and tarred aluminum dish and dried in hot air oven at 105°C with the open lid for 3 hrs. The dish was cooled in a desiccator and weighed. All samples were analyzed in triplicate and the result was expressed in (w.b.) percentage using the formula given below:
![]()
Analysis of Particle Size
The optimized product contained five times more Jackfruit starch (JSS) than the whey protein. So, the particle size distribution in the optimized encapsulated powder was for JSS using laser scattering particle size distribution analyzer LA-950 (Horiba Scientific Instruments, Japan).
Surface Morphology by Scanning Electron Microscope (SEM)
The morphological analysis of the optimized encapsulated powder was done by coating the powder with gold/palladium to a thickness of 27 nm using an SC7620 model mini sputter coater (Quorum Technologies Ltd, West Sussex, UK) followed by SEM analysis using EVO/MA10 model (CarlZeiss, Germany). The SEM was operated at 5000X magnification.
Statistical Analysis
All the experiments were carried out in triplicates and the mean values were used for the optimization using Design-Expert 8.0.7.1 software. The data were analyzed statistically by ANOVA considering p values ≤ 0.05 as statistically significant.
Results and Discussion
Model Fitting for the Optimization
Process optimization for the encapsulation of black carrot anthocyanin was done by RSM taking total anthocyanin content, antioxidant activity and encapsulation efficiency as the responses. The results of the experiments performed as per BBD design are given in Table 1. The regression coefficients, R2, P and F values for the quadratic model of the dependent variables are shown in Table 2. The model for all the responses was found to be significant (p˂0.05) and the R2 value was more than 0.90 as for the good fit of the model it should be more than 0.80 respectively.44 The lack of fit was not significant (p ˃ 0.05) for all the models being analyzed by ANOVA.
Table 2: Regression Coefficients, R2, P and F Values for the Quadratic Model of the Dependent Variables for the Encapsulation of Anthocyanin.
| Anthocyanin content | Antioxidant activity | Encapsulation efficiency | ||
|
Regression coefficient |
2659.766 |
244.060 |
74.632 |
|
|
82.096 |
23.496 |
2.570 |
||
|
57.967 |
13.883 |
1.692 |
||
|
56.923 |
9.030 |
1.245 |
||
|
-197.176 |
-35.115 |
-6.026 |
||
|
45.151 |
34.750 |
1.409 |
||
|
-9.232 |
-17.302 |
-0.941 |
||
|
22.770 |
0.505 |
0.485 |
||
|
-51.027 |
-1.232 |
-0.715 |
||
|
-11.71 |
4.462 |
-0.325 |
||
|
Regression analysis |
R2 |
0.975 |
0.991 |
0.964 |
|
>Adj R2 |
0.944 |
0.980 |
0.918 |
|
|
Pred R2 |
0.817 |
0.965 |
0.769 |
|
|
Model |
F-value |
31.401 |
91.083 |
21.076 |
|
P-value |
˂ 0.0001* |
˂ 0.0001* |
0.0003* |
|
|
Lack of Fit |
F-value |
0.950 |
0.278 |
0.689 |
|
P-value |
0.496* |
0.839* |
0.604* |
*Significant at 0.05 level
Effect of TSS, The Ratio of Whey Protein and JSS and NBRE-15 on the Anthocyanin Content of the Encapsulated Powder
The effect of WPI: JSS, TSS, and NBRE-15 the variables on the anthocyanin content has been depicted through the 3D surface plot (Fig. 1). It was observed that with the increase of total soluble solids (TSS), the anthocyanin content increased but beyond 28 slight drops was observed. The ratio of whey protein and JSS has a positive effect and the anthocyanin content increased considerably. NBRE-15 also imparted positive effect and the increase in the anthocyanin content was observed. With regard to the interaction effect, the interaction of TSS and NBRE-15 has a negative effect with regression coefficient -51.027 while the rest interactions have a positive effect (Table 2).
 |
Figure 1: 3D surface plot for effect of three variables on the MAC.TSS and WPI: JSS (A), TSS and NBRE-15 (B) and WPI: JSS and NBRE-15(C). Click here to View figure |
Effect of TSS, the Ratio of Whey Protein and JSS and NBRE-15 on the Antioxidant Activity of the Encapsulated Powder
The selected variables had a positive effect on the antioxidant activity (Fig. 2). The effects were similar to that on the anthocyanin content. With the increase in TSS, antioxidant activity increased and after then dropped slightly. Unlike TSS, with the increase of the ratio of whey and JSS, the antioxidant activity increased. Similarly, there was a proportionally increase in antioxidant with the increase in NBRE-15. The interaction effects of AB and BC were observed positive while AC had a negative effect.
 |
Figure 2: 3D surface plot for effect of three variables on the antioxidant activity. TSS and WPI: JSS (A), TSS and NBRE-15 (B) and WPI: JSS and NBRE-15 (C). Click here to View figure |
Effect of TSS, the Ratio of Whey Protein and JSS and NBRE-15 on the Encapsulation Efficiency of Encapsulated powder
The response surfaces for the effect of the variables on the encapsulation efficiency are shown in Fig. 3. In this case, also, the three variables effect positively. Encapsulation efficiency increases with the increase in TSS to a certain level and then decreases while with the ratio of whey and JSS there is a logarithmic increase. With NBRE-15 a similar trend to that of the ratio was observed. Regarding the interaction effect, in this case, only AB has a positive effect while the rest AC and BC negatively affects the encapsulation efficiency.
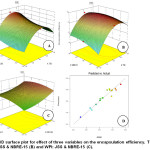 |
Figure 3: 3D surface plot for effect of three variables on the encapsulation efficiency. TSS and WPI: JSS (A), TSS and NBRE-15 (B) and WPI: JSS and NBRE-15 (C). Click here to View figure |
Optimization Constraints and Solution
The optimization of different constraints was done targeting the variables and the responses both to the maximum with high importance. The optimized condition selected for variable was 27.8°Brix (TSS), 1:5 (WPI:JSS) and 0.3% (NBRE-15) having 2766.61 mg/100g dry matter of anthocyanin content, 290.56 µmolTrolox/g dry matter antioxidant activity with encapsulation efficiency of 77.12% at 93.59% desirability. The color was reported to have L*, a* and b* values as 30.61, 29.39 and 0.03 respectively. The moisture content in the optimized encapsulated powder was 4.04%, respectively.
Predicted Model Verification
The predicted model was verified by confirming the actual values against the predicted under the optimum conditions of TSS, the ratio of WPI: JSS and NBRE-15 respectively at 27.8 °Brix, 1:5 and 0.3%. The actual values were found to be significant with the predicted values at the 5% level of significance.
Size Distribution of the Encapsulated Powder
The particle size distribution for the optimized powder containing whey protein isolate, jackfruit starch, and NBRE-15 is shown in Fig. 4. The size distribution for the encapsulated powder is as follows:
![]()
Where, D10 implies that 10% of the particles are below this size 8.71 µm; D50 denotes the median particle size distribution of 21.57 µm; D90 denotes the 90% of the particles are above 21.57 µm, however, below 132.12 µm and Dmean is the average particle size distribution 52.36 µm of the optimized encapsulated anthocyanin powder.
 |
Figure 4: Particle size distribution of the optimized microcapsule (A) and SEM image for surface characterization of the encapsulated powder (B) 2000X magnification (C) 5000X magnification. Click here to View figure |
Surface Morphology Study by Scanning Electron Microscope (SEM)
The SEM image of the encapsulated powder (Fig. 5) shows a complex mixture of encapsulant materials. The powder molecules are slightly oval to globular in shape without any cracks ensuring the safety of core material inside which are similar to reports confirmed by Ersus and Yurdagel.9 The particle size ranged approximately from 2µm to 20µm. The molecules are attached to each other which could be due to the emulsifying property of NBRE-15 in presence of whey protein isolate and jackfruit starch.
Conclusions
The anthocyanin pigment of black carrot was encapsulated using the carrier materials viz., whey protein isolate, jackfruit starch, and NBRE-15. NBRE-15 in presence of whey protein isolate formed the best carrier with the jackfruit seed starch which ultimately gave best encapsulation efficiency with optimum anthocyanin content and antioxidant activity. This was also revealed through the particle size distribution and SEM images for the optimized powder. The proportion of jackfruit starch five times the whey protein isolate with 0.3% NBRE-15 was optimized to be the best carrier material for the encapsulation of anthocyanin pigment.
Acknowledgments
The authors are grateful to the National Agriculture Science Fund (NASF) project (code: 20-09), Government of India for financial support. Authors also thanks ICAR – Indian Agricultural Research Institute, New Delhi, for providing the core material for the encapsulation.
Ethical Approval
This article does not contain any studies with either animals or human participants performed by any of the authors.
Conflict of Interest
The authors declare that they have no conflict of interest.
Authors Contributions
Mr. Avinash Singh Patel design the experiments and conducted the entire research work and write the manuscript under supervision of Dr. Abhijit Kar. Mr. Dipendra Kumar Mahato helped in the qualitative analysis of sample. Dr. Lalit M Bal did the statistical analysis and interpretation of the data.
References
- Gizir A. M., Turker N., Artuvan E. Pressurized acidifiedwater extraction of black carrot (Daucus carotas sp. Sativus var. atrorubens Alef.) anthocyanins. Eur Food Res Technol, 2008; 226:363-370.
CrossRef - Norberto S., Silva S., Meireles M., Faria A., Pintado M., Calhau C. Blueberry anthocyanins in health promotion: A metabolic overview. J Funct Foods, 2013; 5(4):1518-1528.
CrossRef - Alasalvar C., Al-Farsi M., Quantick P. C., Shahidi F., Wiktorowicz R. Effect of chill storage and modifiedatmosphere packaging (MAP) on antioxidant activity,anthocyanins, carotenoids, phenolics and sensory quality ofready-to-eat shredded orange and purple carrots. Food Chem, 2005; 89: 69-76.
CrossRef - Algarra M., Fernandes A., Mateus N., deFreitas V., EstevesdaSilva J. C., Casado J. Anthocyanin profile andantioxidant capacity of black carrots (Daucus carota L. ssp. sativusvar. Atrorubens Alef.) from Cuevas Bajas, Spain. J Food Comp Anal 2014; 33:71-76.
CrossRef - Fernandes I., Faria A., Calhau C., de Freitas V., Mateus N. Bioavailability of anthocyanins and derivatives. J Funct Foods, 2014; 7:54-66.
CrossRef - Kamiloglu S., Pasli A. A., Ozcelik B., VanCamp, J., Capanoglu, E. Colour retention, anthocyanin stability andantioxidant capacity in black carrot (Daucus carota) jams and marmalades: Effect ofprocessing, storage conditions and in vitro gastrointestinal digestion. J Funct Foods, 2015; 13:1-10.
CrossRef - Cevallos-Casals B. A., Cisneros-Zevallos L. Stability of anthocyanin-based aqueous extracts of Andean purple corn and red fleshed sweet potato compared to synthetic and natural colorants. Food Chem, 2004; 86(1), 69-77.
CrossRef - Mazza G., Miniati E. Anthocyanins in fruits, vegetables and grains. (Boca Raton, FL: CRC Press), 1993; pp. 362.
- Ersus S., UnalYurdagel U. Microencapsulation of anthocyanin pigments of black carrot (Daucus carota L.) by spray drier. J Food Eng, 2007; 80:805-812.
CrossRef - Kirca A., Ozkan M., Cemeroglu B. Effects of temperature, solid content and pH on the stability of black carrot anthocyanins. Food Chem, 2007; 101:212-218.
CrossRef - LiuB., Wang, Y., Yang F., Wang X., Shen H., Cui H. Wu D. Construction of a controlled-release delivery system for pesticides using biodegradable PLA-based microcapsules. Colloids Surf B: Biointerfaces, 2016; 144:38-45.
CrossRef - Patel A. S., Pandey A. K. Fortification of Limonia acidissima Linn. fruit powder to develop the phenolic enriched herbal biscuits. J Biores Eng Technol, 2014; 1:74-85.
- Patel A. S., Pradhan R. C. Quality ranking of bottle gourd seed cake powder incorporated biscuits using fuzzy analysis of sensory attribute. BIOINFOLET-A Quart J Life Sci, 2015;12(4a):901-908.
- Pradhan R. C., Patel A. S., Mishra S. Physico-chemical properties of bottle gourd kernel. J Agric Eng, 2015; 52(4):28-34.
- Duhan S., Kar A., Nain L., Patel A. S., Dash S. K. Development of continuous flow microwave and hot water bath system for destruction of spoilage microorganisms in food. Ind J Agric Sci, 2017; 87(2):210-214.
- Ghosh P., Pradhan R. C., Patel A. S., Kar A., Mishra S. Physicochemical characteristics of Syzygium cumini fruit. Curr Res Nutr Food Sci, 2017; 5(1):25-35.
CrossRef - Mohapatra D., Patel A. S., Kar A., Deshpande S. S., Tripathi M. K. Effect of Different Processing Conditions on Proximate Composition, Anti-oxidants, Anti-nutrients and Amino Acid Profile of Grain Sorghum. Food Chem, 2018; 271:129-135.
CrossRef - Patel A. S., Pradhan R. C., Kar A., Mohapatra D. Effect of Fortification of De-oiled Bottle Gourd (Lagenaria siceraria) Seed on the Functional and Chemical Characteristics of the Biscuit: A Nutritional Assessment. Curr Res Nutr Food Sci, 2018; 6(3).
- Jafari S. M. Encapsulation efficiency of food flavors and oils during spray drying. Dry Technol, 2008; 26(7):816-835.
CrossRef - Gouin S. Microencapsulation: industrial appraisal of existing technologies and trends. Trends Food Sci Technol, 2004; 15:330-347.
CrossRef - Cai Y. Z., Corke H. Production and properties of spray-dried Amaranthus betacyanin pigments. J Food Sci, 2000; 65(6):1248-1252.
CrossRef - Murali S., Kar A., Patel A. S., Kumar J., Mohapatra D., Dash S. K. Spray dried encapsulation of rice bran oil in soya protein isolate – tapioca starch complex using response surface methodology. Ind J Agric Sci, 2016; 86 (8):984-91.
- Dhakane J. P., Kar A., Patel A. S., Khan I. Effect of soy proteins and emulsification-evaporation process on physical stability of lycopene emulsions. Int J Chem Studies, 2017; 5(5):1354-1358.
- Murali S, Kar A., Patel A. S., Mohapatra D., Krishnakumar P. Optimization of process conditions of rice bran oil encapsulation using spray drying by response surface methodology. Int J Food Eng, 2017; 13(4):25-35.
- Patel, A., Balunkeswar Nayak. “Mobilization of lipophobicity of cellulose nanocrystals (CNCs): An efficient encapsulation of phycobiliproteins.” In abstracts of papers of the American Chemical Society, vol. 256. 1155 16TH ST, NW, Washington, DC 20036 USA: Amer Chemical Soc. 2018.
- Murali S., Kar A., Mohapatra D., Kalia P. Encapsulation of black carrot juice using spray and freeze drying. Food Sci Technol Int, 2014; 0(0):1-9.
- Huq T., Khan A., Khan R. A., Riedl B., Lacroix M. Encapsulation of probioticbacteria in biopolymeric system. Crit Rev Food Sci Nutr, (2013); 53(9):909-916.
CrossRef - Mahdavee K. K, Jafari S. M., Ghorbani M., Kakhki M. Application of maltodextrin and gum Arabic in microencapsulation of saffron petal’s anthocyanins and evaluating their storage stability and color. Carbohydr Polym, 2014; 105:57-62. doi: 10.1016/j.carbpol.2014.01.042.
CrossRef - Souza J. M., Caldas A. L., Tohidi S. D., Molina J., Souto A. P., Fangueiro R. Zille A. Properties and controlled release of chitosan microencapsulated limonene oil. Rev Bras Farmacogn, 2014; 24(6):691-698.
CrossRef - Faridi A., Jafari S. M., Assadpoor E. and Mohammadi A. Nano-encapsulation ofsaffron extract through double-layered multiple emulsions of pectin and whey protein concentrate. J. Food Eng, 2015; 165:149-155.
CrossRef - Arslan S., Erbas M., Tontul I., Topuz A. Microencapsulation of probiotic Saccharomyces cerevisiae var. boulardii with different wall materials by spray drying. LWT – Food Sci Technol, 2015; 63:685-690.
CrossRef - Silva J. M., Reis R. L., Mano J. F. Biomimetic extracellular environment based on natural origin polyelectrolyte multilayers. Small, 2016; 12(32):4308-4342. doi: 10.1002/smll.201601355
CrossRef - Ocloo F. C. K., Bansa D., Boatin R., Adom T., Agbemavor W. S. Physico-chemical, functional and pasting characteristics of flour produced from Jackfruits (Artocarpus heterophyllus) seeds. Agric Biol J North Amer, 2010; 1(5):903-908.
CrossRef - Karadbhajne S.V., Sawant Y. Comparison of physico-chemical properties of modified jackfruit starch with maize starch. Int J Chem Tech Res, 2014; 6(1):487-494.
- Narkhede S. B., Bendale A. R., Jadhav A. G., Patel K., Vidyasagar G. Isolation and evaluation of starch of Artocarpus heterophyllus as a tablet binder. Int J Pharm Tech Res, 2011; 3(2):836-840.
- Flores F. P., Singh R. K., Kong F. Physical and storage properties of spray-dried blueberry pomace extract with whey protein isolate as wall material. J Food Eng, 2014; 137:1-6.
CrossRef - Hundre S. Y., Karthik P., Anandharamakrishnan, C. Effect of whey protein isolate and β-cyclodextrin wall systems on stability of microencapsulated vanillin by spray–freeze drying method. Food Chem, 2015; 174:16-24.
CrossRef - Mahfoudhi N., Sessa M., Chouaibi M., Ferrari G., Donsi F., Hamdi S. Assessment of emulsifying ability of almond gum in comparison with gum arabic using response surface methodology. Food Hydrocoll, 2014; 37:49-59.
CrossRef - Vasile F. E., Martinez M. J., Ruiz-Henestrosa V. M. P., Judis M. A., Mazzobre M. F. Physicochemical, interfacial and emulsifying properties of a nonconventional exudate gum (Prosopis alba) in comparison with gum arabic. Food Hydrocoll, 2016; 56:245-253.
CrossRef - Krishnan S., Bhosale R., Singhal R. S. Microencapsulation of cardamom oleoresin: Evaluation of blends of gum arabic, maltodextrin and a modified starch as wall materials. Carbohydr Polym, 2005; 61(1):95-102.
CrossRef - Apak A., Gorinstein S., Bohm V., Schaich K. M., Ozyurek M., Guclu K. Methods of measurement and evaluation of natural antioxidant capacity/activity (IUPAC Technical Report). Pure Appl Chem, 2013; 85:957-998.
CrossRef - Patel A. S., Khan I., Kar A. A technique of micro capsulation by spray-drying of nutrient elements: A review. NISCAIR Public, 2016; 24(2): 164-172.
- Patel A. S., Kar A., Khan I. Process for development of β-carotene Nanocomposites with ɷ-fatty acids. 2017.
- Joglekar, A. M., and May, A. T. Product excellence through design of experiments. Cereal Food World, 1987; 32:857-868.

