Introduction
Trianthemaportulacastrum L. is a herbaceous plant, growing annually. This plant commonly found in a moist area, which spreads on the ground not more than 4-6 ft.6 In India, it is commonly grown as a wild vegetable. This edible plant is a vital source of fibres, proteins, riboflavin, potassium, sodium and iron.13 It is also considered as a weed which has many active compounds such as alkaloid, flavonoids, tannins etc. These compounds are also called as allelochemicals, which may stimulate or suppress the growth of neighbouring plants.26 Study of these allelochemicals and its effects on the interaction of plants is called allelopathy. This is now an upcoming branch of biology which involves the study of allelochemicals and its influence on plant distribution, the building of plant biodiversity and its conservation, evolution of mix crop system in nature and community formation.33 By exuding these allelochemicals and sharing the same ecological conditions such as moisture, nutrients, light, space and air these weeds pose the growth and development of neighbouring plants.34 Depend upon the type of weed species, its causative effect on seed germination and seedling growth is varied.8 Plant parts of weed are also equally potential to exert their impression on other plants.4,1 Trianthemais a leafy vegetable with the active wild components. Many workers observed noteworthy observations during allelopathic interaction of this plant. The yield of many economically important plants like cotton, maize, wheat, sorghum, sunflower and mungbean is suppressed due to the presence of Trianthema.2, 25
A method of intercropping is followed during cultivation of fenugreek and Trianthema. A huge area in Vasai (Maharashtra, India) is under this mix cultivation. But in the field, this notorious plant had caused noticeable damage to the fenugreek yield. Considering this on field observations, the present study is conducted. During this study, allelopathic effect of Trianthema. on fenugreek seed germination, seedling growth and on some enzymes of antioxidant metabolism is evaluated.
Material and Methods
Fresh and matured plants of Trianthema. were collected from the local field of Vasai. Plant parts (seeds, stems and leaves) were separated and sundried for 15 days. Leachates of seed, stem and leaf of Trianthema. were prepared by following ratio 1/15 (w/v). These leachates were prepared in distilled water and kept for 24hr. Afterwards, these extracts were filtered through Whatman filter paper No.1. Authorised fenugreek seeds were procured from the market. According to ratio 1/15 (w/v), fenugreek seeds were soaked for 24 hr in seed, stem and leaf leachates of Trianthema.
For germination studies, pretreated seeds of fenugreek were kept in three sets of Petri plates for 96hr. Ten millilitres distilled water was poured in each Petri plate. Germination count was taken for each day and radical, coleoptile length was measured at 96 hr.
Mean germination time and germination index (GI) were calculated by following the method of 5
Mean germination time = ΣDn\Σn
Where n is the number of seeds, which were germinated on day D, while D is the number of days, counted from the beginning of germination.
Method9 was employed for studying enzyme peroxidase. The activity of catalase was determined by following method of17 as described by.27 Method of29 was adopted for determining the activity of ascorbate peroxidase. Enzyme super oxide dismutase was determined by using method11. Soluble proteins were estimated by the method.16 The activity of DPPH was estimated using the method described by.28 Content of saponin was studied using HPTLC finger printing with slight modification in the method.3
Result and Discussion
Changes in plants at morphological, biochemical, physiological and molecular level occurred due to environmental conditions. Allelopathy influences other neighbouring plants to change their growth pattern and metabolism. Such changes are also reported in the present study. Germination percentage (Graph 1), fresh weight (Graph 2) and mean germination time of fenugreek seeds (Graph 3) is affected after treatment with leachates of Trianthema. More significant reduction in the above parameters was noticed in case of leaf leachate treated fenugreek seeds. Seeds of rice were showing similar germination and fresh weight pattern when pre- treated with root, shoot, leaf, seed and whole plant extract of plant Trianthema.14 Many secondary metabolites such as phenols, flavonoids are present in this plant which are responsible for germination retardation. The activity of protease is vital for the seed to hydrolyse the proteins, which crucial for imbibition and for water uptake. Soluble phenolic compounds are largely present in leaf leachate of Trianthema, which may be responsible for disturbances in seed protease activity. This ultimately leads in more decrease in seed germination of fenugreek seeds pre-treated with leaf leachate compared to seed and stem leachate.
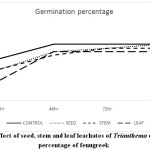 |
Graph 1: Effect of seed, stem and leaf leachates of Trianthema on germination percentage of fenugreek Click here to View Graph |
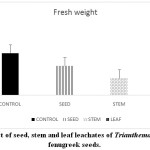 |
Graph 2: Effect of seed, stem and leaf leachates of Trianthemaon fresh weight of fenugreek seeds. Click here to View Graph |
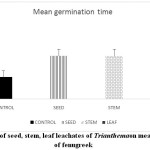 |
Graph 3: Effect of seed, stem, leaf leachates of Trianthemaon mean germination time of fenugreek Click here to View Graph |
Leachates of Trianthema had caused a reduction in coleoptile and radical length of fenugreek seeds (Graph 4 and 5).10 reported decline in germination percentage, plumule and radical length of rice and cowpea when treated with increasing concentration of Acacia auriculiformis leaf leachates. Allelopathic compounds present in the water-soluble form are reported to reduce the activity of GA and IAA.32 This inhibition in hormonal activity results in a decline in cell division and reduction in the growth of a plant.
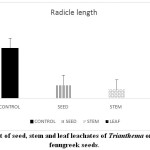 |
Graph 4: Effect of seed, stem and leaf leachates of Trianthema on radicle growth of fenugreek seeds. |
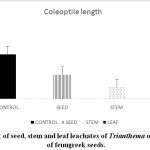 |
Graph 5: Effect of seed, stem and leaf leachates of Trianthema on coleoptiles growth of fenugreek seeds. Click here to View Graph |
Stressful environmental situations are responsible for limiting agricultural productivity, as plants are unable to achieve their maximum genetic strength.18,7 Sensitive cell organelles of plants react quickly to such environmental changes.30 Stressful conditions caused an increase in ROS in plants. These enhanced ROS are scavenged through many enzymatic and non- enzymatic antioxidants. In the present study, the activity of the antioxidant enzyme, peroxidase is decreased in pre-treated fenugreek seeds compared to control (Graph 6). Similar reports are mentioned 24 in Phalaris after treatment with extract of Eucalyptus leaf. Allelopathic effect of Trianthema had caused enhancement in catalase activity of fenugreek seeds (Graph 7). Such kind of enhancement in activity was noticed19 in Black gram seeds after treatment with a lower concentration of leaf extract of Tectona Grandis. In case of fenugreek seeds, as other enzymes are unable to show their antioxidant scavenging activity, accumulation of H2O2 may be caused in the cell. This harmful compound is efficiently catalysed to H2O in peroxisome by increasing the catalase activity in fenugreek. Seed, stem and leaf extract of Trianthemahad caused a decline in enzymatic activity of ascorbate peroxidase in fenugreek seeds (Graph 8). Leaf extract of Trianthemahad major effect than seed and stem extract. The activity of ascorbate peroxidase in germinating wheat seeds was also hampered by treatment with dried shoot extract of Achillea santolina L.20 In this relation,15 noticed that a decline in activity of enzyme catalase and enhancement in APX activity in leaves of cucumber seedlings when prone to stress. Similarly, to compensate for an increased activity of catalase in fenugreek seeds after allelopathic stress, activity of ascorbate peroxidase is decreased. Allelochemicals present in Trianthemaespecially in leaves has caused a decrease in the activity of enzyme superoxide dismutase (Graph 9). According to,21 extravagant amount of reactive oxygen species generated may cause a reduction in the activity of peroxidase and superoxide dismutase. The activity of DPPH in the present study showed a decrease in pre-treated seeds of fenugreek as compared to control seeds (Graph 10). Chilling stress in rice had caused an increase in DPPH activity in tolerant radicles of rice compared to sensitive radicles of rice seedling.12 This indicates that fenugreek seeds are sensitive to allelopathic stress applied by the Trianthama. As noticed on the fields of Vasai, fenugreek seeds are unable to grow and share the same field with Trianthama. Laboratory experiments prove the same. Leaves of Trianthamapossessed more allelopathic compounds than other plant parts.
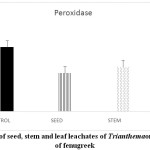 |
Graph 6: Effect of seed, stem and leaf leachates of Trianthemaon enzyme peroxidase of fenugreek Click here to View Graph |
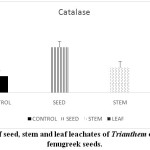 |
Graph 7: Effect of seed, stem and leaf leachates of Trianthem on enzyme catalase of fenugreek seeds. Click here to View Graph |
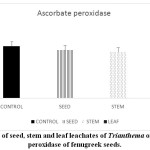 |
Graph 8: Effect of seed, stem and leaf leachates of Trianthema on enzyme ascorbate peroxidase of fenugreek seeds. Click here to View Graph |
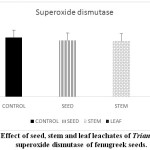 |
Graph 9: Effect of seed, stem and leaf leachates of Trianthema on enzyme superoxide dismutase of fenugreek seeds. Click here to View Graph |
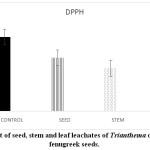 |
Graph 10: Effect of seed, stem and leaf leachates of Trianthema on DPPH activity of fenugreek seeds. Click here to View Graph |
One of the adaptive strategies of plants to cope up with abiotic stress is to synthesise secondary metabolites such as saponins. The saponin content of seeds of fenugreek was found to be reduced due to allelopathic stress of Trianthema leaves, stem and seeds (Plate no.1 and Table no.1). Most noticeable decline in saponin was observed due to the extract of Trianthema leaves. Similarly,31 saponin content in roots, leaves, stems, bulbs, flowers and fruit of Panax ginseng was negatively affected due to abiotic stresses. The combined effect of heavy metals such as Cu and Cd had caused a stressful effect on medicinal plant Gynuraprocumbens (Lour.) Merr. Saponin, Phenolic and flavonoid contents of this medicinal plant were reduced after treating with these metals.22 According to,23 phenolic compounds such as caffeic acid, ferulic acid, M- coumeric acid, P- coumeric acid, syringic acid, venillic acid; and caffeic acid, gallic acid, 4-Hydroxy-3- Methoxybenzoic acid, P-coumeric acid, syringic acid present in Trianthemawhich are responsible for the negative allelopathic effect on plants.
 |
Plate 1: Allelopahic influence of leaves, stem and seeds of Trianthemaon saponin content of fenugreek seeds. Click here to View Plate |
 |
Table 1: Allelopahic influence of leaves, stem and seeds of Trianthema on saponin content of fenugreek seeds. Click here to View Table |
Conclusion
The present study showed that germination ability and seedling growth was badly affected in fenugreek due to the treatment of Trianthema. Antioxidant enzymes such as peroxidase, ascorbate peroxidase, superoxide dismutase and total antioxidant such as DPPH was also reduced after treatment of Trianthema. Leaf lechates of Trianthema were found to be more hazardous than other plant parts. Most vital content of fenugreek i.e. saponin was also affected due to the treatment of Trianthema. Similar kind of observations was noted in the fields of Vasai (Dist. Palghar).
Acknowledgement
Authors are thankful to Head Department of Botany, Viva college (Virar) for providing laboratory facilities.
Conflict of Interest
Authors declare no conflict of interest.
References
- Aziz, A., A. Tanveer, A. Ali, M. Yasin, B.H. Babar and M.A. Nadeem. Allelopathic effect of cleavers (Galiumaparine) on germination and early growth of wheat (Triticum aestivum). Allelopathy J., 2008; 22: 25-34.
- Balayan, R.S. and Bhan, V.M. Competing ability of maize, pearl millet, practices. Crop Res., 1989; 2(2): 147-153.
- Barbara K. -K., Khenifi ,M. L., Piotr M., Messaoud B., Mirosława K.-B. HPTLC determination of diosgenin in fenugreek seeds. Acta Pharm., 2018; 68: 97–107
CrossRef - Economou G., O. Tzakou, A. Gani, A. Yannitsaro and D. Bilalis.Allelopathic effect of Conyzaalbida. Ecolog. 2002; 17: 2021-2034.
- Ellis, R.A. and Roberts, E.H. The quantification of aging and survival in orthodox seeds. Seed and Tech., 1981; 9: 373-409.
- Ghani A. Medicinal plants of Bangladesh chemical constituents and uses. 2nd Ed. The Asiatic Society of Bangladesh, Dhaka. Bangladesh. 2003; 362-363Pp: 502-505
- Gill S.S. and Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem., 2010; 48(12):909-30.
CrossRef - Hamayun M., F. Hussain. S. Afzal and N. Ahmad. Allelopathic effects of Cyperusrotundus and Echinochloacrusgalli on seed germination, plumule and radical growth in maize (Zea mays L.). Pak J. Weed Sci. Res.2005; 11: 81-84.
- Horiguchi T. Mechanism of manganese toxicity and tolerance of plants IV. Effects of silicon on alleviation of manganese toxicity of rice plants. Soil Sci Plant Nutri., 1988; 34:65-73.
CrossRef - Jadhar B.B. and Gayanar, D.G. Allelopathic effects of Acacia auriculiformis on germination of ice and cowpea. Ind. J. Plant Physiol., 1992; 1: 86-89.
- Kakkar P.; Das,B. and Viswanathan, P.N. A modified spectrophotometric assay of superoxide dismutase. Indian J BiochemBiophys., 1984; 2: 130-132
- Kang H.M. and Saltveit, M. E. Effect of chilling on antioxidant enzymes and DPPH-radical scavenging activity of high- and low-vigour cucumber seedling radicals. 2002; 25 (10):1233–1238
CrossRef - Khan N.; Sultana, A.; Tahir,N. and Jamila, N. Nutritional composition, vitamins, minerals and toxic heavy metals analysis of Trianthemaportulacastrum L., a wild edible plant from Peshawar, Khyber Pekhtunkhwa. Pak Afr J Biotechnol., 2013; 12: 6079-6085
CrossRef - Khuram M.; Muhammad A. N.; Tanveer, Z. and Ahmad Z. Allelopathic effect of aqueous extracts of weeds on the germination and seedling growth of rice (Oryza sativa L.). Pak. J. life soc. Sci., 2011; 9(1): 7-12
- Krizek D.T.; Kramer, G.F.; Upadhyaya, A. and Mirecki, R.M. UV-B response of cucumber seedlings grown under metal halide and high pressure sodium/ deluxe lamps. Physiol. Plantarum., 1993; 88: 350- 358.
CrossRef - Lowry O.H.; Rosenbrough, N.J; Farr A. and Randall, R.J.Protein measurement with the folin phenol reagent.J Biol Chem; 1951; 193:265-75.
- Luck H. In: Methods in Enzymatic Analysis II (ed.) Bergmeyer. (Publ.) Academic Press,New York. 1974; Pp 885
- Mahajan S. and Tuteja, N. Cold, salinity and drought stresses: an overview. Arch BiochemBiophys., 2005; 444 :139-158.
CrossRef - Manimegalai A. and Manikandan, T. allelopathic effect of Tectonagrandis leaves extract on antioxidant enzymes in Vigna mungo and Vigna radiate. Asian J. Sci. Tech., 2010; 3 :067-069.
- Maysa M.; Hatata Salama, M. and El-Darier. Allelopathic effect and oxidative stress induced by aqueous extract of Achillea santolina shoot on Triticum aestivum plant. Egypt. J. Exp. Biol. (Bot.)., 2009; 5(0): 131-14.
- Mishra N.P.; Mishra, R.K. and Singhal, G.S. Changes in the activities of anti-oxidant enzymes during exposure of intact wheat leaves to strong visible light at different temperatures in the presence of protein synthesis inhibitors. Plant Physiol., 1993,102: 903–910
CrossRef - Mohd H. I.; Yap, C. K. and Nurul, A. M. Z. Effect of cadmium and copper exposure on growth, secondary metabolites and antioxidant activity in the medicinal plant sambungnyawa(Gynuraprocumbens (Lour.) Merr). Molecules, 2017; 22: 1623.
CrossRef - Muhammad, A.; Asif, T.; Muhammad A. N. and Hafiz, H. A. Comparative allelopathic potential of two aizoaceae weeds against germination of different crops. Pak. J. Weed Sci. Res., 2013; 19(4): 377-391
- Niakan M. and Saberi, K.. Effect of Eucalyptus allelopathy on growth characters and antioxidant enzymes activity in Phalaris weed. Asian J Plant Sci., 2009; 8 (6): 1682-3974
CrossRef - Nayyar M.N., Ashiq, M. and Ahmad, I.. Major weed of Punjab. Manual on Punjab Weeds, 2001; 1: 52-55.
- Putnam A.R. and Wetson, L.A. Adverese impacts of allelopathy in agricultural system. In: Putan, A.R., Tang, S.C. (Edt.). (In.) The science of allelopathy: New York : John Wiely and Sons, Inc, 1986; Pp: 235-239.
- Sadasivum S. and Manikam, A. Biochemical method for agricultural sciences (Publ.) Willey, Eastern Ltd. 1992; Pp:105
- Shimada K.; Fujikawa, K.; Yahara, K. and Nakamura, T. Antioxidative properties of xanthone on the auto oxidation of soybean in cylcodextrin emulsion. J.Agr. Food Chem., 1992; 40: 945–948.
CrossRef - Stepier P.S. and Klobus,G. Antioxidant defence in the leaves of C3 and C4 plants under salinity stress. Plant Physiol., 2005; 125:31-40
CrossRef - Suzuki N.; Koussevitzky, S.; Mittler, R. and Miller, G. ROS and redox signalling in the response of plants to abiotic stress. Plant Cell Environ., 2012; 35(2):259-270.
CrossRef - Szakiel A.; Paczkowski, C. and Henry, M. Influence of environmental abiotic factors on the content of saponins in plants. Phytochem Rev., 2010; 10: 471-491.
CrossRef - Tomaszewski M and Thimann, K.V. Interactions of phenolic acids, and metallic ions, chelating agents on auxin induced growth. Plant physiol., 1966; 41: 1443-1454.
CrossRef - Zhang Kai Mei, Shi Lei and Li Zhen Yu,. Fern allelopathy and its impact on biodiversity.Biodiversity Science, 2004; 12: 466-471.
- Zimdahl R.L. Fundamentals of Weed Science. Third Edition. Academic Press, an imprint of Elsevier. 2007; p 228.


