Introduction
Anthocyanins are the natural pigment present in many flowers, fruits and vegetables and are chiefly responsible for their red, blue or purple colour.1 Research on the application of anthocyanins, due to their potential health benefits in combating several diseases; have attracted great interest in the functional food, nutraceutical and pharmaceutical industries recently. Antioxidant activity of anthocyanins act as an anticarcinogenic agent and are helpful in treating diseases such as neural and cardiovascular illnesses and diabetes.2,3 Most of the industries are using the synthetic food colour have a chemical source that causes adverse health effect. Black carrot anthocyanins can be used as a natural food colourant having antioxidant properties to promote health benefits.4 The black carrot was originated from middle Asia and is considered to be the archetype of all modern orange carrots.5 Carrot, in terms of their nutritional value among 38 other fruits and vegetables has been ranked tenth.6
Five major anthocyanins pigment present in Black carrot are, two nonacylated, cyanidin 3- xylosyl glucosyl galactoside (Cya3XylGlcGal) and cyanidin 3-xylosyl-galactoside (Cya3XylGal), and three derivatives of cyanidin acylated with sinapic acid (cyanidin 3- sinapoyl xylosyl glucosyl galactoside, Cya3SXylGlcGal), ferulic acid (cyanidin 3- feruloyl xylosyl glucosyl galactoside, Cya3FXylGlcGal) and p-coumaric acid (cyanidin 3-p- coumaroyl xylosyl glucosyl galactoside, Cya3pCXylGlcGal).7 Birks8 has been discussed the use of black carrot anthocyanins as the natural colourant in the production of confectionery, jellies, jams and frozen desserts. The factors that affect the colour and stability of anthocyanins are structure and concentration, pH, temperature, light, presence of pigments, self-association, metallic ions, enzymes, oxygen, ascorbic acid, sugar and their degradation products, proteins and sulfur dioxide.9-11
Increasing the demands of nutraceutical food due to increased consumer awareness, researchers are now concentrating to developed food with more functional and nutraceutical properties12-19Among them encapsulation technology is a major area to develop functional ingredients for the fortification in to different food matrices20-24 Encapsulation is a technique to prevent the sensitive materials such as anthocyanins from moisture, heat, light or oxidation.4,25 It is a packaging technique of materials in the form of micro and nanoparticles.26 Because of high perishable nature of black carrot, it is difficult to preserve it as a raw product. Spray drying is a new technique to preserve most of the anthocyanins present in the black carrot in combination with carrier agents for a long time. There are several methods for encapsulation of anthocyanins has been described.27-32 Spray drying has been used as a simple technique in encapsulating water-soluble essence natural aroma and colour.4,25,28 Spray dried black carrot anthocyanin have better heat, light and pH stability, therefore, have enhanced shelf life and stability.5,6,26,33 The present study was conducted to study the storage stability of spray-dried anthocyanin-rich extract from black carrot encapsulated using
Materials and Methods
Black carrot samples for anthocyanin extraction were grown in the Division of Vegetable Science, Indian Agriculture Research Institute, New Delhi and transferred to the Division of Food Science and Post-Harvest Technology, IARI, New Delhi in about 20 kg plastic bags and kept at -20 ºC until extraction.
Juice Extraction
Carrots were manually peeled using a stainless-steel knife and crushed coarsely by using a blender (Waring Laboratory Science Torrington, USA). For the inactivation of polyphenol oxidase activity, the crushed mass was heated to 60ºC in a water bath and cooled rapidly to 45ºC with ice water. The pectinase enzyme (EC3.2.1.1 from Aspergillus niger, 1U/mg, optimum pH 4.5-5.5) was added to the mass at 0.2 ml/kg mixed thoroughly and incubated at 60ºCfor 1 hour.The mass immediately pressed (1800kg/m2) in a hydraulic press (Johnston automation, India). The extracted juice was heated to 90ºC for 1 minute then cool to 5ºC and packed in pre-sterilized amber coloured glass bottles.
Carrier Agent for Spray Drying
Jack fruit seed starch (JSS), Whey protein, Soy protein were obtained from the local market of New Delhi and NBRE-15 (a type of plant source emulsifier developed in the lab, a patent filed) was from National Botanical Research Institute, Lucknow.
Preparation of Feed Mixture
A mix of different carrier agents viz., jackfruit seed starch, soy protein and NBRE-15 (SET -1) and jackfruit seed starch, whey protein and NBRE-15 (SET-2) were used to encapsulate the anthocyanin extract. Previously optimized ratio of JSS:SPI: NBRE-15 and JSS:WPI: NBRE-15 were separately used to prepare the anthocyanin feed mixture at the optimized concentration of 27.3ºBrix (SET-1) and 27.8ºBrix (SET-2).Brix was measured after homogenization of the mixture using digital ultra-turrax homogenizer (IKT®T25) for the stirring period of 30 minutes.4
Spray Drying
The feed mixture was spray dried in a Sono Dry 1000 (SONO-TEK, USA) advance laboratory spray drier with complete unit 650 mm L×550 mm W×1625 mm H and drying chamber diameter size 230 mm. The operating condition was given in the table -1
Table 1: Operating conditions of the Spray drier.
| Parameters | Set Conditions |
|
Inlet temperature |
150ºC±2 (Minimum), 180±2 (Maximum) |
|
Outlet temperature |
50ºC±2 (Min), 80ºC±2 |
|
Sample temperature |
30ºC±2 |
|
Aspirator air flow rate |
50 m3/h |
|
Feed flow rate |
2-2.5 ml/min |
|
Compressor pressure |
115 psi |
Encapsulated anthocyanin powder storage
Encapsulated black carrot anthocyanins powder were stored in amber coloured bottles (ACBs) and in transparent colored bottles (TCBs) with screw caps and placed at 25ºC to determine the quality attribute of encapsulated anthocyanin powder. Degradation study was followed for 9 weeks of storage and the content was analyzed weekly according.34
Moisture Content of Encapsulated anthocyanin powder
The moisture content of the spray dried powder was determined by using a vacuum oven method. 2 g powder samples were measured and kept in the oven at 70ºC and 100 mm Hg vacuum for 7-8 h. After cooling in desiccators, the moisture content in percentage was calculated by using the following equation.
![]()
Colour Measurement
The colour changes in the encapsulated samples were measured with HunterLab colourimeter (LabScan® XE Plus 4500 L, USA). The instrument was calibrated with black and white reference tiles taking as a standard. The color value were expressed as L* (lightness/darkness), a* (red/green), b* (yellow/blue), C (Chroma) and Hº (hue angle).35
C = (a*2 + b*2)1/2 (2)
H° = tan-1 (b*/a*) (3)
Total Anthocyanins Content
The total monomeric anthocyanin was determined by the pH differential method.36 Two buffer systems were prepared- potassium chloride buffer, pH 1 (0.025 M) and sodium acetate buffer, pH 4.5 (0.4 M). The pH was adjusted with HCl. Samples were diluted in pH 1 and pH 5 buffers and the absorbance measurement were made at 510 and 700 nm respectively. Anthocyanins in encapsulated powder expressed as cyanidin-3-glucoside equivalents, as follows:
![]()
Where, A (Absorbance) = pH 1.0 (A510-A700) – pH 4.5 (A510-A700); MW= Molecular weight (449.2 for cyanidin-3-glucoside); DF= Dilution Factor; ɛ= Molar absorptive (26 900 molar extinction coefficient, in L & mol–1& cm–1 for cyanidin-3-glucoside).
The encapsulation efficiency of the anthocyanin was calculated using a previously published method.4
Antioxidant Activity
Antioxidant activity in encapsulated black carrot powder was determined by CUPRAC method developed by Apaket et al.,37 measures the copper (II) reducing ability of polyphenols, Vitamin C and E. For determination of antioxidant activity 100μl supernatant samples (2-5 g samples were extracted in 10-12 ml 80% ethanol and centrifuge at rpm 10000 for 20 min) were mixed with 1 ml 10-2 M copper chloride, 1 ml 7.5×10-3 M Neocuproine (MW= 208.26 g), 1 ml ammonium acetate buffer (pH 7) and 1 ml distilled water in the test tubes. The final volume reached 4.1 ml. The absorbance of the samples was taken at 450 nm after 30 minutes against a reagent blank in the UV –Vis spectrophotometer (Jasco V-670, Japan). The calculation was done using the following equation:
Antioxidant capacity (μ mol Trolox/g)=(A / ɛTR) (Vf / Vs) DF (Vt/m) (5)
Where,A= Absorbance, ɛTR= Molar absorptivity of Trolox (1.67×104), Vf = Final volume made (4.1 ml); Vs = Sample volume taken from diluted extract (ml), DF= Dilution factor, m= weight of the sample (g)
Result and Discussion
The initial moisture content of spray dried black carrot powder was 4.36 % and 5.02 % for the SET-1 and the SET-2 respectively. It was found that the increase in feed flow rate from 2 to 2.5 ml/ min, the moisture content in the encapsulated powder were also increased and reached 4.85 % for SET-1 and 5.42 % for the SET-2. Ersus and Yurdagel38 were also reported that increasing in feed flow rate, increase the moisture content.
During the storage period, the L* values increase significantly for SET-1 and for SET-2, however, values of a* and b* declined with respect to days interval. The same results were reported by Kamiloglu et al.,35 Fresh encapsulated black carrot showed L*, a* and b* values of 32.20, 28.21 and -1.60 for the SET-1. However, for the SET-2 these values were reported to be 34.32, 24.21 and -1.66 respectively. On the other hand, the C values were 30.77 for STE-1 and 29.96 for SET-2 respectively. Our samples had a negative Hº value which ranged -17.61 (for SET-1) to -14.56 (for SET-2) corresponding to a bluish hue for the fresh encapsulants. It was observed that L* values increased as the* and b* values declined every week during storage. The decline in a* values was an indication of the reduction of anthocyanin content and shifted towards bluish hue with a negative Hº value for both the sets. SET-1 had higher retention of a* values as compared to SET-2, showing it had a higher capacity to prevent anthocyanin reduction during storage (table 1 and 2). Amber coloured bottles (ACBs) were found to be more effective to prevent colour degradation over transparent coloured bottles (TCBs). The samples had to fall b* value and attained a higher negative value during week intervals, indicated increased in blueness. Skerede39 reported a decrease in the b* factor of black carrot syrups during storage.
Table 2: Changes in the colour of SET-1 during storage.
|
Days |
SET-1 (ACBs) |
SET-1(TCBs) |
||||||||
|
L* |
a* |
b* |
C |
Hº |
L* |
a* |
b* |
C |
Hº |
|
|
0 |
32.20 |
28.21 |
-1.60 |
30.77 |
-17.61 |
32.20 |
28.21 |
-1.60 |
30.77 |
-17.61 |
|
7 |
32.25 |
28.11 |
-1.80 |
31.32 |
-15.59 |
34.60 |
27.02 |
-1.82 |
30.33 |
-14.82 |
|
14 |
32.33 |
28.01 |
-2.07 |
32.29 |
-13.50 |
34.64 |
26.65 |
-2.09 |
31.01 |
-12.72 |
|
21 |
32.41 |
27.85 |
-2.26 |
32.95 |
-12.29 |
34.66 |
26.52 |
-2.31 |
31.85 |
-11.45 |
|
28 |
32.60 |
27.61 |
-2.38 |
33.27 |
-11.57 |
34.72 |
26.31 |
-2.66 |
33.38 |
-9.85 |
|
35 |
32.71 |
27.40 |
-2.60 |
34.16 |
-10.50 |
34.78 |
25.73 |
-2.81 |
33.62 |
-9.12 |
|
42 |
32.80 |
27.33 |
-2.78 |
35.05 |
-9.79 |
34.83 |
25.68 |
-2.96 |
34.44 |
-8.63 |
|
49 |
33.02 |
27.30 |
-2.94 |
35.94 |
-9.24 |
35.02 |
25.88 |
-3.09 |
35.42 |
-8.33 |
|
56 |
33.12 |
27.12 |
-3.08 |
36.60 |
-8.76 |
35.38 |
26.04 |
-3.18 |
36.15 |
-8.14 |
|
63 |
33.23 |
27.02 |
-3.28 |
37.77 |
-8.19 |
35.51 |
26.19 |
-3.30 |
37.08 |
-7.89 |
Note: ACBs (Amber colored bottles), TCBs (Transparent colored bottles).
Table 3: Changes in the color of SET-2 during storage.
|
Days |
SET-2(ACBs) |
SET-2(TCBs) |
||||||||
|
L* |
a* |
b* |
C |
Hº |
L* |
a* |
b* |
C |
Hº |
|
|
0 |
34.32 |
24.21 |
-1.66 |
26.96 |
-14.56 |
34.32 |
24.21 |
-1.66 |
29.95 |
-14.56 |
|
7 |
34.55 |
24.55 |
-1.91 |
28.19 |
-12.82 |
34.66 |
24.74 |
-1.98 |
28.66 |
-12.46 |
|
14 |
34.84 |
24.67 |
-2.11 |
29.12 |
-11.66 |
34.95 |
25.02 |
-2.18 |
29.77 |
-11.44 |
|
21 |
34.98 |
24.79 |
-2.28 |
29.98 |
-10.84 |
35.11 |
25.28 |
-2.36 |
30.84 |
-10.68 |
|
28 |
35.31 |
24.98 |
-2.45 |
30.98 |
-10.16 |
35.45 |
25.55 |
-2.51 |
31.85 |
-10.14 |
|
35 |
35.42 |
25.26 |
-2.67 |
32.38 |
-9.42 |
35.8 |
25.79 |
-2.75 |
33.35 |
-9.34 |
|
42 |
35.87 |
25.41 |
-2.88 |
33.70 |
-8.78 |
35.97 |
25.94 |
-2.89 |
34.29 |
-8.93 |
|
49 |
35.37 |
25.64 |
-3.02 |
34.76 |
-8.45 |
36.12 |
26.10 |
-3.02 |
35.22 |
-8.60 |
|
56 |
33.12 |
27.12 |
-3.08 |
36.60 |
-8.76 |
35.38 |
26.04 |
-3.18 |
36.15 |
-8.14 |
|
63 |
33.23 |
27.02 |
-3.28 |
37.77 |
-8.19 |
35.51 |
26.19 |
-3.30 |
37.08 |
-7.89 |
Note: ACBs (Amber coloured bottles), TCBs (Transparent coloured bottles).
The anthocyanin content was highly influenced by different coating agents. In case of SET-1 anthocyanin content was found to be maximum 2969.66 mg/100 g of dry matter with encapsulation efficiency 87.72%. On the other hand, SET-2 had low encapsulation efficiency i.e. 75.36 % with anthocyanin content 2752.91 mg/100 g of dry matter (Fig. 1). Ersus and Yurdagel38 were reported anthocyanin content in black carrot 2721.61±5.92 mg/100 g of dry matter.
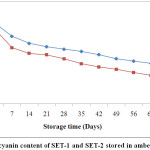 |
Figure 1: Anthocyanin content of SET-1 and SET-2 stored in amber coloured bottles. Click here to View Figure |
ACBs were reported to be more effective in retaining anthocyanin content over TCBs during storage (Fig. 2). At the end of the storage period, anthocyanin retention in SET-1 and SET-2 stored in ACBs were found 49.98 % and 38.04 %. However, those stored in TCBs had retention of 35.15 % for SET-1 and 34.56 % for SET-2. Fig. 1 and Fig. 2 were showing the reduction pattern in anthocyanin content during storage in TCBs and ACBs.
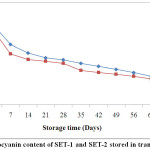 |
Figure 2: Anthocyanin content of SET-1 and SET-2 stored in transparent bottles. Click here to View Figure |
The reduction in the anthocyanin content may be due to the presence of heat stable form of polyphenol oxidase or peroxidase.4,33 It may also a result of the conversion of anthocyanin glycosides to chalcones, resulting in phenolic acids and aldehydes due to hydrolytic reactions.35 Black carrots contain cyanidine based anthocyanin and have vicinal hydroxyl groups. Anthocyanin of this group is expected to more prone to degradation.33 During storage at 25ºC, the soy protein (SET-1) was reported to be better encapsulating agent than whey (SET-2) containing the encapsulating agent.
Structure plays a key role in the determination of the antioxidant activity of anthocyanins and highly correlated with anthocyanin content and total phenolic compound of food products.4,16 The antioxidant activity of encapsulated black carrot was reported to be 344.3 μmoltrolox/100g dry matter (for SET-1) and 282.3 μmoltrolox/100g dry matter (for SET-2) by using the CUPRAC method. Algarra40 were reported antioxidant activity in black carrot by using DPPH and FRAP methods in their study, were low (17.6–240 and 86.4–182 μMTE/100 g fw for DPPH and FRAP, respectively) as compared to our samples. Kamiloglu35 reported antioxidant activity (1720±149 and 5617±357 mg TE/100 g DW for DPPH and FRAP, respectively). The differences in the antioxidant activity depend on various factors like climatic conditions, cultivar, growth conditions and stage of harvesting. The storage temperature plays an important role in determining the stability of the antioxidant. The encapsulated black carrots powders were stored at 25ºC, thus a rapid reduction in the antioxidant activity during storage (Fig. 3 & Fig. 4).
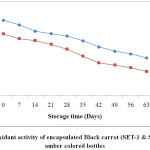 |
Figure 3: Antioxidant activity of encapsulated Black carrot (SET-1 & SET-2) stored in amber coloured bottles. Click here to View Figure |
Kamiloglu et al.,35 were also in the agreement that the more loss in antioxidant activity occurred in black carrot jam and marmalade stored at 25ºC as compared to those stored at 4ºC. The reduction in the antioxidant activity could be due to the presence of oxygen in the headspace of the bottles. At the end of storage, SET-1 showed higher retention i.e. 50% in the antioxidant activity as compared to SET-2 with maximum retention 38.04 stored in ACBs. On the other hand, samples stored in TCBs had retention of 40.14 % and 30.93 % for SET-1 and SET-2 respectively. The reduction in the antioxidant could also be due to exposure of the samples in the light (for ACBs).
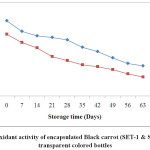 |
Figure 4: Antioxidant activity of encapsulated Black carrot (SET-1 & SET-2) stored in transparent coloured bottles. Click here to View Figure |
Conclusion
For spray drying black carrot anthocyanin, SET-1 containing soy protein with jack fruit seed starch and NBRE-15 gave highest anthocyanin content and antioxidant capacity powder after drying. In order to minimize the colour loss, anthocyanin content and antioxidant activity these products should be stored in amber coloured bottles with minimum head space. SET-1 had higher efficiency to prevent the loss of colour, anthocyanin and antioxidant activity during storage.
Acknowledgements
The authors are grateful to the National Agriculture Science Fund (NASF) project (code: 20-09), Government of India for financial support. Authors also thanks ICAR – Indian Agricultural Research Institute, New Delhi, for providing the core material for the encapsulation.
Ethical Approval
This article does not contain any studies with either animals or human participants performed by any of the authors.
Conflict of Interest
The authors declare that they have no conflict of interest.
Authors Contributions
Mr. Brij Bhushan Mishra conducted the analysis of collected samples. Mr. Avinash Singh Patel conducted the design of the experiments and supported Mr. Mishra during analysis. Mr. Mishra has written the manuscript. Dr. Abhijit Kar interpreted the experimental data and reviewed and revised the manuscript.
References
- Gizir A. M., Turken, N., Artuvan, E. Pressurized acidified water extraction of black carrot (Daucus carotassp.sativus var. atrorubensAlef.) anthocyanins. Eur Food Res Technol, 2008;226:363-370.
CrossRef - Tsai P., McIntosh J., Pearce P., Camden B., Jordan B. R. Anthocyanins and Antioxidant Capacity in Roselle (Hibiscus Sabdariffa L.) Extract. Food Int Res,2002;35:351-356.
CrossRef - Nichenamentla S. N., Taruscio T. G. Barney D. L. Exon J. H. A Review of the Effects and Mechanisms of Polyphenolics in Cancer. Crit Rev Food Sci Nutr,2006; 46:161-183.
CrossRef - Kar A., Mahato D. K., Patel A. S., Bal L. M. The encapsulation efficiency and physicochemical characteristics of anthocyanin from black carrot (Daucus carota Ssp. sativus) as affected by encapsulating materials. Current Agric Res, J. 2019; 7(1).
CrossRef - Kamiloglu S., Pasli A.A., Ozcelik B., Camp J.V., Capanoglu E. Influence of different processing and storage conditions on in vitro bioaccessibility of polyphenols in black carrot jams and marmalades. Food Chem, 2015; 186:74-82.
CrossRef - Alasalvar C., Al-Farsi M., Quantick P.C., Shahidi F.Wiktorowicz R. Effect of chill storage and modified atmospheric packaging (MAP) on antioxidant activity, anthocyanins, carotenoids, phenolics, and sensory quality of ready- to-eat shredded orange and purple carrots. Food Chem, 2005;89:69-76.
CrossRef - Netzel M., Netzel G., Kammerer D.R., Schieber A., Carle R., Lloyd Simons L., Bitsch I., Bitsch R., Konczak I. Cancer cell antiproliferation activity and metabolism of black carrot anthocyanins Innovative. Food SciEmergTechnol,(2007;8:365–372.
CrossRef - Birks S. The potential of carrots. FoodManufact, 1999;47(4):22-23.
- Francis F. J. Food colorants: anthocyanins. CRC Criticl Rev Food Sci Nutrit,1989; 28(4):273-315.
CrossRef - Mazza G., Miniati E. Anthocyanins in fruits, vegetables and grains. London: CRC Press.1993.
- Rodriguez-Saona L. E., Giusti M. M., Wrolstad R. E. Color and pigment stability of red radish and red fleshed potato anthocyanins in juice model systems. J Food Sci, 1999; 64:451-456.
CrossRef - Patel A. S., Pandey A. K. Fortification of Limoniaacidissima Linn. fruit powder to develop the phenolic enriched herbal biscuits. J BioresEng Technol, 2014; 1:74-85.
- Patel A. S., Pradhan R. C. Quality ranking of bottle gourd seed cake powder incorporated biscuits using fuzzy analysis of sensory attribute. BIOINFOLET-A Quart J Life Sci, 2015;12(4a):901-908.
- Pradhan R. C., Patel A. S., Mishra S. Physico-chemical properties of bottle gourd kernel. J Agric Eng, 2015; 52(4):28-34.
- Duhan S., Kar A., Nain L., Patel A. S., Dash S. K. Development of continuous flow microwave and hot water bath system for destruction of spoilage microorganisms in food. Ind J Agric Sci, 2017; 87(2):210-214.
- Ghosh P., Pradhan R. C., Patel A. S., Kar A., Mishra S. Physicochemical characteristics of Syzygiumcumini fruit. Curr Res Nutr Food Sci, 2017; 5(1):25-35.
CrossRef - Patel A. S., Pradhan R. C., Kar A., Mohapatra D. Effect of Fortification of De-oiled Bottle Gourd (Lagenariasiceraria) Seed on the Functional and Chemical Characteristics of the Biscuit: A Nutritional Assessment. Curr Res Nutr Food Sci, 2018; 6(3).
CrossRef - Mohapatra D., Patel A. S., Kar A., Deshpande S. S., Tripathi M. K. Effect of Different Processing Conditions on Proximate Composition, Anti-oxidants, Anti-nutrients and Amino Acid Profile of Grain Sorghum. Food Chem, 2018; 271:129-135.
CrossRef - Patel A. S., Kar A., Pradhan R. C., Mohapatra D., Nayak B. Effect of baking temperatures on the proximate composition, amino acids and protein quality of de-oiled bottle gourd (Lagenariasiceraria) seed cake fortified biscuit. LWT Food Sci Technol, 2019; 106: 247-253.
CrossRef - Yan K., Lu Z., Lee H. W., Xiong F., Hsu P. C., Li Y., Cui Y. Selective deposition and stable encapsulation of lithium through heterogeneous seeded growth. Natur Eneg, 2016; 1(3):16010.
CrossRef - Desai T., Shea L. D. Advances in islet encapsulation technologies. NaturRev Drug Discover, 2017; 16(5):338.
CrossRef - Browne S. H., Peloquin C., Santillo F., Haubrich R., Muttera L., Moser K., … Blaschke T. F. Digitizing Medicines for Remote Capture of Oral Medication Adherence Using Co‐encapsulation. Clin Pharmac Therapeut, 2018; 103(3):502-510.
CrossRef - Khojasteh D., Kazerooni N. M., Marengo M. A review of liquid droplet impacting onto solid spherical particles: A physical pathway to encapsulation mechanisms. J Ind Eng Chem, 2018.
CrossRef - Layne R. E., Cundy S. M., Bebb D. A. U.S. Patent Application No. 10/213,328.2019.
- Jafari S. M., Assadpoor E., He, Y., Bhandari B. Encapsulation efficiency of food flavours and oils during spray drying. Dry Technol, 2008; 26(7):816-835.
CrossRef - Khazaeia K.M., Jafaria S.M., Ghorbania M., Kakhki A.H. Application of maltodextrin and gum Arabic in microencapsulation ofsaffron petal’s anthocyanins and evaluating their storage stability and color. Carbohydr Polym,2014; 105:57-62.
CrossRef - Murali S., Kar A., Patel A. S., Kumar J., Mohapatra D., Dash S. K. Spray dried encapsulation of rice bran oil in soy protein isolate – tapioca starch complex using response surface methodology. Ind J Agric Sci, 2016; 86(8):984-91
- Patel A. S., Khan I., Kar A. A technique of micro capsulation by spray-drying of nutrient elements: A review. NISCAIR Public, 2016; 24(2): 164-172.
- Patel A. S., Kar A., Khan I. Process for development of β-carotene Nanocomposites with ɷ-fatty acids. 2017.
- Dhakane J. P., Kar A., Patel A. S., Khan I. Effect of soy proteins and emulsification-evaporation process on physical stability of lycopene emulsions. Int J Chem Studies, 2017; 5(5):1354-1358.
- Murali S, Kar A., Patel A. S., Mohapatra D., Krishnakumar P. Optimization of process conditions of rice bran oil encapsulation using spray drying by response surface methodology. Int J Food Eng, 2017; 13(4):25-35.
CrossRef


