Introduction
Mustard-rapeseed is the important crop and presently ranked as the world’s leading oilseed crop in terms of area and production. Among the species, Brassica napus and Brassica rapa are regarded as “Rapeseed” while Brassica juncea is “Mustard”. In Bangladesh mustard-rapeseed is the most important edible oilseed crop . However, the total annual production of rapeseed and mustard in worldwide is 68 million tons of seeds from an area of 34.33 million hectares (FAO STAT 2015-2016, faostat.fao.org). Approximately 0.302 million hectares of area were under cultivation of mustard-rapeseed out of 14.22 million hectares in Bangladesh. Bangladesh is one of the important mustard-rapeseed growing country in South Asia after India producing 359 thousand tons in 2014-15 (FAO STAT 2015-2016). Till to date, the most widely cultivated crop species in the crucifer family (Brassicaceae) is mustard-rapeseed. Many factors are associated with the poor yield of mustard-rapeseed in Bangladesh. Diseases have been identified as one of the major factors (Ahmed, 1992).1 Because of an strong bottle-neck in selection for canola quality i.e. double-low (00) seed quality (zero erucic acid, low glucosinolate content) during breeding in the last 3 decades, cultivated mustard-rapeseed varieties in worldwide have a relatively narrow genetic basis and are lacking of a broad spectrum of disease resistance (Obermeier et al. 2013).19 Brassica oilseeds are suffered from thirty diseases (Khan, 2011).11 Common diseases of mustard-rapeseed are Aternaria leaf spot, Stem rot, Stem canker, Clubroot, White rust, Light leaf spot, Downy mildew, Turnip Mosaic Virus (TuMV) and Turnip Yellows Virus (TuYV) (Snowdon et al. 2006).20 Among these diseases Alternaria leaf spot is considered as one of the major diseases due to their wide distribution and yield losses to Brassica crops worldwide (Khan et al., 2010; Khan, 2011; Meah et al., 2002)10,11,16 including Bangladesh. On the other hand, in India this disease causes an average yield loss of 40-70%(Vishwanath and Kolte, 1997)21 and in Bangladesh this yield loss up to 60(Ahmed and Ahmed, 1994; Meah and Hossain, 1988).2,15
Alternaria leaf spot disease is described by the formation of spot on the infected leaves and stems and in siliquae during maturing stage of infected plants. This disease may reduce photosynthesis capacity as grey or black lesion developed on the affected leaves and siliquae and enhance immature ripening. Besides leaf infection, the infection also leads to deteriorate the quality of the seeds i.e. seed size, seed colour, oil contents and germination capacity (Khan et al. 2010).10 Moreover A. brassicae and A. brassicicola are seed borne pathogens and these pathogen can be passed on to next generation by direct infection of developing seeds in siliquae, resulting severe damage in seedlings mostly in the nursery beds (Kubota et al. 2003).14 Yield loss of up to 46-47% in yellow sarson and 35-38% in mustard may occur by this Alternaria leaf spot or leaf blight disease (Vishwanath and Kolte, 1997; Khan et al. 2010; Khan, 2011).21,10,11 However, due to the lack of resistance variety against A. brassicae/A. brassicicola in Brassicaceae family, Alternaria leaf spot is considered the most destructive and widespread fungal disease of Brassica (Ghose et al. 2008).6 As the increase of area cultivated with mustard-rapeseed and lack of resistance in current Brassica cultivars, infection by pathogen such as Alternaria spp. has become a major constrain to mustard-rapeseed production worldwide including Bangladesh. Since Alternaria leaf spot is increasingly critical in oil production, resistant varieties need to be developed as to control this disease and to increase seed production and to enhance sustainable crop production without perilous chemical control. However, In any of the disease resistance breeding program, the first approach is to quickly screen all the available germplasm including local races, improved variety and exotic genetic stocks through traditional techniques in controlled condition in the green house, or by field evaluation. Moreover, resistance breeding is the promising initiative for the long term control of diseases as fungicides could not provide effective field control of A. brassicae/A. brassicicola. Intriguingly, no comprehensive report on resistance screening for Alternaria leaf spot/Black leaf spot of mustard-rapeseed in Bangladesh have been reported. In view of the facts, the present research work has been carried out to develop an effective inoculation method with A. brassicicola on mustard-rapeseed and to screen genotypes of mustard-rapeseed resistance against A. brassicicola through the developed techniques in Bangladesh.
Materials and Methods
Plant Materials
Twenty seven (27) mustard-rapeseed genotypes with control were used in this resistant screening study. The seeds were collected from Department of Genetics and Plant Breeding (GPB), Bangladesh Agricultural University (BAU), Bangladesh Agricultural Research Institute (BARI), Bangladesh Institute of Nuclear Agriculture (BINA) and Sher-E-Bangla Agricultural University (SAU). This screening populations comprised of Brassica campestris /Brassica rapa, Brassica juncea, and Brassica napus species which are commonly cultivated in Bangladesh (Table 1). The seeds were sown in soil using plastic pots in a net house of Seed Pathology Centre (SPC), Bangladesh Agricultural University, Mymensingh-2202, Bangladesh and the entire experiments were conducted during the period from October, 2015 to March, 2016.
Table 1: Genotypes of mustard-rapeseed used in this study.
|
Name of the Brassica species |
Name of the Brassica genotypes |
Collected Organization |
Number of genotypes |
| Brassica rapa | Shampad, Sonali, BARI sarisha-6,9,12,14,15, Kallaynia, Safal, Agrani, Tori-7, BINA Sarisha-6, SAU-1 | BAU, BINA and SAU |
13 |
| Brassica juncea | Daulat, Rai-5, BARI Sarisha 10 & 11, BINA Sarisha-8 | BARI and BINA |
5 |
| Brassica napus | BARI Sarisha-7,8,13, BINA Sarisha-4,5,9 & 10, GPB (BAU)-Line 1 & 2 | BARI, BINA and GPB (BAU) |
9 |
Collection, Isolation and identification of Alternaria brassicicola isolate
Isolate of Alternaria brassicicola was obtained from infected mustard seeds which were collected from farmers stored seeds. Standard blotter method (ISTA, 1996)9 was used for the isolation of this Alternaria pathogen. In this blotter method, 25 mustard seeds were placed on blotter paper (moistened with water drop) in plastic petridishes. Then the petridishes were incubated at the incubation room at 20-250C. After 7-10 days of incubation pathogenic structures (mycelia and conidia) on seeds were observed under stereo binocular microscope. Then the infected seeds were transferred on Potato Dextrose Agar (PDA) medium for 7-10 days in the incubation room to allow the fungus to grow and the concern pathogen was detected by preparing slide and comparing the morphological characters (Figure 1).
Fungal block was transferred to the centre of a fresh PDA plate using a sterilized block cutter. The plates were sealed with parafilm and all the isolates were then grown at 24˚C with alternate 12/12 h UV light and darkness for 10 days. Sequential culturing from fungal stock was done for 4-6 times to get a pure culture which was used for inoculation (Figure 1). All of these process was done within laminar air-flow cabinet.
Inoculum Preparation
A pure culture of A. brassicicola grown on PDA medium was developed from stock culture for artificial inoculation. Conidia of 10 days old culture were collected by washing the pure culture of A. brassicicola with double distilled water and the filtered through cotton cloth. The concentration of the conidial suspension was determined using a hemocytometer and adjusted to 5×104 conidia ml-1.
Methods of Inoculation
Two different inoculation techniques were followed in the present study.
Detached Leaf Inoculation
The detached leaf inoculation was performed according to Doullah et al. (2006)3, in brief, 3rd and 4th true leaves i.e. two different leaves were collected from each of 30 days old plants. Three replications were maintained for each of the genotypes including one control. Leaves were then washed with distilled water and remove moisture by tissue paper, and placed on petridishes having three layers of moistened blotter paper with distilled water. Then the conidial suspension sprayed on the detached leaf using hand sprayer and the petridishes were wrapped with parafilm. The concentration of the conidial suspension was maintained at 5×10⁴ conidia ml-1. After inoculation, the Petridishes containing inoculated leaves were marked and incubated for 3 days at 24˚C in incubator with alternate cycle of light/darkness . One Japanes B. rapa resistance variety, Edononatsu was used as resistant control. BARI-Sharisha-7 was used as susceptible check. Then the disease severity was observed after 72 hrs of inoculation.
Seedling Inoculation Test
In case of seedling inoculation test, 30 day-old plants were used and the inoculum of A. brassicicola was sprayed on the foliar surface of the plants according to Doullah et al. (2006)3. All the genotypes received same amount of suspension and covered with polythene bag having small pores for aeration. After that the inoculated plants were kept in a net house after covering with polythene bags for creating high humidity within the micro climate. Japanes B. rapa variety, Edononatsu and BARI-Sharisha-7 were used as resistant and susceptible control, respectively as before. The inoculum suspension contained 4×105 conidia/ml throughout the test. Distance between control and inoculated seedlings maintained in the net house. Then the disease severity was observed after 72 hrs of inoculation.
Assessment of Disease Symptoms
Development of Alternaria spot/black leaf spot on inoculated detached 3rd & 4th leaves as well as seedlings were assessed by a disease severity scale consisting of 10 classes according to King, (1994).13 Disease symptoms on detached leaves or seedlings were evaluated after 72 hrs of inoculation. Leaf area infection for each of the inoculated plant was considered for assessment of disease severity.
Statistical Analysis
The experiment was performed in a completely randomized design (CRD) with three replicates for both detached leaf and seedling inoculation. In case of seedling inoculation one detached leaf either it is 3rd or 4th for one plant has been considered one replication. In case of seedling inoculation one pot containing 2-3 plants considered as one replication in this study. Statistical analysis for phenotypic data was carried out with SPSS statistical software.
Results
Detached Leaf Inoculation
In case of detached leaf inoculation, 3rd and 4th leaf of 30 days old were collected from each of the 27 mustard-rape seed varieties. Then the detached leaves were inoculated with the conidial suspension (5×104 conidia ml-1) of A. brassicicola and then incubated them at 24˚C for 72 hrs. After 72 hrs of inoculation, the inoculated susceptible mustard variety BARI Sharisha-7 produced typical black spot caused by A. brassicicola on the leaf blade (Figure 2). On the other hand, the resistant Japanese B. rapa variety Edononatsu produced no spot on the leaf blade after 72 hrs of inoculation by A. brassicicola suspension. This result indicates that detached leaf-inoculation was successful.
Seedling Inoculation
In case of seedling-inoculation method, 30 days old seedlings of 27 mustard-rapeseed genotypes were inoculated with A. brassicicola suspension (5×104 conidia ml-1) and the disease symptoms was observed after 72 hrs of inoculation. The inoculated susceptible mustard variety BARI Sharisha-7 produced typical Alternaria leaf spot symptom. However, the seedlings of resistance Japanese B. rapa variety Edononatsu produced no spot on the leaf surface after inoculation. This result indicates that seedling-inoculation technique also successful for screening assessment.
Effect of leaf age on disease development
After inoculation with A. brassicicola, older leaves of mustard-rapeseed showed severe symptoms compared to the younger leaves for both tolerant and susceptible varieties in most of the cases. Variation in disease development was evident among leaves at the 3rd or 4th leaf position (Figure 3). This result indicates that the success of detached leaf inoculation was affected by the age of leaf. Here, the disease severity index of 3rd leaf and 4th leaf of the inoculated mustard-rapeseed genotypes showed a strong positive correlation (R2=0.956) (Figure 4).
Effect of different inoculation methods on disease development
Disease development was observed in 27 cultivars of mustard-rapeseed following the detached leaf and seedling inoculation test. In the present study, the disease expression on detached leaf was higher among the genotypes compared to the seedling inoculation technique (Figure 5; Table 2). However, there was a correlation was present between the disease expression of detached leaf inoculation and seedling inoculation method (Figure 6). This result indicating that detached leaf inoculation will be more effective and reliable for resistance screening of mustard-rapeseed genotypes.
Evaluation of genotypes for resistance
A total of 27 cultivars of mustard-rapeseed were screened for resistance to A. brassicicola by two different inoculation techniques, of which only one cultivar belong to Brassica juncea species showed borderline resistance and significant differences in resistance were found among these cultivars. (Figure 7, Table 2).
Table 2: Mean disease score of two different screening techniques for Alternaria leaf spot disease caused by Alternaria brassicicola in 27 mustard-rapeseed varieties.
|
Variety name |
Brassica species |
Average Disease Severity |
|
|
Detached leaf (3rd leaf) |
Seedling |
||
| BARI Sharisha-7BARI Sharisha-8BARI Sharisha-13BINA Sharisha-4BINA Sharisha-5GPB-1GPB-2BINA Sharisha-9BINA Sharisha-10 |
Brassica napus
|
8.506. 758.506. 757.505. 007.257. 508.25 |
7.506. 508.006. 507.005. 007.007. 508.00 |
| DaulatRai-5BARI Sharisha-10BARI Sharisha-11BINA Sharisha-8 |
Brassica juncea
|
7.256. 006.255. 754.00 |
6.005. 005.506. 004.00 |
| SampadTori-7Sonali SorishaBARI Sharisha-6BARI Sharisha-9BARI Sharisha-12BARI Sharisha-14BARI Sharisha-15KallyaniaSafalAgraniSAU-1BINA Sharisha-6Edononatsua |
Brassica rapa |
6.757. 007.256. 757.508. 757.505. 257.756. 506.257. 506.252. 00 |
6.506. 757.006. 757.507. 007.006. 007.506. 005.507. 506.002. 00 |
| LSDb 0.48 0.62 | |||
aJapanese Brassica rapa cultivars; bLSD (0.05): Least Significant Difference
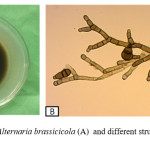 |
Figure 1: Pure culture of Alternaria brassicicola (A) and different structures of Alternaria brassicicola (B) |
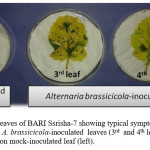 |
Figure 2: Detached leaves of BARI Ssrisha-7 showing typical symptoms of Black leaf spot disease on A. brassicicola-inoculated leaves (3rd and 4th leaves; right) and no Symptoms on mock-inoculated leaf (left) |
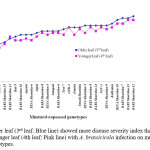 |
Figure 3: Older leaf (3rd leaf: Blue line) showed more disease severity index than the younger leaf (4th leaf: Pink line) with A. brassicicola infection on mustard-rapeseed genotypes. |
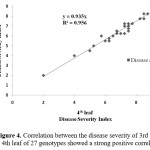 |
Figure 4: Correlation between the disease severity of 3rd leaf and 4th leaf of 27 genotypes showed a strong positive correlation. |
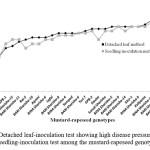 |
Figure 5: Detached leaf-inoculation test showing high disease pressure compared to seedling-inoculation test among the mustard-rapeseed genotypes |
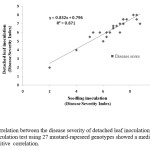 |
Figure 6: Correlation between the disease severity of detached leaf inoculation and seedling inoculation test using 27 mustard-rapeseed genotypes showed a medium strong positive correlation. |
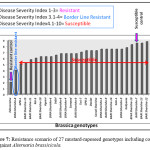 |
Figure 7: Resistance scenario of 27 mustard-rapeseed genotypes including control against Alternaria brassicicola. |
Discussion
Alternaria leaf spot or black leaf spot caused by Alternaria brassicicola is one of the vital diseases of Brassica crops worldwide including Bangladesh. The disease has been an increasing threat to mustard-rapeseed production for the last couple of decades in Bangladesh. Owing to that disease resistance breeding is the promising approach for the durable and effective control of this disease. Moreover effective field control with fungicides is not feasible as significant number of alternate host present during the growing season. No complete resistance is known in the available cultivated varieties against A. brassicicola in Bangladesh. Owing to that, development of inoculation techniques and the screening of mustard-rapeseed germplasm exist in Bangladesh will be a promising approach for the identification of resistant variety against A. brassicicola in Bangladesh.
In the present study, two inoculation techniques such as detached leaf and seedling inoculation techniques have been used for validation of the test using cultivated varieties in the country and to screen for resistance to A. brassicicola for 27 mustard-rapeseed genotypes. The aim of this study was to develop an effctive inoculation technique with A. brassicicola and to identify resistant genotypes in the cultivated mustard-rapeseed. In this experiment, disease expression level developed by artificial inoculation following two methods considering all cultivars was positively correlated (R2 = 0.871; n = 27) meaning that these artificial inoculation techniques are enable to screening various levels of resistance. In addition, incubation environment in the detached leaf inoculation technique do not affect the disease expression. These findings are in conformity with the results of Doullah et al., (2006)3 where they also showed a positive correlation between the disease expression level of detached leaf and seedling inoculation techniques using 14 & 42 Brassica varieties. In this experiment detached leaf inoculation showed clear visible black spot symptoms on inoculated leaf within 36 hours and the results from the detached leaf technique are come reliable under consistent physical environment.Similar type of results reported by Doullah et al., (2006)3 where they mentioned the detached leaf inoculation technique as suitable for screening of mustard-rapeseed genotypes because of having clear symptoms on the leaves within 24 hrs. Doullah and Okazaki (2015)5 used detached leaf and seedling inoculation test for the resistance screening of 281 B. rapa cultivars and detached leaf inoculation showed consistent results in two years compared to the seedling inoculation test. Nowakowska et al., (2016)18 used several parameters like leaf age, leaf position, inocula concentration and incubation temperature under controlled conditions with two methods (detached leaf and seedling inoculation) to screen resistant variety of mustard against Alternaria blight. Their results showed that either of the method can be used for the A. brassicicola resistance breeding considering crucial response to the pathogen. They also mentioned that the detached leaf bio-assays for Alternaria resistance as more effective than the field tests and two interspecific hybrids were identified that might be used in resistance breeding programs. Khan et al., (2012)12 evaluated the relative performance of several artificial inoculation techniques such as foliar spray, soil application, agarose gel method, and seed treatment with A. brassicae and A. brassicicola for screening of resistance genotypes of Indian mustard. In their study they evaluated that the foliar spray was the most promising and effective method of inoculation to developed distinct and severe disease symptoms on host plant. Doullah et al., (2009)4 used spray method for the artificial inoculation of B. rapa leaves and pods for resistance screening against A. brassicicola which was more effective method and easy to assess the results. Giri et al., (2013)7 described five artificial inoculation techniques on detached leaf viz. spraying, wounding, infiltration, spore suspension drop and spore suspension drop along with agarose for the establishment of disease symptoms on Indian mustard (Brassica juncea) plants by Alternaria brassicae and they suggested that spore suspension drop along with agarose inoculation method was ideal as this ensured the inoculum on the target site. However, the detached leaf inoculation technique showed clear symptoms rapidly and easy to use for disease resistance screening on a large scale maintaining standard physical condition within shortage period of time. Therefore, the detached leaf inoculation method is recommended for screening test to minimize the number of mustard-rapeseed genotypes required for the field test.
In this experiment, the older leaves (3rd leaves) of one month-old susceptible mustard-rapeseed plants were optimal for inoculation and showed severe diseased symptoms than the younger leaves (4th leaves). In most cases older leaves were more susceptible for infection compared to younger leaves due to the opportunistic parasitic behavior of the pathogen A. brassicicola, which requires comparatively a weakened host or plant tissue for infection. This finding also corroborates the findings of earlier researchers. Doullah et al., (2006)3 described that 3rd or 4th true leaves from one month-old plants are most favorable for inoculation and 3rd leaf showed more disease severity compared to the 4th leaf after 24 hrs of inoculation with A. brassicicola. Mridha and Wheeler (1993)17 showed that more A. brassicae infections happened in older B. napus plants. King (1994)13 found that both the youngest and oldest plants of B. oleracea resistant genotypes were less susceptible to A. brassicicola.
Among the 27 Bangladeshi mustard-rapeseed cultivars, all most all the cultivars expressed susceptible reaction to A. brassicicola except one cultivar, BINA Sharisha-8, as having border line resistance in this study. Doullah and Okazaki (2015)5 found that in a resistance screening of 281 genotypes of mustard-rapeseed in two different years by detached leaf and seeding inoculation techniques, two cultivars ‘Edononatsu’ and ‘Saroi’ showed high levels of resistance in both the years following the mentioned inoculation techniques, whereas some genotypes exhibited borderline resistance and most of them were susceptible to the black spot disease. In a resistance screening using 52 Brassica rapa genotypes, the detached leaf inoculation method effectively distinguished between different levels of resistance amongst genotypes and screened two B. rapa cultivars namely Saroi and Edononatsu as highly resistance and five cultivars have borderline resistance to A. brassicicola among the genotype used (Doullah et al., 2006)3. Humauan et al. (2013)8 investigated among 31 varieties of mustard-rapeseed and found 1 Resistant, 12 moderately resistant, 17 moderately susceptible and rest 1 showed susceptible against Alternaria leaf spot disease.
Conclusion
Alternaria leaf spot or black leaf spot caused by Alternaria brassicicola is the most important diseases of mustard-rapeseed in Bangladesh, and causing enormous yield loss every year. But the chemical fungicide is still the sole management practice at the farmers’ level as none of the high yielding variety is completely resistant against this pathogen. Owing to that a total of 27 mustard-rapeseed cultivars were selected for resistance screening test. Two inoculation techniques viz. detached leaf and seedling inoculation were evaluated in this resistance screening program. A significant positive correlation was found between the results from the seedling inoculation and the detached leaf inoculation method. Moreover, the detached leaf inoculation techniques was the most suitable for disease resistance screening of mustard-rapeseed cultivars as it was very easy to screen plants for resistance on a large scale within shortage period of time and to maintain standard physical environment. The older leaves (3rd leaf) of 30 day-old plants were showed severe disease symptoms than the younger leaves (4th leaf) of mustard-rapeseed. All most all the mustard-rapeseed cultivars used in this study expressed susceptible reaction to A. brassicicola except BINA Sharisha-8, as it showed border line resistance.
Based on the results of the this study it may be summarized that for resistance breeding program for this disease, there is no potential resistant cultivar exist in Bangladesh and consequently it will be necessary to develop resistant or tolerant cultivar against A. brassicicola through traditional as well as molecular breeding programme by using our high yielding varieties and wild races or resistant genotypes collected from international oilseed research organizations. However, this resistance screening experiment also needs to perform under field conditions or in nursery beds where large numbers of seedlings of mustard-rapeseed genotypes can be accommodate at a time.
Disclosure Statement
No potential conflict of interest was reported by the authors.
Acknowledgements
This work was supported by Bangladesh Agricultural University Research System (BAURES) [Project No. 2015/121/BAU] to Dr. Muhammed Ali Hossain, Department of Plant Pathology, BAU. The authors would like to acknowledge to Prof. Dr. Keiichi Okazaki (Plant Breeding lab, Niigata University, Japan), Bangladesh Agricultural Reasearch Institute (BARI), Bangladesh Institute of Nuclear Agriculture (BINA) and Department of Genetics & Plant Breeding, Bangladesh Agricultural University, Mymensingh for providing the seeds of mustard-rapeseed genotypes.
Funding
This work was funded by Bangladesh Agricultural University Research System (BAURES) [Project No. 2015/121/BAU].
References
- Ahmed, H.V., 1992. Diseases of oil seed crops in Bangladesh. Bangladesh Phytopathological Society. Gazipur.
- Ahmed, M.U., Ahmed, H.U., 1994. Disease Management, Recommendation and Future Plan of Oilseeds Crop in Bangladesh. In Proc. workshop on transfer of technology of CDP crops under Research-Extension Linkage program. pp. 46-56.
- Doullah, M.A.H., Meah, M.B., Okazaki, K., 2006. Development of an effective screening method for partial resistance to Alternaria brassicicola (dark leaf spot) in Brassica rapa. Eur J Plant Pathol. 116: 33-43.
CrossRef - Doullah, M.A.U., Meah, M.B., Mohsin, G.M., Hassan A., Okazaki, K. 2009. Evaluation of resistance in Brassica rapa to dark pod spot (Alternaria brassicicola) using the in vitro detached pod assay. SABRAO J Breed Genet. 41(2): 101-113.
- Doullah, M.A.U., Okazaki, K., 2015. Evaluation of Resistance to Dark Leaf Spot (Alternaria brassicicola) in Brassicae rapa. Int J Expt Agric. 5(2): 5-14.
- Ghose, K., Dey, S., Barton, H., Loake, G.J., Basu, D. 2008. Differential profiling of selected defense-related genes induced on challenge with Alternaria brassicicola in resistant white mustard and their comparative expression pattern in susceptible India Mustard. Mol. Plant Pathol. 9(6):763-775.
CrossRef - Giri, P., Taj, G., Kumar, A. 2013. Comparison of artificial inoculation methods for studying pathogenesis of Alternaria brassicae (Berk.)Sacc on Brassica juncea(L.) Czern. (Indian mustard). Afr J Biotechnol. 12(18): 2422-2426.
- Humauan, M.R., Khalequzzaman, K.M., Akhter, B. 2013. Screening Of Rapeseed-Mustard Varieties/Lines Against Alternaria Leaf Blight Disease In Ishurdi. Annual Report of Regional Agricultural Research Station, Ishurdi, Pabna.
- ISTA 1996: International Rules for Seed Testing. Seed Science and Technology 4: 3-49.
- Khan, M.R., Khan, M.M., Mohiddin, A.F. 2010. Evaluation of some indigenous germplasm of black mustard against Alternaria brassicicola under artificial inoculation. Indian Phytopath. 63: 51-54.
CrossRef - Khan, M.M. 2011. Alternaria Blight of Mustard A Real Farmer Headache. Lap Lambert Academic Publishing.Germany.pp.100.
- Khan, M.M., Khan, M.R., Mohiddin, A.F. 2012. The relative performance of different Inoculation methods with Alternaria brassicae and A. brassicicola on Indian mustard. Plant Pathol J. 11(3): 93-98.
- King, S.R. 1994: Screening, selection and genetics of resistance to Alternaria diseases in Brassica oleracea.Ph.D.thesis, Cornell University, Ithaca, New York.
- Kubota, M., Abiko, K., Nishi, K. 2003: Effect of cultivation conditions of cabbage plugs on sooty spot disease. Bulletin of the National Institute of Vegetable and Tea Science. Japan. 2: 1-8.
- Meah, M.B., Hossain, I. 1988. Screening of germplasm of oilseeds against some diseases and their chemical control. Proc. BAU Res. Sys. Workshop. pp: 52-59.
- Meah, M.B., Hau, B., Siddique, M.K. 2002. Relationships between disease parameters of Alternaria blight (Alternaria brassicae) and yield of mustard. J Plant Dis Prot. 3: 243- 251.
- Mridha, M.A.U., Wheeler, B.E.J. 1993. In vitro effects of temperature and wet periods on infection of oilseed rape by Alternaria brassicae. Plant Pathol. 42: 671–675.
CrossRef - Nowakowska, M., Wrezesinska, M., Kaminski, P., Nowicki, M., Lichocka, M., Tartanus, M., Kozik, E.U. 2016. Screening for Alternaria brassicicola resistance in the Brassiceae: Bio-assay optimization and confocal microscopy insights into the infection process. PeerJPrePrints (http://doi.org/10.7287/peerj.prints.1360v2/).
- Obermeier, C., Hossain, M.A., Snowdon, R.J., Knufer, J., von, Tiedemann, A., Friedt, W. 2013. Genetic analysis of phenyl propanoid metabolites associated with resistance against longisporum in Brassica napus. Mol Breed. 31:347-361.
CrossRef - Snowdon, R.J., Luhs, W., Friedt, W. 2006. Oilseed rape. In: Kole C. (ed) Genome Mapping and Molecular Breeding. Vol. oilseeds. Springer Verlag, Heidelberg, New York/ Berlin/ Tokyo, pp.55-114.
- Vishwanath, Kolte, S.J. 1997. Variability in Alternaria brassicae: Response to host genotypes, toxin production and fungicides. Indian Phytopathol. 50: 373-381.


