Introduction
Egg parasitoids of the genus Trichogramma sp. are important natural enemies that have been successfully used in biological control programs for the management of Lepidopteran pests. These pests infest corn, rice, sugarcane, cotton, vegetables, sugar beets and fruit trees.22,16 Approximately 210 species of Trichogramma have been identified, among which 30 are documented in India. However, only 12 have been utilized for pest management practices.22 Among the Trichogramma species, T. chilonis is the widely used species in several countries and agro ecosystem, where Lepidopteran pests occur naturally. These parasitoids attack the egg stage of their hosts, preventing them from reaching the larval stage, responsible for crop damage.6 As a result, they have proved to be promising candidate in integrated pest management (IPM) approaches worldwide. However, a huge impediment to effective integrated pest management practices is the frequent incompatibility of biological and chemical control measures. Although some classes of pesticide are generally designed to be selectively harmful against one or a few target pest populations, others with broad activity spectra negatively influence other pests and beneficial species, including egg parasitoids. This interference of biological control agents by indiscriminate use of insecticides causes resurgence of more resistant target pests and outbreaks of secondary pests. Intensive toxicological studies have showed undesirable effects of pesticide applications on Trichogrammatids such as reduced parasitism in T. exigum Pinto and T. pretiosum Riley on Heliothis zea Boddie and Manduca sp. in pyrethroid treated fields.5 Intoxication from insecticide application has also resulted in reduced emergence and longevity of the emerged Trichogrammatids.15,11,27,9,26 Development of insecticide resistant natural enemies, which could be introduced in IPM practices, was attempted.7 The resistant parasitoid released into the field would mate with susceptible parasitoid, and, over subsequent generations resulting to a larger proportion of resistant hybrid that could assist in the release and management strategies of the resistant strain in the field. Subsequently, natural enemies with a proven potential to resist insecticides tend to hinder the manifestation of resistance in pest population, over time.12 By genetic enhancement, about 15 species of parasitoids and predators of insects and mites that are resistant to insecticide applications in the field have been established, with the most classical case being the strains of predatory phytoseiid mites.3,8 Employing laboratory selection, azinphosmethyl resistance levels was increased 7.5 fold in the aphid parasitoid, Trioxys pallidus Haliday12 and endosulfan resistance was 15.1 fold in the T. chilonis.14 Some natural enemy populations have demonstrated appreciable levels of resistance to insecticides in the field and can survive field at application rates.20,2 Since resistance is as a result of altered genomic changes in natural populations, a full discernment of the evolution of this phenomenon requires an accurate knowledge of its genetic basis21 thus leading to a better estimation of resistance risk in populations. In this study, selection responses of the adult stage of the parasitoid T. chilonis Ishii to monocrotophos, using Corcyra cephalonica, a major laboratory host, was investigated. The aim this study was to assess the level of tolerance of T. chilonis for insecticide application and determine the inheritance of monocrotophos resistance to understand the potential use of T. chilonis as a bio-control agent.
Materials and Methods
Rearing of insect
A parasitized card (2×2 cm) of resistant “R” and susceptible “S” eggs of T. chilonis was obtained from the National Bureau of Agricultural Insect Resources (NBAIR), Bangalore. The “S” culture parasitoid was maintained in the laboratory for the past five years without exposure to insecticide and the “R” culture parasitoid was collected from the field and selected with (monocrotophos) pesticide in the laboratory for 10 generations. After emergence, the parasitoids were reared and maintained in the laboratory on Corcyra cephalonica (Staiton) eggs (killed by ultraviolet radiation) at 26±1oC, RH 60± 5%, LD 14: 10.
Insecticide
Monocrotophos (Milphos 36% EC, Excel Industries, India), an organophosphate insecticide, with molecular formula C7H14NO5P, and international Union for Pure and Applied Chemistry (IUPAC) name dimethyl (E)-1-methyl-2-(methylcarbamoyl) vinylphosphate, was used for determining the lethal concentration values (LC50) at varying concentrations of insecticide at five serial dilutions (1/2 dilution). The control treatments were done using water.
Ensuring homozygosity of parental strains
Interbreeding and selection was done to minimize heterozygosity at all resistance loci, before genetic analysis studies were undertaken. To facilitate crosses and selection, the parasitized eggs of C. cephalonica ‘S’-strain and ‘R’-strain were allowed to mate freely after emergence of the adult stage. All parasitized eggs were gently dislodged from the card and each egg was confined in a glass vial (one egg per vial) for emergence. Following emergence, the adults were mass produced by providing them with C. cephalonica eggs. This was done in order to raise enough progeny for treatment with monocrotophos. The insecticide solution was prepared, reducing by ½ starting from the dosage of 1.4mL, in five serial dilutions . Monocrotophos was uniformly applied on the inner sides of the cylindrical glass tube (20 × 4 cm) using an atomizer. The tubes were then kept to dry in a shade after which, one end of the dried glass tube was tightly closed with double-layered long black cloth. About 200 adults were released in each glass tube. The mortality was noted after 6 and 24 hours of constant exposure. The resistant factor was determined by dividing LC50 value of the resistant strain by that of susceptible strain.
Genetic analysis of resistant strain
T. chilonis adults were sorted out based on their sexes. Males and females of both “R” and “S” strains were allowed to cross freely in order to produce hybrid females.The resultant females were provided with C. cephalonica eggs to obtain sufficient progeny to be subjected to monocrotophos. The F1 inter-strain (R



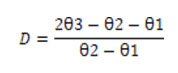
out LC50 values.To determine the degree of dominance (D) of the resistant factor, the formula of [23] was employed.
In which, log10 (LC50) of the susceptible strain, θ2= log 10 (LC50) of the resistant strain and = log10 (LC50) of the heterozygous cross 1 (R

Table 1: F1 crosses and backcrosses of various Trichogramma chilonis population.
| Strain | Monocrotophos |
| F1 reciprocal crosses | Cross 1 – R × S × S Cross 2 – R Cross 2 – R × S × S |
| Backcrosses | F1 cross 1 × RP × RP  F1 cross 2 F1 cross 2 × RP × RP F1 cross 1 F1 cross 1 × SP × SP F1 cross 2 F1 cross 2 × SP × SP F1 cross 1 F1 cross 1 × SP × SP F1 cross 2 F1 cross 2 × SP × SP F1 cross 1 F1 cross 1 × RP × RP F1 cross 2 F1 cross 2 × RP × RP |
Mode of inheritance of resistance (Backcrosses)
“R” and “S” parental strains were backcrossed with the F1 hybrid to determine mode of inheritance as depicted in Table 1. C. cephalonica eggs were provided to the crosses for multiplication to get sufficient progeny, which upon emergence, were exposed to monocrotophos at five serial dilutions (1/2 dilution) from 1.4mL to work out LC50 values. The mortality was recorded after 6 and 24 hours of constant exposure.
Statistical analyses
All experiments including the LC50values of the tolerant and susceptible strains, and genetic level of tolerance were repeated three times and performed in triplicate. The data obtained on mortality were subjected to probit analysis, employing a statistical program SPSS version 15. The data were transformed to log base 10 before probit analysis and antilog of calculated values gave actual LC50 and LC90. The fiducial limits, slope, chi-square (χ2) and regression equation were also computed.
Results
Following repeated laboratory selection episodes of T. chilonis with monocrotophos, inheritance of insecticide resistance was studied in the bioassay experiment with sequential decrease in dose of insecticide (1.4mL to 0.0875mL). The LC50 value for resistant strain was 0.346 mL, while 0.114 mL was reported for the susceptible strain and the reciprocal F1 crosses (cross 1 R





Table 2: Toxicity of monocrotophosto the susceptible, resistant and F1 reciprocal hybrid of T. chilonis.
| Strain LC50 95%CL Slope ± SE df χ2 Regression(μL) Upper Lower |
| “S” 0.114 0.095 0.132 3.400±0.246 13 40.23 y = -3.203+ 3.400x |
| “R” 0.346 0.218 0.545 2.189±0.106 13 247.98 y = -1.009+ 2.189x |
| F1 0.419 0.357 0.491 2.944±0.107 13 84.986 y = -1.111+ 2.944x |
The monocrotophos resistance appeared to be complete dominant with degree of dominance (D) value of 1.629. This was verified with the results from LC50 values of F-1 reciprocal crosses, which were comparatively greater than the LC50 values of resistant and susceptible parents. Thus, it could be inferred from non-overlap fiducial limit test that there was a notable difference between susceptible and resistant and between susceptible and F-1 crosses. The resistance factor (Rf) of resistant strain and F 1 crosses was represented by 3.04 and 3.675 folds, respectively, over susceptible strain. Genetic analyses of resistant strain which involved backcrossing the F1 hybrids from cross 1 and cross 2 to their homozygous parents yielded a progeny, whose LC50 values indicated increase in percentage of tolerance with increased adult survival than individual resistant strain. This was confirmed from the LC50values of backcrosses, which were greater than the LC50 values of resistant, susceptible and reciprocal F1 progeny as depicted in (Table 3 and Table 4).
Table 3: Toxicity of monocrotophos to the resistant strain with backcrosses of T. chilonis.
| Strain LC50 95% CL Slope ± SE df χ2 Regression(μL) Upper Lower |
Cross 1F1 ×RP ×RP 0.785 0.624 1.037 2.734±0.134 13 114.89 y = – 0.288+2.734xF1 0.785 0.624 1.037 2.734±0.134 13 114.89 y = – 0.288+2.734xF1 ×RP ×RP 0.684 0.593 0.803 2.297±0.018 13 39.289 y = – 0.379+2.297x 0.684 0.593 0.803 2.297±0.018 13 39.289 y = – 0.379+2.297x |
Cross 2F1 ×RP ×RP 0.920 0.765 1.161 1.749±0.098 13 34.723 y = – 0.063+1.749xF1 0.920 0.765 1.161 1.749±0.098 13 34.723 y = – 0.063+1.749xF1 ×RP ×RP 0.491 0.397 0.619 1.869±0.090 13 70.677 y = – 0.577+1.869x 0.491 0.397 0.619 1.869±0.090 13 70.677 y = – 0.577+1.869x |
Table 4: Toxicity of monocrotophos to the susceptible strain with backcrosses of T. chilonis
| Strain LC50 95% CL Slope ± SE df χ2 Regression(μL) Upper Lower |
Cross 1F1 ×SP ×SP 0.533 0.382 0.773 1.339±0.075 13 115.57 y = – 0.336+1.339x F1 0.533 0.382 0.773 1.339±0.075 13 115.57 y = – 0.336+1.339x F1 ×SP ×SP 0.616 0.488 0.806 2.295±0.100 13 118.012 y = – 0.483+2.295x 0.616 0.488 0.806 2.295±0.100 13 118.012 y = – 0.483+2.295x |
Cross 2F1 ×SP ×SP 0.711 0.576 0.915 1.944±0.088 13 88.328 y = – 0.288+1.944x F1 0.711 0.576 0.915 1.944±0.088 13 88.328 y = – 0.288+1.944x F1 × SP × SP 0.522 0.418 0.672 2.211±0.108 13 74.729 y = – 0.624+2.211x 0.522 0.418 0.672 2.211±0.108 13 74.729 y = – 0.624+2.211x |
Discussion
The selection of various beneficial insects for resistance to insecticides has been undertaken over the past years, with mixed results. In the resistant strain of T. japonicum, another species of Trichogramma, there was a remarkable increase in the LC50 values in response to methamidophos (0.8892 ppm), fenvalerate (8.6511 ppm), and metaphos (0.0592 ppm) insecticides. However, treatment with mipcrin, showed a significant decrease in LC50 (0.1103 ppm) when treated for 36-43 generations.29 A 15 -fold increase in T. chilonis tolerance to endosulfan after 341 laboratory selection episodes with LC50 values for resistant, susceptible and F1 being 1074.96, 70.91 and 604.96, respectively, was reported.14 In this investigation, T. chilonis tolerance to monocrotophos was found to have increased up to 3.04-folds after 10 cycles of selection with insecticide in the laboratory. Repeated laboratory selection of the parasitoid to the insecticide would have contributed to the development of resistance in some parasitoid, which survived due to their distinct genetic makeup. The offspring of these survivors carried the resistance genome, and increased in greater proportion with each subsequent generation of the population. In Amblyseius nicholsi Ehara, resistance to phosmet was as a result of a single semi-dominant gene as reported in.13 In a generalist pteromalid parasitoid, Anisopteromalus calandrae, malathion resistance was inherited as incompletely dominant trait controlled by a single gene.1 Genetic analyses of backcrosses of endosulfan resistant strains of T. chilonis indicated the role of a single semi-dominant gene in inheritance of resistance.14 In this study involving same species, mode of monocrotophos inheritance was complete dominance, which indicated that resistance varied with the resistant colony to particular insecticides. The other mode of inheritance, polygenic, was observed in the laboratory selected resistance to azinophosmethyl in the parasitoid Trioxys pallidus Haliday4 and predatory mite Phytoseiulus persimilis Athias-Heuriot.10 It has been widely suggested that laboratory selection programme for insecticide resistance will likely result in a polygenic pattern of inheritance due to small incremental increase in pesticide resistance in the selection process over time.21 However, in the present study, the likelihood of a single gene involvement in the determination of resistance was not discounted. The selection coverage of susceptible population has been identified to determine the choice of a specific mode of resistance, either monogenic or polygenic. While selection within this population inclines towards polygenic resistance, selection outside the population, on the other hand, results in monogenic response that is characterized by single gene mutations.21 Single gene controlled resistance develops and spreads rapidly when compared to polygenic resistance.25,21 Over time, resistance quickly manifests to new areas through migration of the resistant insect as demonstrated in a study of the organophosphate resistance genes of C. pipiens.19,24 This observed dominance in resistance to monocrotophos in the selected strain of T. chilonis will help in the establishment and stability of the selected strain in the field, where insecticide with the same mode of action is frequently sprayed to control pests. Thus, this strain can be profitably incorporated into IPM strategies as effective and complement to chemical insecticide. Although conversely, it is important to point out that this study was carried out under laboratory conditions where the parasitoid was subjected to a constant pesticide pressure, under field conditions pesticides might have their negative impact lightened because the biological control agents can benefit from natural shelters or avoid sprayed areas. Furthermore, sunlight degradation plays an important role in the field that also helps to decrease the impact of pesticides on the beneficial arthropods observed in laboratory.8
Conclusion
Inheritance of monocrotophos resistance in the laboratory selected strain of T. chilonis was studied. While the resistant strain showed an LC50 value of 0.346 mL, the susceptible strain displayed an LC50 of 0.114 mL. Based on the genetic analysis, it was evident that the F1 cross exhibited a complete dominance response to monocrotophos, with degree of dominance (D) of 1.629. The resistant factor for resistant and F1 strains were 3.04 and 3.675 folds over susceptible strain, respectively.These results assert that the resistant strain can be compatible in IPM practices in various crops where insecticide use is higher.
Conflict of interest
No competing interests.
Acknowledgements
This research was supported by grant from the University Grants Commission (UGC), New Delhi, India (Grant No: MRP – Major- BIOC-2013-37866, Government of India).
References
- Baker J. A, Perez-Mendoza J and Beeman R. W. Inheritance of malathion resistance in the parasitoid Aninosopteramalus calandrae (Hymenoptera: Ptetomalidae). J Econ Entomol. 1997;902)304-308.
CrossRef - Baker J. E and Weaver D. K. Resistance in field strains of the parasitoid Anisopteromalus calandrae (Hymenoptera: Pteromalidae) and its host, Sitophilus oryzae (Coleoptera: Curculionidae), to malathion, chlorpyrifos-methyl, and pirimiphos-methyl. Biol. Control. 1993;33):233–242.
CrossRef - Beckendorf S. K and Hoy M. A. Genetic improvement of arthropod natural enemies through natural hybridization or genetic engineering techniques, In Hoy, M. A and Herzog, D. C (Eds) Biocontrol in Agricultural IPM systems, Academic, Orlando. 1985;167-187.
- Brown E. J, Cave F. E and Hoy M. A. Mode of inheritance of azinphosmethyl resistance in a laboratory-selected strain of Trioxys pallidus. Entomologia Experimentalis et Applicata. 1992;63:229-2361.
CrossRef - Campbell C. D, Walgenbach J. F and Kennedy G. G. Effects of parasitoids on lepidopterous pests in insect-treated and untreated tomatoes in Western North Carolina. J Econ Entomol. 1991;84:1662.
CrossRef - Carvalho G. A, Reis P. R, Moraes J. C, Fuini L. C, Rocha L. C. D and Goussain M. M. Effect of pesticides used on tomato crop (Lycopersicon sculentum Mill.) on Trichogramma pretiosum Riley (Hymenoptera: Trichogrammatidae). Science and Agrotechnology. 2002;26:160–166.
- Croft B. A. Arthropod biocontrol agents and pesticides. John Wiley and Sons Inc. New York NY, USA. 1990;460
- Denholm I, Devine G. J and Williamson M. S., “Insecticide resistance on the move” Science. 2002;2975590):2222-2223.
CrossRef - Desneux N, Decourtye A and Delpuech J. M. The sublethal effects of pesticides on beneficial arthropods. Annu Rev Entomol. 2007;2:81-106.
CrossRef - Fournier D, Pralovorio M, Trottin-Caudal Y, Coulon J, Malezieux S and Berge J. B. Artificial Selection for resistance to Methidathion in Phytoseiulus persimilis A.H. BioControl. 1987;322)209-219.
- Geraldo A. C, Paulo R. R, Luiz C.D.R, Jair C. M, Loriney C. F and Carvalho C. E. Side-effects of insecticides used in tomato fields on Trichogramma pretiosum (Hymenoptera, Trichogrammatidae). Acta Scientiarum. Agronomy Maringá. 2003;25:275-279.
- Hoy M. A and Cave F. E. Toxicity of pesticides used on walnuts to a wild and azinphosmethyl-resistant strain of Trioxys pallidus (Hymenoptera: Aphidiidae). J Econ Entomol. 1989;82:1585–1592.
CrossRef - Huang M. D, Xiong J. J and Du T.Y. The selection for and genetical analysis of phosmet resistance Amblyseius nicholsi. Acta Entomologica Sinica. 1987;30:133.
- Jalali S. K, Singh S. P, Venkatesan T, Murthy K. S and Lalitha Y. Development of endosulfan tolerant strain of an egg parasitoid Trichogramma chilonis Ishii (Hymenoptera:Trichogrammatidae). Project Directorate of Biological Control, Bangalore. Indian J. Exp. Biol. 2006;44:584-590.
- Kawamura S, Takada Y and Tanaka T. “Effects of various insecticides on the development of the egg parasitoid Trichogramma dendrolimi (Hymenoptera: Trichogrammatidae)”. J Econ Entomol. 2001;94:1340–1343.
CrossRef - Li, L. Y. “Worldwide use of Trichogramma for biological control on different crops: a survey”. In: Wajnberg, E.; Hassan, S.A. (eds) Biological control with egg parasitoids. CAB International, Oxon, UK. 1994;37–53.
- Mona A. S. Effect of some insecticides on the immature stages of the egg parasitoid Trichogramma evanescens West. (Hym. Trichogrammatidae). Egypt. Acad. J. Biol. Sci. 2010;31):31-38.
- Oppenoorth F. J. Biochemistry of insecticide resistance. Pestic Biochem Physiol. 1984;22:187–193.
CrossRef - Polson K. A, Rawlins S. C, Brogdon W. G and Chadee D. D “Organophosphate resistance in Trinidad and Tobago strains of Aedes aegypti” Journal of American Mosquito Control Association. 2010;64)403-410.
CrossRef - Rathman R. J, Johnson M. W, Roseheim J. A and Tabashnik B. E. Carbamate and pyrethroid resistance in the leaf miner parasitoid Diglyphus begini (Hymenoptera: Eulophidae). J Econ Entomol. 1990;836)2153–2158.
CrossRef - Roush R. T and McKenzie J. A. “Ecological genetics of insecticide and acaricide resistance” Annu. Rev. Entomol. 1987;32:361-380.
CrossRef - Smith S. M. “Biological control with Trichogramma: advances, successes, and potential of their use”. Annu Rev Entomol. 1996;41:375–406.
CrossRef - Stone B. F. A formula for determining degree of dominance in cases of monofactorial inheritance of resistance to chemicals. Bulletin World Health Organization. 1968;38:325-326.
- Tabashnik B. E and Johnson M. W. Evolution of pesticide resistance in natural enemies. In (Bellows TS & TW Fisher TW (eds): Handbook of biological control: Principles and applications of biological control Academic Press, New York. 1999;673–689.
- Tabashnik B. E. “Computer stimulation as a tool for pesticide resistance management,” in Pesticide Resistance: Strategies and Tactics for Pesticide Resistance Management, National Academy Press, Washington, DC, USA. 1986;195–203.
CrossRef - Vianna U.R, Pratissoli D, Zanuncio J.C, Lima E.R, Brunner J, Pereira F.F and Serrão J E. Insecticide toxicity to Trichogramma pretiosum (Hymenoptera: Trichogrammatidae) females and effect on descendant generation. Ecotoxicology. 2009;18:180-186.
CrossRef - Vogt H and Brown K. (eds). Working Group “Pesticides and Beneficial Organisms.” In: Proceedings of a meeting at Debe, Poland, September 27-30, Bulletin OILB/SROP. 2006;29-100;120.
- Whitten M. J and Hoy M. A. Genetic improvement and other genetic considerations for improving the efficacy and success rate of biological control. In: TS Bellows & TW Fisher (Eds) Handbook of biological control: Principles and applications of biological control (eds), Academic Press, New York. 1999;271–296.
CrossRef - Xing H, Huang L. K, Fen L. Y, Zhi M. Q, Ying L. L and Chu Z. L. Preliminary study on the selection for insecticide resistant strain of Trichogramma japonicum Ashmed. Trichogramma and other egg parasitoids. II International Symposium, Guangzhou (China). Nov. 10-15. Ed Institut National de la Recherche Agronomique, Paris 1988. (Les Colloques de I’INRA. 1986;43:411.

