Introduction
Phosphorus (P) is a primary key nutrient for plant growth.1 Although phosphorus is abundant in various soil most of the P is not usually readily available to plants.2 The reason for the decrease in P availability is the formation of less soluble Fe, Al or Ca phosphates due to contact with phosphate anions in soil.3-4 Hence, the regular appliance of substantial amounts of chemical fertilizers which contain soluble P is required to attain greatest plant efficiency.5 However, manufacture of conventional P fertilizer contain chemical handing out of insoluble high-grade mineral phosphate ore, which is based upon an energy-intensive treatment with sulfuric acid at elevated temperature, which is an expensive process and have potential to cause environmental damage.6-7 Chemical fertilizer has some harmful impacts on the physicochemical and biological properties of soil which includes its structure, composition, and microflora.8-9 There is an interesting, easy and quick way is the direct application of low-grade rock phosphate (RP) to assess P availability to the soil leads to minimize pollution and decrease the expenses of chemical treatment. Phosphate rocks (PR) available naturally have been accepted as a valuable option for P fertilizers.10 It is estimated that in India, there are nearly 260 million tons of phosphate rock deposits are present and this stuff will afford a cheap source of phosphate fertilizer for crop production.11 As reported by IFDC and Sanchez et al. in recent years, attention has been given to PR direct use.12 The other element present in PR, such as Ca, is an advantage to improve soil physical and chemical properties and to contribute to plant nutrition.13 The present experiment, therefore, aimed towards the evaluation of effects of low-grade phosphate rock on the growth of maize plants grown in the pot.
Materials and Methods
Lowgrade phosphate rock was collected from Hindustan Zinc Ltd., Udaipur. The phosphates rock was of the sedimentary source and is called as low-grade because of its low P content. The soil used for the pot experiments was red lateritic, which was collected from the premises of CSIR-IMMT; Bhubaneswar, India at a distance downward of 0–30cm. Seeds of Maize (Zea mays L.) were obtained from the National Seed Corporation, Bhubaneswar.
Physicochemical study
Before analysis, the samples were air-dried and sieved through a 2 mm screen. The pH (1:2w/v) and Electrical conductivity, EC (1:2w/v) of the samples were calculated according to McLean14 and Rhoades.15 Bulk density was determined as described by Blake and Hartge.16 Water holding capacity of samples was measured using the standard protocol by Panda et al.17 Wakley and Black method[18] determined organic carbon. The digestion of samples was carried out in H2SO4 for determination of total nitrogen by using the Micro Kjeldahl method, and for P and K the ternary solution (HNO3: H2SO4: HClO4 = 10:1:4 with volume) was used. Available nitrogen (N), phosphorus (P) and potash (K) were analyzed according to the standard protocol.19-20
For assessment of heavy metals, 1 g of the sample was digested through 1:4 (nitric: perchloric acid) for about 24 h. The samples were remained there to digest till a clear solution obtained. When the sample got fully digested it was filtered and an additional distilled water was added to get the required dilution. These filtered samples were used directly to determine heavy metal concentration by AAS.21
Experimental set-up
The experiment was done in Red plastic pots having 20 cm height, and 25 cm in diameter. A random block design with three replicates was used in each trial with 2, 4, 6, 8 and 10g of PR per kg of soil. Control was also maintained with soil only (Table 1). Before sowing, maize seeds were surface sterilized using 98% ethanol, 2% sodium hypochlorite followed by washes in sterile distilled water. The pot was watered sufficiently and regularly with stored rainwater throughout the experimental period. The pots were kept at 250-300C in natural photoperiod for 11-12 hr.
Table 1: Details of Growth Substrate (S = Soil, Pr = Phosphate Rock).
| Treatment | Mixed by w/w ratio |
| T0 | S (1kg) |
| T1 | S (1kg)+PR(2g) |
| T2 | S (1kg)+PR(4g) |
| T3 | S (1kg)+PR(6g) |
| T4 | S (1kg)+PR(8g) |
| T5 | S (1kg)+PR(10g) |
Measurements
The measurement of the percentage of seed germinate have monitored at the commencement of the assays. Arbitrary samples of the plants were taken after harvest (51 days after sowing) from each replicate. Shoots and roots were carefully separated for different growth and biochemical parameters such as root length, shoot length, fresh weight and following this the plant parts (roots and shoots) were set aside in an oven running at 600 for 24 hr, and the dry weights were recorded in grams. For leaf area measurement completely prolonged clean leaves of plants were sampled randomly from each replicate. After extraction with methanol in an opaque container for 24 h the photosynthetic pigments were determined. For pure methanol, the concentrations of chlorophyll-a and chlorophyll-b were calculated using following equations given.22
[Chlorophyll-a]= (16.75 × A665.2) − (9.16 × A652.4)
[Chlorophyll-b]= (34.09 × A652.4) − (15.28 × A665.2)
The pheophytin and carotenoid content were quantified spectrophotometrically following the standard protocol.23 Using bovine serum albumin as standard the foliar protein content was analyzed by following standard protocol.24
The original mineral concentrates of phosphate rock
The EDXRF (Energy Dispersive X-ray Fluorescence) technique using a Philips PW 1480 X-ray spectrometer has used to know the chemical composition of the original concentrates relating to major elements. The results from the EDXRF analysis are shown in Table 2.
Table 2: Mineralogical And Chemical Composition Of The Original Phosphate Rock (Edxrf) (N = 3, Mean±Se)
| Components | Value |
| Al (%) | 2.83±0.02 |
| Si (%) | 16.65±0.06 |
| S (%) | 1.56±0.04 |
| K (%) | 0.15±0.02 |
| Ca (%) | 29.89±1.27 |
| Ti (%) | 0.22±0.002 |
| Fe (%) | 3.32±0.0001 |
| V (ppm) | 11.2±2.7 |
| Mn (ppm) | 40.6±0.4 |
| Cu (ppm) | 47.0±2.7 |
| Zn (ppm) | 220.5±1.3 |
| As (ppm) | 6.1±0.003 |
| Sr (ppm) | 695.0±12.9 |
| Ba (ppm) | 100.9±4.3 |
| La (ppm) | 23.7±0.3 |
Statistical Analysis
The experiments were carried in triplicates for each treatment, and the data shown in the tables and figures are mean ± SEM (Standard Error of Mean) of three replicates.
Results and Discussion
Soil and phosphate rock characteristics
Main chemical properties of the sample were given in table 3. It was observed that the soil is acidic, low in fertility than the PR and electrical conductivity in samples was 0.11 and 0.99 dS m-1 correspondingly which is a evaluate of soluble salt present and shows the amount of macro-and micronutrients. The organic carbon was found to be a higher value (0.09%) in comparison to PR (0.001%) whereas available nitrogen content was extremely low (0.002ppm) in PR than soil. Phosphate rock has significantly higher content of total and available P (8.8% and 12.3 ppm) compared to the normal soil (1.8% and 4.2 ppm) indicates that the soils required supplemental P. The available K was high in normal soil (6.18 ppm) than PR while total K found slightly more in PR. The environmentally available Na, Mg, Cr, Co, Ni and Pb content in PR is very high compared to soil.
Table 3: Main Chemical Properties Of Soil And Phosphate Rock (N = 3, Mean ± Se
| Parameters | Soil | Phosphate rock |
| pH | 5.62±0.1 | 7.81±0.9 |
| EC (dS/m) | 0.11±0.01 | 0.99±0.001 |
| Bulk density (g/cm3) | 1.037±0.01 | 2.7±0.007 |
| Organic carbon(%) | 0.09±0.02 | 0.001±0.02 |
| WHC (%) | 34.42±0.01 | 60±4.9 |
| Total N (ppm) | 0.98±0.002 | 560±12.9 |
| Total P (%) | 1.8±0.004 | 8.8±2.9 |
| Total K (%) | 0.06±0.0002 | 0.39±0.001 |
| Available N (ppm) | 0.28±0.01 | 0.002±0.0005 |
| Available P (ppm) | 4.2±0.01 | 12.3±0.01 |
| Available K (ppm) | 6.18±0.09 | 2.7±0.9 |
| Na (ppm) | 4.04±0.002 | 8300±12.9 |
| Mg (ppm) | 1.25±0.005 | 5900±24.9 |
| Cr (ppm) | 0.317±0.0001 | 207±10.7 |
| Co (ppm) | 0.160±0.0002 | 13.6±3.9 |
| Ni (ppm) | 0.009±0.0004 | 78.1±20.6 |
| Pb (ppm) | 0.066±0.002 | 6.6±0.7 |
Crop Response
The treatments investigated and the responses of plant growth parameters have been summarized in figure 1 and 2. Germination % in 6, 8 and 10 g of PR shows highest value followed by control and 2 g PR amended soil (Fig. 1 A). Further, root and shoot length status in soil treated with 8 g PR is markedly highest and all other treatments have the higher value than control soil except treatment having 10 g of PR in the soil. As far as charging of PR is concerned, significantly higher fresh and dry weight, protein content was obtained with 8g charged PR than no-PR (control) treatment.
An exception has seen in the case of leaf area, the control and 8 g PR carrying plant show similar results while other have higher value except for T5 (carrying 10g) PR showing lowest value. Amongst the sources similar trends in relative effectiveness regarding photosynthetic pigment (Chl-b and Chl-c) were observed due to 8g RP per kg of soil except for Chl-a, pheophytin, and carotenoids, there has a random value in each treatment.
Results showed that there was nearly increase in each parameter of plant growth when the plants were fertilized by 8 g phosphate rock per kg of soil as compared to unfertilized, plants fertilized by 2, 4, 6 and 10 g of phosphate rock. This may be attributed to the higher amount of P available to the plant from raw phosphate rock than normal soil. Parameters like mean and standard error mean (SEM) were measured in between the value of control and T4, statistical significance among the means was established through Student’s t-test. P< 0.05.
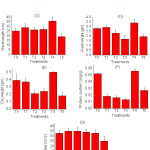 |
Figure 1: Response of plant parameters (Zea mays L.) and effectiveness of different treatments (n = 3, Mean±SE) |
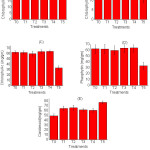 |
Figure 2: Photosynthetic pigment content in various treatments (n = 3, Mean±SE) |
Conclusions
This experimental work shows that rock phosphate could be an effective amendment for Zea mays L. by direct application to the soil. Phosphate rock in a proper ratio (8g/kg) of soil can be utilized as a potential alternative for phosphatic fertilizers. The outcome shows that the PR has an agronomic significance for maize cultivation and crop yield which may help in the eradication of problems encountered with food shortage and crop production economy.
Acknowledgments
The authors wish to acknowledge the Director CSIR-IMMT, Bhubaneswar, Odisha, India, for providing necessary facilities to carry out this study.
References
- Yadav H., Fatima R., Sharma A., Mathur S. Enhancement of applicability of rock phosphate in alkaline soils by organic compost. Applied Soil Ecology. 2017;113:80-85.
CrossRef - Klaic R., Plotegher F., Ribeiro C., Zangirolami T.C., Farinas C.S. A novel combined mechanical-biological approach to improve rock phosphate solubilization. International Journal of Mineral Processing. 2017;161:50-58.
CrossRef - Fontes M.P.F., Weed S.B. Phosphate adsorption by clays from Brazilian Oxisols, relationships with specific surface area and mineralogy. Geoderma. 1996;72(1-2):37-51.
CrossRef - Mendes G.D., De Freitas A.L.M., Pereira O.L., Da Silva I.R., Vassilev N.B., Costa M.D. Mechanisms of phosphate solubilization by fungal isolates when exposed to different P sources. Annals of Microbiology. 2014;64(1):239-249.
CrossRef - Gyaneshwar P., Kumar N., Parekh L.J., Poole P.S. The role of soil microorganisms in improving P nutrition of plants. Plant and Soil. 2002;245(1):83-93.
CrossRef - Vassilev N., Medina A., Azcon R., Vassileva M. Microbial solubilization of rock phosphate on media containing agro-industrial wastes and effect of the resulting products on plant growth and P uptake. Plant and Soil. (;287(1-2):77-84.
CrossRef - Vassilev N., Vassileva M. Biotechnological solubilization of rock phosphate on media containing agro-industrial wastes. Applied Microbiology and Biotechnology. 2003;61(5-6):435-440.
CrossRef - Reddy M.S., Kumar S., Khosla B. Biosolubilization of poorly soluble rock phosphates by Aspergillus tubingensis and Aspergillus niger. Bioresource Technology. 2002;84(2):187-189.
CrossRef - Koliaei A.A., Akbari Gh A.., Armandpisheh O., Labbafi M.R., Zarghami R. Effects of phosphate chemical fertilizers and biologic fertilizers in various moisture regimes on some morphological characteristics and seeds performance in maize. Asian Journal of Agricultural Sciences. 2011;3:223-234.
- Zapata F., Zaharah A.R. Phosphorus availability from phosphate rock and sewage sludge as influenced by the addition of water-soluble phosphate fertilizer. Nutrient Cycling in Agroecosystems. 2002;63(1):43-48.
CrossRef - Fertilizer Association of India (FAI), Fertilizer Statistics, 2001-02, The Fertilizer Association of India, New Delhi. 2002
- IFDC. Proceedings of Seminar on Phosphate Rock for Direct Application, International Fertilizer Development Center, Muscle Shoals, Alabama, USA. 1978.
- Ahn P.M. Tropical Soils and Fertilizer Use, Longman. UK. 1993.
- McLean E.O. Soil pH and Lime Requirement,,In: Page, A.L. (Ed.), Methods of Soil Analysis, Part 2, Chemical and Microbiological Properties, 2nd ed. Agronomy No: 9, ASA, SSSA, Madison, Wisconsin. 1982
- Rhoades J.D., Salts, In: Page, A.L. (Ed.), Methods of Soil Analysis, Part 2, Chemical and Microbiological Properties, 2nd ed. Agronomy No: 9, ASA, SSSA, Madison, Wisconsin. 1982;167–179.
- Blake G.R., Hartge K.H. Bulk Density, In: Klute, A. (Ed.), Methods of Soil Analysis, Part 1, Physical and Mineralogical Methods, 2nd ed. : ASA, SSSA, Agronomy No: 9. Madison, Wisconsin. 1986;363–375.
- Panda S.S, Basu A., Dhal N.K. Effects of Chromium Ore Tailings on Growth and Physiological Activities of Mesua Ferrea l. Soil and Sediment Contamination. 2016;25(5):563-572.
CrossRef - Allison L.E. Organic Carbon. In: Methods of Soil Analysis. Part II. In: Black C.A. (ed.). American Society of Agronomy. 1965;1367-1376.
- Jackson M.L., Soil Chemical Analysis. Prentice Hall of India Private Limited, New Delhi. 1973.
- Black C.A., Evans D.D., White J.L., Ensminger L.E, Clark F.E. In: Methods of soil Analysis: Chemical and Microbiological properties, Agronomy 9, Part II Medison, Wisconsin, USA. 1965.
- Chapman H.D. Methods of Analysis for Soils, Plant and Water, vol. 4034. Division of Agricultural science, University of California, Berkeley. 1982.
- Lichtenthaler H.K., Buschmann C. Chlorophylls and carotenoids: measurement and characterization by UV–VIS spectroscopy. Current Protocols in Food Analytical Chemistry. 2001;F4.3.1–F4.3.8.
- Porra R.J., Thompson W.A., Kriedmann P.E. Determination of accurate extinction coefficients and simultaneous equations for assaying chlorophylls a and b extracted with four different solvents: verification of the concentration of chlorophyll standards by atomic absorption spectroscopy. Biochimica et Biophysica Acta (BBA) – Bioenergetics. 1989;975(3):384–394.
CrossRef - Lowry O.H., Rosebraugh N.J, Furr A.L, Randall R.J. Protein measurement with folin phenol reagent. The journal of Biological Chemistry. 1951;193:265-275.

