Introduction
Rauvolfia, a genus of family apocynaceae, are shrubs or under shrubs or occasionally trees. R. serpentina is a well-known drug yielding plant, commonly used as an antidote for snake bites. It has number of other medicinal properties because it contains numbers of alkaloids viz. ajmalicine, chandrine, rauwolfinine, reserpine, serpagine, serpentine, serpentinine, tetraphyllicine, yohimbine, etc. R. tetraphylla is also of high medicinal value and is used in high blood pressure, stomach ailments, fever, insomnia, etc. Alkaloids such as ajmaline, reserpine, serpentine, tetraphyllin, tetraphyllicine, yohimbine, etc. are present. Both of these species of Rauvolfia cannot be propagated immediately after harvest due to the dormancy of seeds.1 Dormancy is not just associated with the absence of capacity of germination; rather it is a characteristic of the seed that determines the conditions required for germination.2 There are five classes of dormancy viz. physiological, morphological, morphophysiological, physical and combinational.3
As both R. serpentina and R. tetraphylla have high medicinal value, they are very much important for pharmaceutical industries. Seed germination studies on medicinal plants have proved to be useful in developing appropriate conservation strategies.4 The demand for medicinal plants is increasing in both developing and developed countries because of products being non-narcotic, having no side effects, easily available at affordable prices and sometimes the only source of health care available to the poor. Thus, the objective of this study was to develop some effective methods that would help to break dormancy in both the species of Rauvolfia and provide suitable conditions required for their germination and easy propagation.
Materials and Methods
The study was conducted in the department of Botany, Gauhati University, Guwahati.
Seed Source
Mature fruits of Rauvolfia serpentina and R. tetraphylla were collected from the plants grown in botanical garden of the Department of Botany, Gauhati University, Guwahati during the month of July, 2011. These seeds were allowed to dry in shade and kept in sealed cellophane paper bags at room temperature for further experiments. A total of 500 seeds were randomly collected from each species for the experiment.
Viability of Seeds
Viability of the collected seeds was determined by using tetrazolium technique5 and by dissection microscope.6
Pretreatments and Experimental Conditions
The experiment was conducted in two sets. In the first set, seeds were treated with H2O2, KNO3, KH2PO4 and GA3. These four chemicals were taken at three different doses i.e., 3%, 5% and 7% solutions of H2O2, KNO3 and KH2PO4; whereas GA3 was prepared as solutions of 100 ppm, 200 ppm and 300 ppm. The second set of experiments consisted of treatment with conc.H2SO4 and moist chilling. Seeds were treated with conc. H2SO4 for 5 minutes; whereas moist chilling was done for 7 days, 14 days and 21 days. Surface sterilization of seeds was done prior to experiment by soaking them in 3% sodium hypochlorite solution for 5 minutes and subsequently rinsing thoroughly with sterilized distilled water. Three replicates of 30 seeds were used for each treatment. The treated and untreated seeds were placed in sterile petridishes lined with two sterile Whatman no.1 filter papers moistened with sterile distilled water to ensure adequate moisture for the seeds. Further, each of these treatments were carried out at four controlled and constant temperature regimes viz., 25oC, 30oC, 35oC and 40oC. All the experimental sets of seeds were incubated at normal day/night photoperiod. The observations were recorded daily up to 60 days. Seeds with 2 mm radical emergence were considered germinated. Total germination percentage (Gt) was calculated by using the following formula-
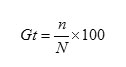
Where, n= total no. of germinated seeds at the end of the experiment and N= total no. of seeds used for germination test.7
Statistical Analysis
All the experiments were conducted in a randomized block design (RBD) with three replications for each treatment combinations. The data collected were then statistically analyzed by two-way analysis of variance technique (P<0.01).
Results and Discussion
Seed Viability
The viability of seeds of R. serpentina and R. tetraphylla was 67% and 82% respetively. However, strong seed dormancy was observed in both the experimental plants. The results of tetrazolium test were not in accordance with the seed germination percentage of the two species of Rauvolfia.
Effect of Pretreatments on Germination Percentage of R. Serpentina and R. Tetraphylla Seeds
The analysis of variance showed that different chemicals significantly affected seed germination in R. serpentina and R. tetraphylla. Similarly different temperatures and doses of chemicals also affected seed germination significantly. On the other hand, the interaction effect of chemical and dose and chemical and temperature were significant both in R. serpentina and R. tetraphylla. The interaction effect of temperature and dose was, however insignificant in R. serpentina but significant in R. tetraphylla (Table: 1 and 2).
Table 1: Analysis of variance for seed germination in R. serpentina.
| Sources of variation |
Degrees of freedom |
Sum of squares | Mean square | Calculated ‘F’ value | Table value of ‘F’ at 1% |
| Chemical (A) |
3 |
8039.97 | 2679.99 | 467.71** | 5.09 |
| Temperature (B) |
3 |
381.26 | 127.08 | 22.18** | 5.09 |
| Dose (C) |
2 |
142.85 | 71.42 | 12.46** | 6.01 |
| Chemical X Dose |
6 |
1141.77 | 190.29 | 33.21** | 4.01 |
| Chemical X Temperature |
9 |
886.35 | 98.48 | 17.18** | 3.37 |
| Temperature X Dose |
6 |
59.6 | 9.93 | 1.73n.s | 4.01 |
* Highly significant (P<0.01)
n.s= not significant
Table 2: Analysis of variance for seed germination in R. tetraphylla.
| Sources of variation |
Degrees of freedom |
Sum of squares | Mean square | Calculated ‘F’ value | Table value of ‘F’ at 1% |
| Chemical (A) |
3 |
10410.98 | 3470.33 | 13993.27* | 5.09 |
| Temperature (B) |
3 |
171.10 | 57.03 | 229.96* | 5.09 |
| Dose (C) |
2 |
508.73 | 254.36 | 1025.65* | 6.01 |
| Chemical X Dose |
6 |
947.51 | 157.92 | 636.77* | 4.01 |
| Chemical X Temperature |
9 |
59.96 | 6.66 | 26.85* | 3.37 |
| Temperature X Dose |
6 |
14.38 | 2.40 | 9.68* | 4.81 |
*= P<0.01
N.S= not significant
The highest germination percentage of R. serpentina seeds in control was 11.27 at an incubation temperature of 30oC (Fig: I). The germination percentage at 30oC increased to 34.94 and at 35oC increased to 33.33 when the seeds were treated with 3% H2O2 (Fig: I). Further increase in dose of H2O2, decreased the germination percentage (Fig: I). On the other hand, R. tetraphylla seeds showed low rate of germination than the germination of seeds set for control (Fig: II). The excessive levels of oxidants may result in severe cellular damage.8 Though, antioxidant enzymes (AOE) eliminate the effect of active oxygen species (AOS).9 But in this case, the activities of AOE may not be sufficient in scavenging of excess amount of AOS.
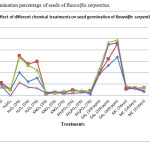 |
Figure 1: Germination percentage of seeds of Rauvolfia serpentina. |
Seeds treated with KNO3 in R. serpentina showed maximum germination percentage of 4.24 at a dose of 7% KNO3 at 35oC which was low as compared to control sets (Fig: I). Whereas, R. tetraphylla seeds showed maximum germination percentage of 52.70 at a dose of 3% KNO3 at 30oC which was higher than the control sets (Fig: II). But as the dose of KNO3 increased to 5% and 7% the germination percentage decreased (Fig: II).
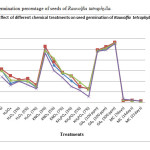 |
Figure 2: Germination percentage of seeds of Rauvolfia tetraphylla. |
The low germination percentage observed in R. serpentina may be due to high percentage of KNO3 treatment. Paul et al.,10 treated R. serpentina seeds with 1% KNO3 for 24 hours which was effective in improving the germination percentage and thus high dose of KNO3 was not effective in this case. On the other hand, germination percentage improved in R. tetraphylla seeds to some extent but as the dose of KNO3 increased germination percentage decreased. High dose of KNO3 was not effective in breaking seed dormancy of Chenopodium album seeds as compared to low dose.11 0.5% KNO3 treatment gave better results as compared to high dose of this chemical in chickpea seeds12. KH2PO4 was not effective in either of the two species (Fig: I and II). The results of this work are in accordance with the results obtained by Paul et al.10 in case of R. serpentina seeds. Seed priming with 0.5% KH2PO4 gave better results as compared to its high dose in chickpea seeds12. Corn seeds primed with KH2PO4 resulted in advanced metabolic processes and higher germination percentage and germination rate as compared with unprimed seed13.
The seeds of R. serpentina and R. tetraphylla achieved maximum germination percentage when these were treated with GA3 (Fig: I and II). A maximum germination of 48.65% in R. serpentina and 56.66% in R. tetraphylla was achieved when treated with a dose of 300ppm of GA3 respectively at 35oC and 30oC of incubation temperature. GA3 was the most effective chemical that showed good response in increasing the germination percentage of both the species. The results of our work are similar to those of Baskin and Baskin14 where they found positive effect of soaking of Osmorhiza claytonia seeds in GA3 solution. It is reported that GA3 is effective in breaking the non-deep physiological dormancy, but it does not overcome the deep physiological dormancy3.
Two functions for gibberellins (GA) during seed germination have been proposed. First, GA increases the growth potential of the embryo. Secondly, GA is necessary to overcome the mechanical restraint conferred by the seed-covering layers, by weakening of the tissues surrounding the radicle15, 16. Scarification of R. serpentina and R. tetraphylla seeds with concentrated sulphuric acid (conc. H2SO4) showed no improvement in germination (Fig: I and II). Trivedi and Kumari17 also did not observe any germination improvement in R. serpentina seeds. In our experiment, acid scarified seeds reached a maximum level of germination of 5.27% in R. serpentina and 25.04% in R. tetraphylla which was low as compared to the untreated seeds i.e., respectively 11.27% and 31.26%. The failure of germination of acid scarified seeds was due to damage caused to the seeds or embryo of seeds for a longer period18. Cold stratification treatment for 7 days, 14 days and 21 days failed to show any improvement in the germination percentage of R. serpentina and R. tetraphylla seeds. The germination percentage decreases as compared to control and it was due to the lethal effect of cold stratification on the seeds. It has been reported by Ren and Tao19 that cold stratification cause lethal effect on viable seeds. Favourable conditions of moisture and temperature directly affected embryo growth and ultimately germination of seeds of Pastinaca sativa and Conium maculatum which exhibited morphological dormancy20. The role of four different temperature regimes were also observed in addition to all other treatments and it was found that optimum seed germination temperatures were 35oC and 30oC for R. serpentina (Fig:I) and R. tetraphylla (Fig:II) respectively. Seeds of different species have different degrees of temperature requirements. Bewley and Black21 stated that, the most optimum temperature for germination is generally the one at which a given number of seeds achieve their maximum per cent of germination, over a period of time.
From the above results of the present work it is concluded that R. serpentina and R. tetraphylla have non-deep physiological dormancy. Though GA3 broke dormancy of seeds of these two species to a significant extent, but it could not break dormancy of all seeds. This indicated the presence of another kind of dormancy in addition to non-deep physiological dormancy. The other kind of dormancy might be the coat-induced dormancy, as the seed coats were very hard in both these species. Hence, further work is necessary to break the dormancy of all viable seeds and to determine the type of non-deep physiological dormancy.
Acknowledgements
The first author is thankful to the UGC, New Delhi for financial support in the form of Maulana Azad National Fellowship.
References
- Dey A. and De J.N., Ethnobotanical aspects of Rauvolfia serpentina (L). Benth.ex Kurz. in India, Nepal and Bangladesh. Journal of Medicinal Plant Research, 5(2), 144-150 (2011).
- Fenner M. and Thompson K., The ecology of seeds. Cambridge, UK: Cambridge University Press, (2005).
CrossRef - Baskin J.M. and Baskin C.C., A classification system for seed dormancy. Seed Science Research, 14, 1-16 (2004).
CrossRef - Kandari L.S., Rao K.S., Chauhan K., Maikhuri R.K., Purohit V.K., Phondani P.C. and Saxena K.G., Effect of presowing treatments on the seed germination of two endangered medicinal herbs of the Himalaya (Angelica glauca Edgew and Pleurospermum angelicoides Wall. Ex DC. Benth. Ex C.B. Clarke). Proc. Indian Nat. Sci. Acad., 73, 11-16 (2007).
- Grabe D.F., Tetrazolium testing handbook for agricultural seeds. Association of Official Seed Analysts, Contribution No.29 to the Handbook of seed testing, (1970).
- ISTA. International Seed Testing Association. International Rules for Seed Testing. Bassersdorf, Ch-Switzerland, (2003).
- Farashah H.D., Afshari R.T., Sharifzadeh F. and Chavoshinasab S., Germination improvement and α-amylase and β-1, 3-glucanase activity in dormant and non-dormant seeds of Oregano (Origanum vulgare). AJCS., 5(4), 421-427 (2011).
- Cavusoglu K. and Kabar K., Effects of hydrogen peroxide on the germination and early seedling growth of barley under NaCl and high temperature stresses. Eur Asia J Bio Sci., 4, 70-79 (2010).
CrossRef - Breusegem F.V., Vranova E., Dat J.F. and Inze D., The role of active oxygen species in plant signal transduction. Plant Science, 161, 405-414 (2001).
CrossRef - Paul D., Paul N.K. and Basu P.K., Seed germination response of Rauvolfia serpentina Benth. to certain physical and chemical treatments. J. bio-sci., 16, 129-131(2008).
- Tang D., Hamayun M., Ko Y., Zhang Y., Kang S. and Lee I., Role of Red light, Temperature, Stratification and Nitrogen in Breaking Seed Dormancy of Chenopodium album L. J. Crop Sci. Biotech., 11(3), 199-204 (2008).
- Sarwar N., Yousaf S. and Jamil F.F., Induction of salt tolerance in chickpea by using simple and safe chemicals. Pak J Bot., 38, 325-329 (2006).
- Soleimanzadeh H., Effect of seed priming on germination and yield of corn. IJACS., 5(4), 366-369 (2013).
- Baskin C.C. and Baskin J.M., Non-deep complex morphophysiological dormancy in seeds of Osmorhiza claytonia (Apiaceae). Am J. Bot., 78, 588-593 (1991).
CrossRef - Kucera B., Cohn M.A. and Leubner-Metzger G., Plant hormone interactions during seed dormancy release and germination. Seed Sci Res., 15, 281-307 (2005).
CrossRef - Finch-Savage W.E., Cadman C.S., Toorop P.E., Lynn J.R. and Hilhorst H.W., Seed dormancy release in Arabidopsis Cvi by dry after-ripening, low temperature, nitrate and light shows common quantitative patterns of gene expression directed by environmentally specific sensing. Plant J., 51, 60-78 (2007).
CrossRef - Trivedi M.P. and Kumari R., Ethno-botanical and Germinational Aspects of Rauvolfia serpentina (L.) Benth. Ex Kurz. Our Nature, 9, 176-178 (2011).
- Wang Y.R., Hanson J. and Mariam Y.W., Effect of sulphuric acid pretreatment on breaking hard seed dormancy in diverse accessions of five wild Vigna species. Seed Science and Technology, 35, 550-559 (2007).
CrossRef - Ren J. and Tao L., Effects of different pre-sowing seed treatments on germination of 10 Calligonum species. Forest Ecology and Management, 195, 291-300 (2004).
CrossRef - Scholten M., Donahue J., Shaw N.L. and Serpe M.D., Environmental regulation of dormancy loss in seeds of Lomatium dissectum (Apiaceae). Annals of Botany, 103, 1091-1101 (2009).
CrossRef - Bewley J.D. and Black M., Seeds. Physiology of Development and Germination. 2nd Ed. New York: Plenum Press, (1994).
