Introduction
Crop productivity is influenced by three fundamental categories: environmental, biological, and technical factors. Among these factors, pollination also play a crucial role in successful reproduction where one-third of the global food crops depend on animal pollination.1,2 Pollination enhances the fruit and seed quality and also increases the productivity of 70% of 1330 tropical crops3 and 85% of 254 crops in Europe.4 Likewise, many of the plantation crops such as cardamom, coffee and vegetable crops are dependent on pollination.5-8 Amomum subulatum demonstrated higher yields with an increased abundance of bumblebees and bees in plantations.9,10 Coffea arabica relies on both wind and insect pollination, which has been shown to enhance coffee productivity by 50%11. Similarly, experimental augmentation of insect pollinators in apple orchards significantly improved fruit set and yield.12,13
The primary significance of increasing yield lies in its potential to alleviate poverty substantially. This is especially important in places like Sikkim, where a large portion of the people depends on agriculture for their livelihood and security.14 Among the various crops grown, Sikkim mandarin is a key cash crop.15 However, its reported decline in yield over the years poses a significant threat to the livelihoods of farmers who rely on it. Pollination, with its proven capacity to enhance crop yields,10 could play a pivotal role in reversing the decline in Sikkim mandarin productivity. The declining productivity of mandarin in the eastern Himalayas is a major concern and has consistently been linked to pest and disease outbreaks, insufficient availability of planting material, and inadequate management practices.16,17 While pollination is critical for improving yields, the challenges associated with Citrus crops are more complex compared to other fruit crops.
Citrus has diverse pollination requirements, resulting in conflicting reports about its response to pollination. Citrus varies from self-fertile to complete self-sterile where pollens must be transferred to such self-sterile flowers from other compatible types for greater yield.1 In some cases, the transfer of pollen between flowers within the same cultivar or species enhances fruit production, while in others, self-pollination using a flower’s own pollen is sufficient for optimal yield1. Citrus, in particular, has been identified as a crop with minimal or no reliance on insect pollination1. However, domestication over the years with the practice of grafting18 has resulted in the formation of hybrids. Moreover, domestication also results in changes in crop species when compared to their wild progenitors.19-21 The ‘Interdonato’ lemon has been identified as a citron × lemon hybrid.22 Additionally, pollination involving other Citrus hybrids can result in citron hybrids when propagated through seeds.18 Therefore, the varieties do not remain entirely static irrespective of its asexual reproduction23 and the likelihood that its pollination requirements have changed overtime cannot be overlooked. Lemon trees isolated from bees produced only one-fourth of the fruit compared to those subjected to cross-pollination.24 In oranges, honeybees contributed to a 31% increase in fruit set, a 22% increase in fruit weight, a 33% increase in juice content, and a 36% increase in seed number.25 However, bees had no effect on the production of “Valencias”26 whereas cross-pollinating it with “Pearl” tangelo pollen was found to boost its fruit set and seed number. Pollination issues are notably pronounced in the mandarin and mandarin-hybrid complex.1 While mandarins exhibit some degree of apomixis,27 certain varieties are self-incompatible, sterile, parthenocarpic, or produce defective pollen.28,29 The yield of ‘Clementine’ tangerines increased significantly when cross-pollinated by bees with ‘Dancy’, ‘Temple’, ‘Duncan’, or other seedy cultivars.30 Additionally, cultivars such as ‘Lee’, ‘Page’, ‘Nova’, and ‘Robinson’,31 as well as ‘Orlando’, ‘Minneola’, and ‘Osceola’, were identified as self-incompatible.32 The ‘Hyuganatsu’ mandarin, on the other hand, was found to be self-sterile but cross-fertile.33 Such vast variation in requirements of pollination in citrus and mandarin cultivars makes it hard to ascertain the breeding system and pollination biology of mandarin cultivars. Therefore, this study investigates the following queries in light of the dynamic character of mandarin cultivars regarding their pollination biology and breeding system: (i) Are mandarins self-compatible? (ii) How reliant on pollination are mandarins? (iii) What is the Mandarin’s reproductive success rate? (iv) Can problems with crop yield be resolved with a better knowledge of pollination biology and the breeding system?
Materials and Methods
Study site
The study was carried out in an orchard located in Dzongu, in the northern district of Sikkim, India (27° 28.423’ N, 88° 30.602’ E, 1151 m a.s.l.), during the peak blooming period at the onset of spring in March 2021 and 2022. The research site experiences rainfall of 211.16 mm per year with temperatures ranging from a minimum of 14.70 °C to a maximum of 23.82 °C. The subtropical forest type of the study site is home to numerous species of Alnus nepalensis, Albizia spp., Macaranga spp., Juglans regia, and Myriocarpa spp.
Field data collection
Pollination activity
An observation period of the day was determined after preliminary observations were taken. Accordingly, a plot with thirty open flowers and seven healthy trees were chosen.34 Only when a visitor comes into contact with the stigma and anther while foraging is it deemed a pollinator. For seven days (168 hours in both years) during the peak flowering season, from 6th March – 11th March 2021, and 10th March –17th March 2022, the pollinators’ frequency of visits inside the plot was recorded at hourly intervals beginning at 0600–1800 h. For one week, the seven healthy trees that were chosen for the study were watched one after the other.
Pollination treatments
Hand-pollination experiments were used to evaluate the pollinator reliance and breeding system of mandarin oranges. Out of seven healthy trees, five sets of flower buds were chosen at random and bagged before anthesis and anther dehiscence. Ribbons of various colours were used to identify each pair. The first set (N=100) was bagged without emasculation in order to verify autogamy. On the day of anthesis, the bags were opened, and pollen from the newly dehisced anther of the same flower was used to fertilize the flowers. Following emasculation, the second batch (N=100) was bagged in order to check for apomixis. The third set (N=100) was bagged after emasculation for geitonogamous self-pollination. The bags were opened, the emasculated flowers were pollinated with pollen from another flower on the same plant, and the bags were then sealed again on the day of anthesis. The fourth set (N=100) was bagged similarly after emasculation, but the blooms were pollinated with pollen from a flower on a separate plant before being rebagged in order to evaluate xenogamous pollination. To demonstrate open pollination, the fifth set (N=100) was not treated.34 A separate set of flowers was enclosed in bags, and a pollinator was allowed a single visit before the flowers were re-bagged to assess pollination efficiency (PE).34 On November 2, 2021, and November 12, 2022, the mature fruits for each set were harvested. The percentage of fruit set for each set of flowers during the fruiting season was recorded in order to assess the breeding experiment’s outcomes.
Breeding System
The breeding system of the species was analyzed using the index of self-incompatibility (ISI),35 which is calculated as the ratio of fruit set in self-pollinated flowers to fruit set in cross-pollinated flowers. Fully self-compatible species have an ISI of 1 or higher, moderately self-compatible species have an ISI of 0.2 but less than 1, and fully self-incompatible species have an ISI of <0.2 or less than 1.35
Pollinator dependency
Pollinator dependency (PD) was computed by deducting the proportion of fruit set attained by autogamy from the percentage of fruit set attained through open pollination.36 When the PD index is zero, there is no reliance on pollinators; when it is 100, there is total reliance on pollinators.
Pollination efficiency
Spear’s pollination index =Pi – Z )/U – Z was used to assess pollination efficiency based on the number of seeds produced per visit34. Pi represents the average number of seeds in the fruit following a single pollinator visit, i, z represents the average number of seeds in the fruit that did not receive any pollinator visits, and U represents the average number of seeds in the fruits that received unrestricted visits.37 The pollinator efficiency index is a number between 0 and 1, where 0 means the pollinator has no effect on the production of fruit or seeds, and 1 means that the pollinator’s contribution is equal to the amount of fruit or seeds produced by flowers that were left open to pollinate.
Pollen limitation
Pollen restriction was evaluated by dividing the fruit set in cross-pollinated flowers by the fruit set in open-pollinated flowers.38 On this scale, zero represents no pollen limitation and one hundred represents total pollen limitation.
Pollen fertility
Pollen fertility was assessed using in-vitro germination utilizing the sitting drop culture method.34 At intervals of 0, 3, 6, 9, and 24 hours following anthesis, a small layer of pollen was applied to a dry microscope slide. The slide was then prehydrated for 30 to 60 minutes in a humidity chamber. At each time point, pollen from four distinct trees was gathered and cultivated in four repetitions. After that, the prehydrated pollen was spread out on a microscope slide in a 20–30 µl drop of regular germination medium. 100 mg/l boric acid and 10% sucrose were combined to create the standard medium. Before being assessed to determine the percentage of germination, the pollen cultures were incubated in the humidity chamber for two hours.
Quantitative and physico-chemical analysis
After gathering fruits from several pollination treatment sets (N=10), morphological characteristics like height, diameter, weight, and quantity of seeds per fruit were measured.39 The juice’s acidity was assessed using acid-base titration, and its total soluble solids (TSS) were measured using a hand refractometer (ERMA Japan).
Statistical analysis
Using R version 3.5.2, a Pearson correlation test was used to investigate the connection between pollinator abundance and environmental factors. Additionally, stepwise multiple linear regression was carried out using IBM Corp.’s SPSS version 18 to predict the effect of environmental factors on pollinator abundance. One-way ANOVA with Post hoc Gabriel tests were used to examine the quantitative characteristics of fruits under various treatments. Spearman’s rank correlation coefficient was used to examine the relationship between pollen viability and time.
Results
Foraging activity
The common honeybee was determined to be the most common pollinator during the observation period. A. cerana regularly returned to the same flowers, and the flowering rhythm was asynchronous. The blooms lasted for three days, after which the petals would drop when they came into contact with a pollinator. The frequency of trips peaked between 10:00 AM and 1:00 PM (Fig. 1), following which there was a slow decrease until foraging halted at 6:00 PM. Foraging activity started at 8:00 AM.
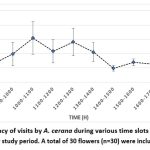 |
Figure 1: The frequency of visits by A. cerana during various time slots throughout the day, recorded over a 7-day study period. A total of 30 flowers (n=30) were included in the study plot. |
Correlation and Multiple linear regression analysis
Temperature, relative humidity, and carbon dioxide had no discernible effects on A. cerana field activities, according to an examination of pollinator abundance on mandarin orange blossoms in relation to environmental factors (Table 1). On the other hand, there was a substantial negative association between temperature and carbon dioxide (r = -0.829, p = 0.01) and a strong link between relative humidity and carbon dioxide (r = 0.964, p = 0.01). Temperature significantly affected A. cerana abundance, according to stepwise multiple regression analysis (p = 0.019, Table 1). An increase of 47 ± 15 individual A. cerana visitors per hour was estimated by the resulting multiple linear regression model, which had an adjusted R2 value of 0.613 for every degree Celsius of temperature increase. Carbon dioxide was not included in the model among the three predictor variables (t = 0.630, p = 0.552).
Table 1: Results of multiple linear regression analysis evaluating the impact of environmental variables on the abundance of A. cerana.
| Variable | β | SE | t value | p value | R2 |
| Temp (°C) | 46.95 | 14.67 | 3.20 | 0.019 |
0.613 |
| RH | 30.47 | 15.74 | 1.93 | 0.101 | |
| CO2 | -0.82 | 1.31 | 0.63 | 0.552 |
β = regression coefficient; SE = Standard Error, R2 = coefficient of determination; RH = Relative Humidity, Temp = Temperature, CO2 = Carbon-dioxide
Fruit set, breeding system and pollinator dependency
One of the main reasons the blossoms attracted pollinators was the strong scent they emitted. Mandarins were found to be moderately self-compatible with an index of self-incompatibility (ISI) of 0.28 and a pollinator dependency of 42%. Fruit set during open pollination was 46%, while fruit set by autogamy and apomixis was 4% and 5%, respectively (Table 2). Pollen fertility, a measure of pollination efficiency, decreased significantly over time (r = 0.001, p<0.005), with fertility percentages of 90.77, 55.56, 40.24, 28.85, and 17.65% at 0, 3, 6, 9, and 24 hours, respectively (Fig. 2).
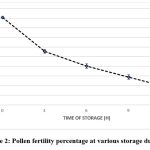 |
Figure 2: Pollen fertility percentage at various storage durations. |
Physico-chemical analysis
The physico-chemical properties of fruits varied significantly across different pollination treatments (Table 2). With the largest fruit set (46.00 ± 3.10%) and seed count (12.00 ± 4.96), open pollination yielded the heaviest fruits (54.65 ± 2.72 g). Cross-pollination produced a lower fruit set (14.00 ± 2.45%) and smaller fruits (40.72 ± 3.65 g). Autogamy produced the smallest fruits (22.56 ± 2.97 g) and the lowest fruit set (4.00 ± 2.29%), while geitonogamous self-pollination produced moderate fruit weight (51.61 ± 4.08 g) and fruit set (31.00 ± 6.48%). Apomixis yielded limited fruit set (5.00 ± 2.45%) seedless fruits. With minor variances, total soluble solids (TSS) and acidity were largely constant among treatments. In contrast to self-pollination and apomixis, these findings show that open pollination increases fruit weight, seed count, and fruit set.
Statistical analysis of pollination effects on fruit traits
ANOVA revealed significant effects of pollination treatments on fruit parameters (Table 3). Fruit set %, fruit weight, and fruit diameter all varied significantly (p < 0.001), suggesting that pollination had a major impact on these characteristics. Significant differences were also seen in fruit height and seed count (p < 0.01). TSS (°Brix) and acidity, on the other hand, did not differ significantly (p > 0.05), indicating that pollination treatments have no effect on these characteristics. These findings highlight the critical role of pollination in fruit development and reproductive success.
Effect of Pollination Treatments on Fruit Characteristics
Significant variations in fruit attributes across pollination treatments were found using the Post hoc Gabriel test (Table 4). When compared to autogamy, open pollination yielded the largest and heaviest fruits, with a considerably higher diameter (13.23 mm) and weight (32.14 g) (p < 0.05). Similarly, open pollination had the highest fruit set percentage, which was substantially higher than that of autogamy (42.00%), geitonogamous self-pollination (15.00%), and cross-pollination (32.00%) (p < 0.05). The tiniest fruits and the lowest fruit set were produced by autogamy, indicating its poor fruit production efficacy. Compared to autogamy and apomixis, the quantity of seeds in open-pollinated fruits was significantly larger (p < 0.05), confirming the importance of cross-pollination in boosting reproductive success. However, TSS and acidity did not differ substantially between treatments (p > 0.05), indicating that pollination mode has no effect on fruit quality attributes. These findings highlight how self-pollination, especially autogamy, results in less fruit development and reproductive output, whereas open pollination maximizes fruit yield and reproductive efficiency.
Table 2: Physico-chemical properties of fruits resulting from different pollination treatments.
| Treatments | Avg. Weight (g) | Avg. Height (mm) | Avg. Diameter (mm) | No. of seeds | TSS (°Brix) | Acidity | Fruit set (%) |
|
Open pollinated |
54.65 ± 2.72 |
41.17 ± 3.30 |
48.67 ± 2.64 |
12.00 ± 4.96 |
10.40 ± 3.01 |
1.13 ± 0.62 |
46.00 ± 3.10 |
| Cross pollinated | 40.72 ± 3.65 | 36.00 ± 4.24 | 42.07 ± 5.13 | 10.00 ± 3.87 | 10.70 ± 2.69 | 1.09 ± 0.39 | 14.00 ± 2.45 |
| Geitonogamous self- pollinated | 51.61 ± 4.08 | 39.09 ± 4.09 | 47.99 ± 4.22 | 10.00 ± 3.87 | 10.80 ± 4.98 | 1.00 ± 0.71 | 31.00 ± 6.48 |
| Autogamy | 22.56 ± 2.97 | 31.25 ± 7.18 | 35.47 ± 5.04 | 5.00 ± 2.45 | 11.10 ± 6.76 | 1.19 ± 0.92 | 4.00 ± 2.29 |
| Apomixis | 47.56 ± 10.96 | 38.17 ± 6.48 | 46.92 ± 9.10 | 7.00 ± 3.92 | 11.20 ± 7.04 | 1.18 ± 0.89 | 5.00 ± 2.45 |
Table 3: Results of ANOVA for fruit parameters resulting from different pollination treatments.
| Fruit parameters | Treatments | Sum of Squares | df | Mean Square | F | Sig. |
| Between Treatments | 6538.832 | 4 | 1634.708 | |||
| Avg. Weight (g) | Within Treatments | 1660.593 | 45 | 36.902 | 44.299 | *** |
| Total | 8199.425 | 49 | ||||
| Between Treatments | 573.387 | 4 | 143.347 | |||
| Avg. Height (mm) | Within Treatments | 1393.755 | 45 | 30.972 | 4.628 | ** |
| Total | 1967.142 | 49 | ||||
| Between Treatments | 1227.578 | 4 | 306.895 | |||
| Avg. Diameter (mm) | Within Treatments | 1591.207 | 45 | 35.360 | 8.679 | *** |
| Total | 2818.785 | 49 | ||||
| Between Treatments | 308.000 | 4 | 77.000 | |||
| No. of seeds | Within Treatments | 760.000 | 45 | 16.889 | 4.559 | ** |
| Total | 1068.000 | 49 | ||||
| Between Treatments | 4.120 | 4 | 1.030 | |||
| TSS (°Brix) | Within Treatments | 1362.600 | 45 | 30.280 | 0.034 | ns |
| Total | 1366.720 | 49 | ||||
| Between Treatments | 0.295 | 4 | 0.074 | |||
| Acidity | Within Treatments | 26.487 | 45 | 0.589 | 0.125 | ns |
| Total | 26.782 | 49 | ||||
| Between Treatments | 13140.000 | 4 | 3285.000 | |||
| Fruit set (%) | Within Treatments | 688.500 | 45 | 15.300 | 214.706 | *** |
| Total | 13828.500 | 49 | ||||
| * p < 0.05; ** p < 0.01; *** p < 0.001; ns = non-significant | ||||||
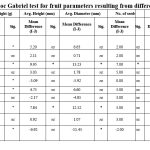 |
Table 4: Results of Post-hoc Gabriel test for fruit parameters resulting from different pollination treatments. |
Discussion
The current study’s findings unequivocally show that the mandarin variety under investigation is dependent on pollinators for successful reproduction and is only partially self-compatible. The asynchronous flowering pattern that mandarins have adapted, which promotes genetic variety within the population and helps guarantee cross-pollination, is responsible for this reliance on pollinators. Flowering phenology is recognized as a crucial factor influencing pollination success,40 with asynchronous flowering being advantageous for rewardless flowers to achieve successful pollination through deceit.41 Throughout the flowering season, a constant flow of pollinator visits is sustained due to asynchronous flowering. Asynchronous flowering has been shown to enhance cross-pollination,26 decrease intraspecific competition for pollinators, and reduce effective population density.42,43 The anthers dehisce asynchronously to compensate for the gradual decrease in pollen fertility. Throughout the flowering season, this method guarantees that there will be a enough quantity of viable pollen grains available for cross-pollination. Plants can sustain a consistent supply of viable pollen by releasing pollen at different times, which improves the likelihood of successful fertilization and reproduction.2 A comparable decrease in pollen fertility over time has been documented in Shogun (C. reticulata) in southern Thailand.44
In contrast to flowers that were bagged after anthesis and left untreated, as well as flowers that were emasculated and bagged, the study showed that open-pollinated, geitonogamous self-pollinated, and xenogamous pollinated flowers produced more fruits. These results underline the vital role of pollinators by strongly implying that the mandarin type under investigation depends on pollen from other blossoms. This supports finding that pollination is necessary for the reproduction of mandarins and their hybrids, as they are self-incompatible.45 Additionally, the data showed that autogamous and open-pollinated fruits differed significantly in diameter and number of seeds, highlighting the significance of cross-pollination for the best possible fruit development. Our results align with the breeding system experiments conducted on Kinnow mandarin.46 Similarly, honeybee pollination has been shown to enhance both the quantity and quality of sweet orange (Citrus sinensis), resulting in heavier, less acidic fruits.47
Our findings demonstrated that social bees belonging to the Apidae family, specifically Apis cerana, are the most prevalent and genuine pollinators of mandarin. A. cerana was reported to be the most common pollinator among the 24 insect species that were reported15. The activity of A. cerana was higher on sunny days, and regression analysis indicated that temperature was a significant predictor of its abundance.48 Similar findings have been reported where the abundance of A. cerana rose during sunny days.15
A. cerana exhibited some pollination-helpful behaviours, like landing on flowers and moving in circles to make sure its abdomen made contact with the stigma. Similar behavioural observations have been made in mandarin orchards in Nepal and India, where A. cerana outperformed A. mellifera in pollination efficiency.15,49 A. cerana also inserted its proboscis into the nectaries and rubbed its belly and thorax against the stigma. This conduct increases the likelihood of successful fertilization by ensuring sufficient pollen deposition on the stigma when paired with frequent visits to the same flower. A. cerana, along with A. dorsata, A. florea, and A. mellifera, has been reported as a pollinator of mandarin oranges in Nepal.49 The pollination efficiency (PE = 0.52) and lack of pollen limitation (0.30) indicate that A. cerana is an important pollinator in a successful fruit set in mandarin oranges. Similar findings have been reported in sweet orange (C. sinensis) where A. cerana contributed to increased seed production and fruit weight.45,46
Ecological and Economic Implications
From an ecological perspective, maintaining pollinator diversity is crucial to reducing pollination deficits and increasing yield.50 Interestingly, while our study found that pollination treatments significantly influenced physical traits like fruit weight and diameter, intrinsic biochemical properties such as total soluble solids (TSS) and acidity remained unaffected. This suggests that effective pollination increases fruit quality yields such as size and weight51 without compromising fruit taste, which is consistent with findings that pollination improves quantitative traits without changing the chemical composition of the fruit.47
Effective pollination has significant economic benefits. Around the world, pollination by flies, honey bees, and native bees boosts agricultural productivity by $235 to $577 billion (USD) a year. A realistic estimate for pollination services in Nepal is $477 million (USD) per year, or 9% of overall agricultural income.52 These numbers highlight how economically necessary it is to incorporate pollination management into farming methods. Since mandarins are partially self-compatible targeted pollination techniques, like protecting natural habitats and encouraging the activity of pollinators like A. cerana, can result in notable increases in fruit yield for mandarin growers in the eastern Himalayas.
Conclusion
In conclusion mandarin (Citrus reticulata Blanco) is a partially self-compatible species based on our study of its pollination biology and breeding system in the eastern Himalaya, India. Asynchronous flower opening promotes open pollination, cross-pollination, and geitonogamous self-pollination by facilitating the transmission of geitonogamous and cross-pollen. Higher fruit set and better fruit quality are results of these processes. The absence of pollen constraint demonstrated the effectiveness of Apis cerana, which emerged as the main pollinator. These results demonstrate that pollinators are essential to mandarins’ higher yield and higher-quality fruit. However, mandarin is a highly heterogenous group and pollination in mandarin is observed to be highly dynamic. Therefore, similar kinds of study should be conducted in other parts of the Himalayas to get a comprehensive understanding of the pollination requirements of mandarin.
Acknowledgement
The authors are highly grateful to Department of Botany, Sikkim University and Sikkim Alpine University for providing lab facilities. The authors are highly indebted to Mr. Tenzing Gyatsho Lepcha for allowing the authors to conduct their studies in his orchard.
Funding Source
The author(s) received no financial support for the research, authorship, and/or publication of this article.
Conflict of Interest
The authors do not have any conflict of interest.
Data Availability Statement
The manuscript incorporates all datasets produced or examined throughout this research study.
Ethics Statement
This research did not involve human participants, animal subjects, or any material that requires ethical approval.
Author contributions
Subhankar Gurung and Arun Chettri: designed the research.
Subhankar Gurung, Arunika Subba and Aita Rani Subba: conducted the experiment.
Aditya Moktan Tamang: analyzed the data.
Subhankar Gurung: wrote the manuscript.
References
- McGregor S.E. Insect pollination of cultivated crop plants. Agric Res Serv, US Dept Agric. 1976;(Agricultural Handbook No. 496).
- Klein A.M., Vaissière B.E., Cane J.H., Steffan-Dewenter I., Cunningham S.A., Kremen C., Tscharntke T. Importance of pollinators in changing landscapes for world crops. Proc R Soc B Biol Sci. 2007;274(1608):303-313.
CrossRef - Roubik D.W. Pollination of cultivated plants in the tropics. FAO Agric Serv Bull. 1995;118:196.
- Williams I.H. The dependence of crop production within the European Union on pollination by honey bees. Agric Sci Rev. 1994;6:229-257.
- Partap U. Pollination management of mountain crops through beekeeping. Trainers’ Resource Book.
CrossRef - Chandel R.S., Thakur R.K., Bhardwaj N.R., Pathania N. Onion seed crop pollination: a missing dimension in mountain horticulture. XXVI Int Hortic Congr: Issues Adv Transplant Prod Stand Establish Res. 2002;631:79-86.
CrossRef - Sinu P.A., Shivanna K.R. Pollination biology of large cardamom (Amomum subulatum). Curr Sci. 2007;92(4):548-552.
- Sinu P.A., Shivanna K.R. Pollination ecology of cardamom (Elettaria cardamomum) in the Western Ghats, India. J Trop Ecol. 2007;23(4):493-496.
CrossRef - Verma S.K. Preliminary studies on the effect of honey bees on the yield of greater cardamom. 1987;25-26.
- Gaira K.S., Rawal R.S., Singh K.K. Variations in pollinator density and impacts on large cardamom (Amomum subulatum) crop yield in Sikkim Himalaya, India. J Asia-Pac Biodivers. 2016;9(1):17-21.
CrossRef - Krishnan S., Kushalappa C.G., Shaanker R.U., Ghazoul J. Status of pollinators and their efficiency in coffee fruit set in a fragmented landscape mosaic in South India. Basic Appl Ecol. 2012;13(3):277-285.
CrossRef - Stern R., Eisikowitch D., Dag A. Sequential introduction of honeybee colonies and doubling their density increases cross-pollination, fruit-set and yield in ‘Red Delicious’ apple. J Hortic Sci Biotechnol. 2001;76(1):17-23.
CrossRef - Ladurner E., Recla L., Wolf M., Zelger R., Burgio G. Osmia cornuta (Hymenoptera Megachilidae) densities required for apple pollination: a cage study. J Apic Res. 2004;43(3):118-122.
CrossRef - Lepcha B., Avasthe R., Singh R., Phukan P., Singh N.J. Enhancing livelihood of tribal farmers of Sikkim through integrated organic farming system: a case study. Indian Res J Extens Educ. 2018;18:84-87.
- Pradhan U., Devy M.S. Pollinators of Sikkim mandarin orange (Citrus reticulata) (Sapindales: Rutaceae). J Threat Taxa. 2019;11(5):13625-13628.
CrossRef - Biswas K.K., Choudhuri S.P., Godara S. Decline of mandarin orange caused by Citrus tristeza virus in Northeast India: conventional and biotechnological management approaches. J Agric Eng Food Technol. 2016;3(3):236-241.
- Gurung N., Sarkar S., Allay S., Meena R., Singh B. Problems and future prospects of Darjeeling mandarin in Darjeeling and Sikkim hills: a review. J Agroecol Nat Resour Manag. 2017;4(3):228-231.
- Nicolosi E, La Malfa S, El-Otmani M, Negbi M, Goldschmidt EE. The search for the authentic citron (Citrus medica): historic and genetic analysis. HortScience. 2005;40(7):1963-1968.
CrossRef - Colunga‐GarcíaMarín P., Estrada‐Loera E., May‐Pat F. Patterns of morphological variation, diversity, and domestication of wild and cultivated populations of Agave in Yucatan, Mexico. Am J Bot. 1996;83(8):1069-1082.
CrossRef - Casas A., Valiente‐Banuet A., Rojas‐Martínez A., Dávila P. Reproductive biology and the process of domestication of the columnar cactus Stenocereus stellatus in Central Mexico. Am J Bot. 1999;86(4):534-542.
CrossRef - Casas A., Otero-Arnaiz A., Perez-Negron E., Valiente-Banuet A. In situ management and domestication of plants in Mesoamerica. Ann Bot. 2007;100(5):1101-1115.
CrossRef - Deng Z.N., Gentile A., Nicolosi E., Domina F., Vardi A., Tribulato E. Identification of in vivo and in vitro lemon mutants by RAPD markers. J Hortic Sci. 1995;70(1):117-125.
CrossRef - Webber H.J., Batchelor L.D. The Citrus Industry. Vol. 1. University of California Press; 1979.
- Glukhov M.M. Honey Plants. 6th ed. Moskva, Gos. Izd-vo Selkhoz Lit-ry; 1955:512.
- Wafa A.K., Ibrahim S.H. Effect of the honeybee as a pollinating agent on the yield of orange. 1960;Jan/Feb.
- Frankie G.W., Haber W.A. Why bees move among mass-flowering neotropical trees. 1983:360-372.
- Iglesias D.J., Cercós M., Colmenero-Flores J.M., et al. Physiology of citrus fruiting. Braz J Plant Physiol. 2007;19:333-362.
CrossRef - Baldwin E.A. Citrus fruit. In: Biochemistry of Fruit Ripening. Dordrecht: Springer Netherlands; 1993:107-149.
CrossRef - Davies F.S., Albrigo L.G., C.A.B. Citrus. International. Wallingford, UK; 1994.
- Oppenheimer H.R. Experiments with unfruitful “Clementine” mandarins in Palestine. 1948:63.
- Hearn C.J., Reece P.C. Pollination needs of Page, Lee, Nova and Robinson citrus hybrids. 1967:19670307745.
- Robinson F.A., Krezdorn A.H. Factors affecting the unfruitfulness of tangelos. 1959:102-207.
- Miwa T. On pollination, fertilization phenomena and problems connected with fruit drop in the Hyuganatsu mandarin. 1951:2.
- Shivanna K.R., Tandon R. Reproductive Ecology of Flowering Plants: A Manual. New Delhi: Springer India;
CrossRef - Zapata T.R., Arroyo M.T.K. Plant reproductive ecology of a secondary deciduous tropical forest in Venezuela. Biotropica. 1978;221-230.
CrossRef - Tur C., Castro-Urgal R., Traveset A. Linking plant specialization to dependence in interactions for seed set in pollination networks. PLoS ONE. 2013;8(10).
CrossRef - Spears Jr E.E. A direct measure of pollinator effectiveness. Oecologia. 1983;196-199.
CrossRef - Larson B.M.H., Barrett S.C.H. A comparative analysis of pollen limitation in flowering plants. Biol J Linn Soc. 2000;69(4):503-520.
CrossRef - Descriptors for Citrus. Rome, Italy: Int Plant Genet Resour Inst. 1999:66.
- Elzinga J.A., Atlan A., Biere A., Gigord L., Weis A.E., Bernasconi G. Time after time: flowering phenology and biotic interactions. Trends Ecol Evol. 2007;22(8):432-439.
CrossRef - Gurung S., Pradhan A., Chettri A. Pollination in an endemic and threatened monoecious herb Begonia satrapis CB Clarke (Begoniaceae) in the eastern Himalaya, India. J Threat Taxa. 2019;11(10):14328-14333.
CrossRef - Bawa K.S. The reproductive biology of Cupania guatemalensis Radlk. (Sapindaceae). Evolution. 1977;31(1):52-63.
CrossRef - Primack R.B. Variation in the phenology of natural populations of montane shrubs in New Zealand. J Ecol.1980;849-862.
CrossRef - Chelong I., Sdoodee S. Pollen viability, pollen germination and pollen tube growth of shogun (Citrus reticulata Blanco) under climate variability in southern Thailand. 2012;2297-2307.
- Sanford M.T. Pollination of citrus by honey bees. University of Florida Cooperative Extension Service, Inst Food Agric Sci. EDIS;
- Manzoor-ul-Haq, Manzoor-ul-Haq, Rafie-ul-Din M., Ghaffar A. Effect of insect pollination on fruit bearing in Kinnow mandarin (Citrus reticulata), and physical and chemical properties of the fruit. 1978;47-49.
CrossRef - Malerbo-Souza D.T., Nogueira-Couto R.H., Couto L.A. Honey bee attractants and pollination in sweet orange, Citrus sinensis (L.) Osbeck, var. Pera-Rio. J Venom Anim Toxins Incl Trop Dis. 2004;10:144-153.
CrossRef - Gaira K.S., Rawal R.S., Singh K.K. Variations in pollinator density and impacts on large cardamom (Amomum subulatum) crop yield in Sikkim Himalaya, India. J Asia Pac Biodivers. 2016;9(1):17-21.
CrossRef
- A report: Cash Crop Farming in Nepal: The importance of pollinator diversity and managed pollination in Citrus. International Center for Integrated Mountain Development; 2003. 49pp.
- Pandey R., Sharma S., Thapa R, et al. Assessing the economic and nutritional value of pollination services in Nepal. Sci Rep. 2024;14:75584. doi:10.1038/s41598-024-75584-x.
CrossRef - Gogoi B., Rahman A. Foraging behavior and effect of Apis cerana pollination on fruit set and yield of Assam lemon (Citrus limon). Indian J Agric Sci. 2007;77(2):120-122.

