Introduction
Chrysanthemum is a prominent ornamental crop which is one of the most commercially important flowers but is highly perishable and vulnerable to post-harvest losses. In commercial markets, the use of chemicals as hydrating solutions in chrysanthemum is common, which is costly and synthetically produced. Concerning its toxicity, there are potential floral preservatives that can be used for chrysanthemum. The chrysanthemum flower technically known as mums, is an important ornamental member of the Asteraceae family that represents as flowers with medicinal value cultivated in the world during the most ancient period.1 It is the second to the rose in terms on the commercial flower industry.2 In the world, there are numerous Chrysanthemum cultivars grown with distinct variations in terms of its popularity and morphology. This ornamental crop is not only praised globally by its economic value but also has a different aesthetic feature with vibrant color and structure with acclaimed therapeutic and medicinal values. In addition, it has a significant role in cosmetic and culinary industries.3,4 Moreover, various genus of this commodity also recognized for its functional impact in the environment that purifies air by capturing air pollutants under indoor condition.5 Currently, the chrysanthemum cut flowers value is economically as second in world next to the rose.6 According to Dirjen Hortikultura7, high value of chrysanthemum must meet the following conditions such as flower stems are strong and long, smooth, clean and beautiful with no stain, can last long after being cut, with little to no damaged in packing, free of diseases or harmful microorganisms with green and fresh leaves.
Water uptake is one of the key factor processes in enhancing shelf life of cut flowers and factors that influence the hydration after harvest. Water is drawn upward as the leaves transpire. In this biochemical process, it is accelerated to impeded by vascular blockage which led to increased opening of the stomates. As transpiration exceeds the water uptake, water deficiency will occur.8 In horticultural products, cut flowers have the great and many potentials to be developed. One of the first cutflowers in the global market which is commonly used as decoration is the Chrysanthemum.9,10 To be accepted in the market, great efforts to sustain the quality traits and extension shelf-life quality of chrysanthemum cut flower is very essential.11,12 Chrysanthemum vase-life is an important parameter for evaluating its physical characteristics which is an important aspect for domestic and export markets.13,14 There are several techniques of extending the vase life of this type of crop which is as important asset for both famers and consumers.15
Valuable characteristics among the various chrysanthemum species determined the differences in terms of commercial value, consumers preferences and quality.16 In the vase life of chrysanthemum, one of its main problems is the yellowing of leaves and easy wilting of petals that causes the failure of flowers to open.17-19 Studies revealed that there are some organic based acids that can extend the longevity and postharvest of cut flowers, this, potential hydrating acids are sources of energy and carbon for cells and are utilized for some biochemical and respiratory cycle pathways that give an important and crucial role in the development and growth of the plant particularly on the transport of electrons.20- 22
The huge demand for flowers crops around the world is high, particularly used in festive celebrations and occasions where the farmers or growers are made force to produce more in less span. In every phase of production, ornamental crops, it is regulated strictly to make sure that the quality of fresh produced flowers are hugely relied on high and extensive applications of agrochemicals with detrimental impact in ornamental industries. Consumers and end users typically wanted their flower crops to be fragrant and colorful, but they are often applied with chemicals. In the phase of postharvest, cut flowers are bombarded with several chemicals in order to maintain freshness and quality including anti-ethylene compounds, acidifying chemical agents, including the various antimicrobial compounds, carbohydrate sources and plant regulators. Many chemicals were observed in cutflower bouquets that could lead to serious health implications and are highly dangerous to be used. Nowadays, techniques in the vase life extension of cut flowers have already been explored and identified; by using the acid based hydrating solutions, it causes the increase of xylem vessel water conductance in cut flower and bacterial population at the same time.8
Other acid-based solutions used as hydrating solutions in cutflowers were found to form iron which has an important function in the transportation of nutrients, that includes reduction in water pH and bacterial proliferation which causes the blocking of xylem vessels in the basal cut portion of flowers.21,23,24 On the other hand, the effect of citric acid on enhancing the shelf life of some cutflowers were significantly observed to have a positive effect which was observed in tuberose and lilium species as reported by Eidyan,25 And Darandeh and Hadavi.21
Materials and Methods
The materials used in the study are containers used in chrysanthemum cut flowers, acid based hydrating solutions used as treatments such as (control- water, coconut vinegar, sugar cane vinegar, apple cider, banana vinegar and calamondin with all dilution rate of 10ml/L of water. Digital weighing scale was also used to measure the weight loss of samples from the start up to the end of the study. A record book was also used to record all the data parameters evaluated in the study such as: Visual Quality Rating: (9- excellent; field fresh; no defects; 7- good; minor defects; 5- fair; moderate defects; limit of marketability; 3- poor; serious defects; limit of fitness for decoration; 1- unfit for decoration); Discoloration (0- no discoloration; 1- 1-25% discoloration; 2- 26-50% discoloration; 3- above 50% discoloration); Wilting (0-no wilting, 2- 1-25% wilting, 3- 26-50% wilting, 4- above 50% wilting); Neck bending (0- no bending, 1- 1-25% bending, 2- 26-50% bending, 3- above 50% bending); Vase life (9.0- excellent; field fresh; no defects, 7.0-8.99- good; minor defects,5.0-6.99- fair; moderate defects; limit of Marketability, 3.0-4.99- poor; serious defects; limit of marketability, 1.0-2.99- unfit for decoration).26,27
Sources and Selection of Samples
Chrysanthemum cut flowers were procured from Brgy. Kapatagan, Digos Davao Del Sur, Philippines with an elevation of 1,907.7 meters. Chrysanthemum cut flowers are graded based on their flower appearance, stem straightness, color, freshness and number of flowers. Flowers are harvested at 3 months old with fully opened flowers. Criteria for selection for good quality cut flowers are as follows: (1) good quality samples; (2) disease free flowers; (3) 12 inches long with 4-5 number of leaves; and (4) half bloom (50% bloom) commercial maturity stage at harvest.
Site of the Experiment
The experiment was conducted at the Postharvest Horticulture Laboratory located at the College of Agriculture, Division of Horticulture, University of Southern Mindanao, Kabacan, North Cotabato, Philippines from August to September 2024. The sample cut flowers were carefully brought to experimental area, there were a total of 10 sample cut flowers used weighing around 170-200 grams per experimental unit. The transparent vases used in the experiment were sanitized by washing in boiling water to avoid contamination of hydrating solutions. Disinfecting the materials kills potential contaminants such as bacteria and pathogens that remain on the surfaces.
Preparation of treatments (acid based-hydrating solutions)
Preparation of various acid based-hydrating solutions were carefully selected and procured from a registered agri-supply market, hydrating solutions with diluted rate were as follows: control- pure distilled water; coconut vinegar (10ml/L of water), sugarcane vinegar (10 ml/L of water), apple cider vinegar (10 ml/L of water), banana vinegar (10 ml/L of water), and calamondin (10 ml/ L of water). The identified acid -based hydrating solutions were diluted in a 1-liter distilled water. Each vase was filled with 500 ml diluted solutions based on assigned treatment per experimental lot samples. After filling the vase, fresh harvested cut flowers (chrysanthemum) were immediately placed in various hydrating solutions for vase life evaluation study. Regular monitoring was investigated based on the morphological and qualitative characteristics being evaluated from the start up to reaching the limit of marketability of all the samples.
Data Analysis and Postharvest Quality Parameters
The significant differences were analyzed using the Likert Scale Test with the data parameters evaluated using the following rating scale with verbal description as follows: Visual Quality Rating: (9- excellent; field fresh; no defects; 7- good; minor defects; 5- fair; moderate defects; limit of marketability; 3- poor; serious defects; limit of fitness for decoration; 1- unfit for decoration); Discoloration (0- no discoloration; 1- 1-25% discoloration; 2- 26-50% discoloration; 3- above 50% discoloration); Wilting (0-no wilting, 2- 1-25% wilting, 3- 26-50% wilting, 4- above 50% wilting); Neck bending (0- no bending, 1- 1-25% bending, 2- 26-50% bending, 3- above 50% bending); Vase life (9.0- excellent; field fresh; no defects, 7.0-8.99- good; minor defects,5.0-6.99- fair; moderate defects; limit of Marketability, 3.0-4.99- poor; serious defects; limit of marketability, 1.0-2.99- unfit for decoration). Lot samples per treatment were evaluated starting from day 1 upto 9 days of vase life storage. The percentage weight loss of samples were also gathered at 2 days using Statistical Tool for Agricultural Research (STAR) software version 2.0 at a 5% level of significance. The differences among treatment means were determined using Tukey’s honest significant difference (Tukey’s HSD).

Results
Vase Life Evaluation Parameters
In terms of the visual quality rating and storage vase life as shown in Fig. 1 and Fig. 5, chrysanthemum cut flowers subjected to calamondin extract (10 ml/1 L of water), coconut vinegar (10 ml/L of water) and sugar cane vinegar (10 ml/L of water) as hydrating solutions obtained the longest day where it reached VQR 3 (limit of marketability) at 10 days of vase life storage, while chrysanthemum cut flowers treated with no application (control), apple cider vinegar (10 ml/L ot water) and banana vinegar (10 ml/ L of water) observed to have the shortest days where it reached VQR 3 (limit of marketability) at 7 days of vase life storage. Moreover, it was also investigated that samples subjected to treatment control (without application), apple cider vinegar, coconut vinegar and banana vinegar resulted in attaining VQR 2 that verbally described with poor and serious defects at 10 days of vase life storage compared to chrysanthemum cut flowers treated with calamondin extract and sugarcane vinegar where most of lot samples retained its marketable features (VQR 3-limit of marketability).
Other parameters observed such as wilting (Fig. 2), discoloration (Fig. 3) and neck bending (Fig. 4) of chrysanthemum cut flowers subjected to various hydrating solutions using calamondin extract (10 ml/L of water) and sugar cane vinegar (10 ml/L of water) hydrating solution for chrysanthemum cut flowers showed slowing deterioration of samples where it reached 26-50% wilting, discoloration and bending at 9 days of vase life storage compared to other cut flowers subjected to without application (control) banana vinegar (10 ml/L of water), coconut vinegar (10 ml/L of water), apple cider vinegar (10 ml/L of water) where it immediately reached 50% above discoloration, wilting and neck bending at 7 days of vase life storage. The results indicate that the application of calamondin extract, and sugar cane vinegar diluted in 1 L of distilled water showed promising results in delaying the senescence and extending the shelf life of chrysanthemum cut flowers based on the results of the data evaluated.
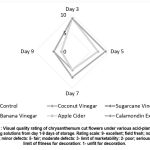 |
Figure 1: Visual quality rating of chrysanthemum cut flowers under various acid-plant based hydrating solutions from day 1-9 days of storage. |
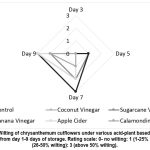 |
Figure 2: Wilting of chrysanthemum cutflowers under various acid-plant based hydrating solutions from day 1-9 days of storage. Rating scale: 0- no wilting: 1 (1-25% wilting): 2 (26-50% wilting): 3 (above 50% wilting). |
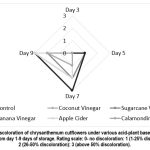 |
Figure 3: Discoloration of chrysanthemum cutflowers under various acid-plant based hydrating solutions from day 1-9 days of storage. Rating scale: 0- no discoloration: 1 (1-25% discoloration): 2 (26-50% discoloration): 3 (above 50% discoloration). |
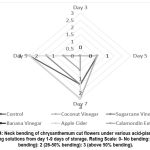 |
Figure 4: Neck bending of chrysanthemum cut flowers under various acid-plant based hydrating solutions from day 1-9 days of storage. Rating Scale: 0- No bending: 1 (1-25% bending): 2 (26-50% bending): 3 (above 50% bending). |
Percentage (%) Weight Loss
In terms of the percentage weight loss evaluated as shown in Table 1, cut flowers subjected to pure water without the addition of acid plant based exact obtained the highest weight loss (8.67%-day 3, 35.57%-day 5, 60.99%-day 7 and 70.12%-day 9) which were consistent until the last day of vase life storage (Day 9). On the among various hydrating solutions evaluated, the least percentage weight loss (1.44%-day 3, 30.37%-day 5, 54.67%-day 7 and 60.23%-day 9) were consistently observed to cut flowers subjected to calamondin extract (10 ml/L of water). The results indicated that the use of calamondin extract diluted in water as hydrating solution can prolong the vase-life and slow the deterioration process of chrysanthemum cut flowers.
Table 1. Percentage (%) weight loss of chrysanthemum cut flowers under various acid-plant based hydrating solutions from day 1-9 days of storage.
| Acid-plant based hydrating solutions | Days of Storage | ||||
| Day 1ns | Day 3* | Day 5* | Day 7** | Day 9** | |
| Control (w/out application) | 0 | 8.67a | 35.37a | 60.99a | 70.12a |
| Coconut vinegar | 0 | 2.32b | 32.70ab | 57.57a | 67.32ab |
| Sugarcane vinegar | 0 | 1.59c | 31.81ab | 57.42a | 66.42b |
| Banana vinegar | 0 | 3.24b | 36.01a | 60.24a | 71.23a |
| Apple cider vinegar | 0 | 2.44ab | 37.99a | 59.24a | 68.73ab |
| Calamondin extract | 0 | 1.44c | 30.37c | 54.67b | 60.23c |
| CV | – | 19.3 | 12.59 | 14.60 | 60.23 |
CV = coefficient of variation; ns = non-significant; * = significant (p < 0.05); ** = highly significant (p < 0.05) different.
Discussion
Vase Life Evaluation
According to the results of the study, several implications that cause the reduction and deterioration of crops are due to lipid oxidation and bacterial growth.28,29 In cut flowers after harvest, increasing the vase life and delaying its senescence process is very important.30 Hence, with the potential of using acid based hydrating solutions (calamondin extract and sugar cane vinegar) in chrysanthemum cut flowers, it can be an easy, cheap and more environment friendly and an organic alternative hydrating solution enhancing its shelf life and postharvest quality. This correlates to studies of Bauters et al.,31 that explains endogenous acid-based hormones found in most plants play a systematic and important function resistance against pathogens and in stresses acclimation which are common in some acid plant-base extracts like in citrus. The freshness of cut flower chrysanthemum is very independent after it is harvested particularly on post-harvest handling and preservations, based on the results of Lintang and Payuk32 and Lintang and Payuk,33 it is shown in their research that the use of hydrating solutions is a factor in producing quality of flowers (varieties of Mustika Kania, Puspita Pelangi and Puspita Nusantara) in terms of the parameters that included diameter, number of half-bloomed flowers, number of florets, and number of blooming flowers.
In addition, Kumar et al.,16 also stated that this hormone is known to delay the ethylene production by suppressing the activities of ACC oxidase and 1-aminocyclopropane-1 carboxylate (ACC) synthase that are known to delay the process of senescence. Moreover, Ramezanian et al.,34 supported that application of naturally occurring substances such as plant-based extracts and oil has been proven control measures in controlling the different bacterial agents and other fungal microorganisms that caused deterioration and postharvest loss of crops like fruits and ornamental cut flowers. According to the study of Van Doorn and Dhorn,8 he emancipated that the contributing factor of cut flower deterioration is due to the limited absorption of the water due to the stem end blockage caused by tyloses, air embolism and bacterial growth.
There was another research that indicated that natural substances or compounds can be an alternative used for chemicals in cut flowers. Mehraj et al.,35 also observed that utilizing citrus extract with sucrose, caused increasing life of cut snowball flowers. Furthermore, some organic acids found in plants have an significant role in prolonging the shelf-life of cut-flowers which are good sources of energy of cells and carbon are used in the biochemical and respiratory cycle pathway within the plant system.20,21
In addition, the ascorbic acid found in citrus that can enhance the postharvest life of cut flower has an important role in plant growth and development particularly in the transport of the electrons.22 It is also associated with some biological activities in plants such as electron transporter, enzyme co-factor and antioxidant at plasma membrane or in the chloroplast.36
The results and findings of the study revealed that the different acid based hydrating solutions significantly affected the vase-life of chrysanthemum cut flowers during 9 days of storage. Among the data parameters evaluated, calamondin extract and sugar cane vinegar diluted in water (10 ml/ L of water) in hydrating solutions were potentially observed in slowing down the process of senescence of harvested cut flowers resulting in longer retention of marketable value and good qualitative traits under vase-life storage. Moreover, in terms of the percentage weight loss, chrysanthemum cut flowers subjected to calamondin hydrating solution observed to have the least percentage weight loss compared to other treatments. With all the correlating studies and results of the experiment, it can be recommended to use calamondin extract and sugar cane vinegar as hydrating based water-solution for prolonging the vase-life of chrysanthemum cut flowers. Moreover, identified acid-plant based hydrating solutions (calamondin extract) can be an alternative solution to be used compared to using other chemical hydrating solutions for extending the vase-life of cut flowers. Furthermore, the results of the study will benefit the chrysanthemum growers, enthusiasts, wholesalers and retailers and other researchers that would like to validate the effects of these acid-plant based hydrating solutions in other cut flowers.
Conclusion and Recommendations
The results and findings of the study revealed that the different acid based hydrating solutions significantly affected the vase-life of chrysanthemum cut flowers during 9 days of storage. Among the data parameters evaluated, calamondin extract and sugar cane vinegar diluted in water (10 ml/ L of water) in hydrating solutions were potentially observed in slowing down the process of senescence of harvested cut flowers resulting in longer retention of marketable value and good qualitative traits under vase-life storage. Moreover, in terms of the percentage weight loss, chrysanthemum cut flowers subjected to calamondin hydrating solution observed to have the least percentage weight loss compared to other treatments. With all the correlating studies and results of the experiment, it can be recommended to use calamondin extract and sugar cane vinegar as hydrating based water-solution for prolonging the vase-life of chrysanthemum cut flowers. Moreover, identified acid-plant based hydrating solutions (calamondin extract) can be an alternative solution to be used compared to using other chemical hydrating solutions for extending the vase-life of cut flowers. Furthermore, the results of the study will benefit the chrysanthemum growers, enthusiasts, wholesalers and retailers and other researchers that would like to validate the effects of these acid-plant based hydrating solutions in other cut flowers.
Acknowledgement
The primary author would like to extend her gratitude to her subject professors as my co-authors Dr. Mark Al-jamie Muttulani and Dr. Lorelyn Joy N. Turnos-Milagrosa for the unwavering support and guidance throughout the conduct of this research.
Funding Sources
The primary author would like to thank DOST-SEI Scholarship for the financial support to pursue her MS Horticulture Program at Graduate School, University of Southern Mindanao, Philippines
Conflict of Interest
The authors do not have any conflict of interest.
Data Availability Assessment
This statement does not apply to this article
Ethics Statement
This research did not involve human participants, animal subjects, or any material that requires ethical approval
Author Contributions
Riza D. Meperanum- Paper writing, data collection, analysis and interpretation.
Mark Al-jamie J. Muttulani- Paper statistical data review and editing, supervision of data collection.
Lorelyn Joy Turnos-Milagrosa- Paper grammar review and editing, supervision of data collection.
References
- De, L.C. and Bhattacharjee, S. K. Ornamental Crop Breeding Aavishkar Publishers, Distributors,, Jaipur India. 2011. Google Scholar.
- Kalia, R. Effect of different concentrations of auxins on the regeneration of Chrysanthemum morifolium plantlets. J. Tec. Res. Appl., 3. 2015, pp. 106-107. Crossref Google Scholar
- Chae, S. C. An up-to-date review of phytochemicals and biological activities in Chrysanthemum spp. Biotechnol. Res. Asia, 13. 2016, pp. 615-623
CrossRef - Zhang, X. , Xie, Y.L. , Yu, X. T., Su, Z. Q., Yuan, J., Li, Y. C., Su, Z. R., Zhan, J. Y. X. Lai, X. P. Protective effect of super-critical carbon dioxide fluid extract from flowers and buds of Chrysanthemum indicum linnen against ultraviolet-induced photo-aging in mice Rejuvenation Res., 18. 2015, pp. 437-448
CrossRef - Irga P., Pettit T., Torpy F. The phytoremediation of indoor air pollution: a review on the technology development from the potted plant through to functional green wall biofilters Environ. Sci. Bio Technol., 17. 2018, pp. 395-415
CrossRef - Hidayah, A. Iis Nur, A., Sulifah A. P. Pengaruh Rebusan Daun Sirih (Piper betle) pada Larutan Perendam terhadap Kesegaran Bunga Potong Krisan (Chrysanthemum indicum L.) dan Pemanfaatannya sebagai Karya Ilmiah Populer. (The effect of Piper betle Leaf Stew in Holding Solution for Chrysanthemum indicum L. Cut Flowers Vase Life and Its Utilization as Popular Scientific Work). UNEJ JURNAL. 2012, I (1): 1-5.
- Dirjen Hortikultura, (2012). Standar Operasional Prosedur (Sop) Pascapanen Bunga Krisan Potong (Dendranthema grandiflora Tzvelev).
- van Doorn, W.G. Water relations of cut flowers. Horticulture Review, 1997. 18: 1-85.
CrossRef - Boukhebti H., Demirtas I., Omar L., and Chaker A. N., Chemical composition, antibacterial activity of essential oil and anatomical study of Chrysanthemum morifolium, Journal of Drug Delivery and Therapeutics. 2020 10, no. 2-s, 7–13, https://doi.org/10.22270/jddt.v10i2-s.4015.
CrossRef - Dole J. M. and Wilkins H. F., Bedding plants: JM dole and HF wilkins, Floriculture Principles and Species. 2005. second edition, Pearson-Prentice Hall, Upper Saddle River, NJ, USA, 916–917.
- Macnish A. J., Leonard R. T., and Nell T. A., Treatment with chlorine dioxide extends the vase life of selected cut flowers, Postharvest Biology and Technology. 2008. 50, no. 2-3, 197–207, https://doi.org/10.1016/j.postharvbio.2008.04.008, 2-s2.0-52949123237.
CrossRef - Redman P. B., Dole J. M., Maness N. O., and Anderson J. A., Postharvest handling of nine specialty cut flower species, Scientia Horticulturae. 2002. 92, no. 3-4, 293–303, https://doi.org/10.1016/s0304-4238(01)00294-1, 2-s2.0-0037074893.
CrossRef - Alkaç O. S., Belgüzar S., and Güneş M., Effects of organic acids, chemical treatments and herbal essential oils on the vase life of cut carnation (Dianthus caryophyllus) flowers, Emirates Journal of Food and Agriculture. 2023. 35, no. 4, 332–341, https://doi.org/10.9755/ejfa.2023.v35.i4.3002
CrossRef - Ketsa S. and Nobuchi T., Histochemical study of vascular blockage in flower stems of orchis in relation to vase life, Agriculture and Natural Resources. 1991. 25, 111–118.
- Nair S. A., Singh V. T., and Sharma V. R. S., Effect of chemical preservatives on enhancing vase-life of gerbera flowers, Journal of Tropical Agriculture. 2003. 41, 56–58.
- Kumar A. A., Effect of Postharvest Preservatives on Vase Life of Chrysanthemum (Dendranthema grandiflora) cv. Hybrid-1, 2016, Depart. Of Flor. and Landscape Architecture, College of Horticulture, Mandsaur, India. Google Scholar
- Abd-Allah A. A. E. T., Mohamed T. A. D., Darwish M. A., Khenizy S. A. M., and Taha M. H., Changes in post-harvest life of cut chrysanthemum as influenced by different holding solutions and two cultivars, Middle East Journal of Agriculture Research. 2019. 8, no. 1, 82–95.
- Doi M., Nakagawa Y., Watabe S., Aoe K., Inamoto K., and Imanishi H., Ethylene-induced leaf yellowing in cut chrysanthemum (Dendranthema grandiflora Kitamura), Engei Gakkai Zasshi. 2003. 72, no. 6, 533–535, https://doi.org/10.2503/jjshs.72.533
CrossRef - Doi M., Aoe K., Watabe S., Inamoto K., and Imanishi H., Leaf yellowing of cut standard chrysanthemum (Dendranthema grandiflora Kitamura) ‘Shuho-no-chikara’ induced by ethylene and postharvest increase in ethylene sensitivity, Engei Gakkai Zasshi. 2003 72, no. 6, 533-535, https://doi.org/10.2503/jjshs.72.533
CrossRef - da Silva, J.A.T. The cut flower: postharvest considerations. Online Journal of Biological Science, 2003. 3: 406-442.
CrossRef - Darandeh, N. and Hadavi, E. Effect of pre-harvest foliar application of citric acid and malic acid on chlorophyll content and post-harvest vase life of Lilium cv. Brunello. Frontiers in Plant Science. 2012, 2: 1-3.
CrossRef - El-Kobisy, D.S., Kady, K.A., Hedani, R.A. and Agamy, R.A. Response of pea plant (Pisum sativum) to treatment with ascorbic acid. Egyptian Journal of Applied Science, 2005. 20: 36-50.
- Hell, R. and Stephan, U.W. Iron uptake, trafficking and homeostasis in plants. Planta, 216: 2003. 541–551.
CrossRef - Nowak, J. and Rudnicki, R.M. Postharvest handling and storage of cut flowers florist, greens and potted plants. Timber Press. 1990. 210 p.
CrossRef - Eidyan,B. Effect ofiron and citric acid foliar applications in combination with nitrogen fertigation on tuberose (Polianthes tuberosa L.). Horticulture. 2010. Karaj, Islamic Azad University, Karaj Branch 75.
- Kader, A. A. Postharvest Technology of Horticultural Crops. 2002. University of California, Davis.
- Lutz, J. M., & Hardenburg, R. E. The commercial storage of fruits and vegetables, and florist and nursery stocks. 1968. USDA, Agricultural Research Service.
- Aziz M, Karboune S. Natural antimicrobial/antioxidant agents in meat and poultry products as well as fruits and vegetables: A review. Crit Rev Food Sci Nutr. 2017; 8398:1-28.
- Shahidi F, Zhong Y. Novel antioxidants in food quality preservation and health Eur J Lipid Sci Technol. 2010; 112: 930-940.
CrossRef - Monterio, J. A., Nell, T.A. and Barrett, J.E. Effect of exogenous sucrose on carbohydrate levels. Flower respiration and longevity of potted miniature rose flowers during postproduction. Postharvest Biology and Technology. 2002. 26: 221-229.
CrossRef - Bauters, L., Stojilkovic B., Gheysen, G. Pathogens pulling the strings: effectors manipulating salicylic acid and phenylpropanoid biosynthesis in plants. Plant Pathol.., 22 .2021., pp. 1436-1448, 10.1111/mpp.13123.
CrossRef - Layuk L & P. Keragaan Masa Pajang Beberapa Varietas Krisan Tipe Spray di Kota Tomohon. Buletin Agrosaintek Sulawesi Utara Volume 1. Nomor 2. Desember 2015. ISSN 2528-519X.
- Layuk L. & P.. Kajian Penggunaan Larutan Holding Dalam MempertahankanMasa Pajang Bunga Potong Krisan Varietas Remix. Prosiding Seminar Nasional Inovasi Pertanian Mendukung Bio-Industri. 9 Oktober .2014. di Manado. BBP2TP, Badan Litbang Pertanian. ISBN 978-602-1280-70-6.
- Ramezanian, A., Azadi, M., Mostowfizadeh-Ghalamfarsa, R. & Saharkhiz, M.J. Effect of Zataria multiflora Boiss and Thymus vulgaris L. essential oils on black rot of ‘Washington Navel’ orange fruit Postharvest Biol. Technol. 2016. 112 152 158
CrossRef - Mehraj, H., Ona, A.F., Taufique, T., Mutahera, S. and Jamal Uddin, A.F.M. Vase life quality improvement of white snowball using vase life extanding solutions. Bangladesh Research Publications. 2013. 8(3): 191-194.
- Conklin, P. Recent advances in the role of biosynthesis of ascorbic acid in plant cell environment. Journal of Plant, Cell and Environment,. 2001. 24: 383-394.
CrossRef

