Introduction
Parthenium hysterophorus L., is a very invasive and toxic herbaceous annual plant in the Asteraceae family. It is known by several names, including whitetop, feverfew, bitter weed, carrot weed, broom bush, and congress grass in India. This plant, which was originally native to the American tropics, especially Central and South America, has rapidly expanded to many different parts of the world, posing serious problems for agriculture, public health, and ecology.
P. hysterophorus L. is a fast-growing, upright, widely branched herb that is either annual or temporary.1 The plant is known for its allelopathic properties, which inhibit the growth of surrounding vegetation and disrupt local ecosystems.2 Conventional medicine has long utilized Parthenium hysterophorus L. to treat a range of illnesses including heart attacks, fevers, wounds, anaemia, and ulcerated sores. The infusion of roots is used to treat diarrhoea, while extracts at lesser concentrations have demonstrated potential as antifungal agents. Though the plant infusion is consumed internally to treat a variety of ailments, it is applied externally for skin complications. Additionally, it appears promising in the fight against hepatic amoebiasis.3 Traditionally, it has been used for treating infertility, menstrual and delivery issues, fever, migraine headache, toothache, stomachache, bug stings, and rheumatoid arthritis. The plant is particularly noted for its anti-inflammatory and analgesic effects, making it useful in the treatment of pain and inflammatory conditions. Infusions of P. hysterophorus are traditionally used to address ailments such as fever, toothache, and headache, showcasing its potential as a natural remedy for symptomatic relief. Additionally, the plant has demonstrated antispasmodic properties, which may be beneficial in managing gastrointestinal disorders. Additionally, P. hysterophorus has been investigated for its potential in reproductive health, particularly in addressing infertility and menstrual complications. Its bioactive constituents, including sesquiterpene lactones, pinenes, and flavonoids, contribute to its diverse pharmacological effects. Due to its bioactive constituents, which include sesquiterpene lactones, pinenes, and flavonoid glycosides, P. hysterophorus has been reported to exhibit a wide spectrum of biological activities. These components also contribute to its pharmacological activities, which include antispasmodic, anti-inflammatory, anticancer, and cardiotonic effects,4 also shows thrombolytic & act as analgesics.5,6 The aerial part of P.hysterophorus also utilized as insecticides and nematicides.7,8
The whole P. hysterophorus plant is rich in flavonoids, which include parthenin, 6-hydroxykaempferol-3,7-dimethyl ether, and stigmasterol. Its flowers yield ambrosanoli, while its leaves yield acids, parthenin, flavonoids, campesterol, and stigmasterol.9 P.hysterophorus essential oils obtained from both aerial and root part contains major compounds like germacrene D, trans-β-ocimene, β-myrcene, carotol, β-caryophylene, bicyclogermacrene, carota-5,8-diene & borneol acetate.10 This research aims to fill this gap by analyzing how different environments and geographic diversity across various districts in Garhwal affect the chemical profile of P. hysterophorus essential oil. Additionally, the study investigates how these phytochemical variations impact its antifungal and allelopathic activities. The primary purpose of this study is the estimation of the biological action of P. hysterophorus L. EO towards weeds and fungi; therefore, it is redundant to elaborate extensively on its biological activity, which should be briefly presented, including only the most important medical effects.
Materials and Methods
Plant material: The aerial parts of P. hysterophorus were collected from Dehradun and Haridwar, Uttarakhand, India during December 2023 and February 2024, respectively (Table 1). Dehradun, at 640 m, has a humid subtropical climate with warm summers and loamy, organic-rich soil supporting mixed deciduous forests. In contrast, Haridwar, at 249.7 m, features a semi-arid climate with hot summers and sandy, low-nutrient soil characterized by open grasslands and shrubs.
Table 1: Details of plant materials collected from different geographical locations of Uttarakhand.
| Essential oil sample site | Latitude(N);
Longitude(E) |
Elevation (m) | Date of collection |
| Naugaon (wild)
Dehradun, Uttarakhand |
30.3882°N; 78.0666° E | 640 | 27 December 2023 |
| Rodi Belwala(wild)
Haridwar, Uttarakhand |
29.9387°N; 78.0653° E | 249.7 | 6 February 2024 |
Essential oil extraction
Approximately 500 g of the fresh aerial parts of the plant, collected from two different districts, were subjected to essential oil extraction using a Clevenger-type apparatus.11 This process lasted for 4-5 h and utilized about 1 L of water (roughly two-thirds of the plant material). Following the extraction, the attained EOs were dried over anhydrous sodium sulfate to eliminate any moisture in the samples. The dried essential oils were then kept at a low temperature (4 °C) in a refrigerator for subsequent study.
GC-MS analysis
The phytochemical constituents of the essential oils was determined using GC-MS with a Perkin Elmer GCMS-SQ8 instrument, equipped with a PE5 column (30.0 m × 250 μm i.d., 0.25 μm film thickness). A 1μL of the oil sample was injected, with the injector temperature maintained at 280 °C. Helium aided as the carrier gas, maintaining a flow rate of 1 mL/min and a split ratio of 50:1. The GC oven temperature program in progress at 50 °C for 3 min, then increased to 200 °C at a rate of 3 °C/min, tracked by a ramp of 6 °C/min to 250 °C, where it was seized constant for 2 min and then for an additional 11 min. To identify the compounds in the essential oils, their mass fragmentation patterns and retention index (RI) values were compared with those in the NIST (version 2.1) and WILEY (7th edition) mass spectral libraries.12 Experimental retention indices were obtained by injecting a homologous series of n-alkanes (C7–C20). The compounds quantification was performed by normalizing the peak areas and expressing each as a % of the total peak area.
Biological Activities
Allelopathic activity
The allelopathic activity of essential oil isolated from P. hysterophorus L. was evaluated against hybrid radish (Raphanus raphanistrum sub sp. sativus) seeds using the methodology proposed by Sahu and Devkota.13 Essential oil solutions were produced in an aqueous solution containing 1% Tween-20 at different doses (50,100, 150, 200µL/mL) to evaluate the suppression of seed germination. Before the experiment, the radish (Raphanus raphanistrum sub sp. sativus) seeds were surface sterilized for fifteen min. in a NaOCl 5% solution. Each petriplate contained seven sterile radish seeds and the bottom was covered with filter paper to maintain the ideal moisture content for germination. After that, the plates were filled with 4 millilitres of each concentration of the tested sample, and the seeds were incubated for 24 h at 25±1 °C to allow them to germinate. When all of the seeds in the control group sprouted, the experiment was over. By contrasting the allelopathic activity with distilled water (negative control) and the widely used herbicide paraquat (positive control) at different doses 50,100, 150 and 200 µL/mL, we were able to determine its effectiveness. The following formulas were used for calculation.
| Inhibition of seed germination
% Inhibition =100 × (1- Gt/Gc) Where, Gt: Number of seeds germinated in treatment Gc: Number of seeds germinated in control |
Inhibition of shoot length
% Inhibition =100 × (1- Ct/Cc) Where, Ct: shoot length in treatment Cc: shoot length in control |
Inhibition of root length
% Inhibition =100 × (1- Rt/Rc) Where, Rt: root length in treatment Rc: root length in control |
Antifungal activity
The antifungal activity of essential oils was evaluated against Curvularia lunata and Colletotrichum lindemuthianum, which were isolated from naturally infected plant samples collected from agricultural fields and identified based on morphological and microscopic characteristics using standard taxonomic keys. C. lunata formed dark brown to black velvety colonies on Potato Dextrose Agar (PDA), while C. lindemuthianum produced white to gray colonies with a dark center. Microscopically, C. lunata conidia were curved, multi-septate, and brown with an enlarged central cell, whereas C. lindemuthianum had cylindrical to fusiform, hyaline conidia arranged in slimy masses, with identification confirmed using standard references.14,15 Fungal cultures were revived on PDA plates and incubated at 26 ± 2 °C for one week before preparing assay discs from seven-day-old cultures. Essential oils at 50, 100, 150, and 200 μL/mL were aseptically introduced onto PDA plates, with a control plate maintained for comparison. Antifungal activity was assessed by measuring inhibition zones around fungal colonies, and radial growth inhibition was calculated relative to the control. Carbendazim at equivalent concentrations served as a standard, with inhibition percentages determined using McKinney’s method.16
Inhibition (%) = 100 x (X-X/Y)
Where, X : Growth in control, Y : Growth in treatment.
Statistical Analysis
For the statistical analysis, two-way or three-way analysis of variance (ANOVA) was employed, followed by Duncan’s Multiple Range Test (DMRT) to assess the differences between treatment means. These statistical procedures were performed using RStudio (version 2021.09.2). A p-value of less than 0.05 was measured statistically significant, representing that the experiential differences among treatment means were unlikely due to random variation. the SRPLOT (http://www.bioinformatics.com.cn/en?keywords=heatmap) was used for principal component analysis (PCA). These advanced analytical methods were applied to the chemical and biological analysis data of the essential oils. PCA facilitated the identification of key components that significantly contributed to the variance within the dataset, while circular heat map clustering provided a visual overview, pinpointing the most critical features and their relationships within the data.
Results and Discussion
Terrestrial and hilly ecosystems are greatly impacted by an altitudinal shift from mean sea level. Variations in altitude along with atmospheric conditions result in equivalent variations in temperature, relative humidity, water availability, wind speed, and length of sunshine. Numerous eco-physiological processes in plants are thus impacted by these alterations in the external environment, or abiotic variables. Consequently, shifting ecological niches are predicted to impact the constituents and content of plant volatiles.17 In the present study, two wild populations of P. hysterophorus were collected in December 2023 and February 2024 from different habitats at altitudes ranging from 249.7 meters to 640 m. The hydro-distilled essential oils average yields were 1.5% for the plant material from Dehradun and 1.3% for Haridwar. The chemical compounds of these EOs were analyzed using GC-MS. The study exposed a total of 26 and 35 compounds, accounting for approximately 83.18% and 74.89% of the total EOs composition for the Dehradun and Haridwar samples, respectively (Table 2). The results showed that oxygenated sesquiterpenes dominated all essential oils. β-cyperone, found in varied quantities in all studied essential oils, was the main chemical ingredient. For Dehradun sample, other significant constituents included saussurea lactone (7.81%), sclareol (6.78%), 2,4a,5,8a-tetramethyl-1,2,3,4,4a,7,8,8a-octahydronaphthalen-1-ol (6.68%), and 2,3-bornanediol (6.13%). Minor constituents present in the Dehradun essential oil included longifolenaldehyde (4.03%), undec-10-ynoic acid, tridec-2-yn-1-yl ester (3.46%), Bicyclo[8.2.0]dodecan-11-one, 12,12-dichloro- (1R*,10S*)- (2.96%), hydroxycitronellal (2.89%), and isolongifolol (2.73%). For Haridwar sample, the major constituent identified was nootkatone-11,12-epoxide, comprising 8.38% of the essential oil. This was followed by 6-(1-hydroxymethylvinyl)-4,8a dimethyl 3,5,6,7,8,8a-hexahydro-1H-naphthalen-2-one (7.98%), α,α-di-T-butyl-O-methoxybenzyl alcohol (7.17%), and curcumenol (6.06%). Other minor constituents included 8-acetyl-5,5-dimethyl-nona-2,3,8-trienoic acid, methyl ester (5.30%), 5-(7a-isopropenyl-4,5-dimethyloctahydroinden-4-yl)-3-methyl-pent-2-enal (5.11%), 5-phosphatricyclo[6.1.1.0(2,6)]dec-2(6)-ene, 5,9,9-trimethyl- (3.49%), valerenic acid (3.26%), 1-isopropenyl-4-methyl-1,2-cyclohexanediol (3.00%), 4-hydroxymenthol (3.00%), 7-(1.3-dimethylbuta-1,3-dienyl)-1,6,6-trimethyl-3,8-dioxatricyclo[5.1.0.0(2,4)]octane (1.94%), 4-hydroxy-β-ionone (1.79%), and 2,7-octanedione, 4,4-dimethyl-3-[2-(1-hydroxy-1-methylethyl)-3-methyl-3-butenylidene (1.66%).
The analysis reveals a significant differences among the essential oils studied. Dehradun sample was notably rich in β-cyperone (compound 21), whereas Haridwar sample contained a higher concentration of nootkatone-11,12-epoxide (compound 36). Additionally, both EOs shared several common compounds (compounds 3-5, 8-10, 12-14, 16, 20, 21, 23), while significant differences were observed in other compounds (compounds 1,2, 6, 7, 11, 17-19, 24-48) (Table 1). Moreover, Dehradun sample has higher contents of β-cyperone, neoclovene oxide, α-terpineol, sclareol, saussurea lactone, longifolenaldehyde, isolongifolol, 1-isopropenyl-4-methyl-1,2-cyclohexanediol, p-menth-3-ene, 2,3-bornanediol, and 3-cis-(1,1-dimethylethyl)-4,cis-methoxycyclohexanol, each exceeding 1.0% of its composition compared to Haridwar sample. This data underscores the notable similarity in the overall profiles of Dehradun and Haridwar sample, despite these differences in specific compound concentrations. It is deduced that altitude gradient, geographical differences, environmental factors, and meteorological circumstances could be the cause of the changes in chemical composition.
Table 2: Comparative chemical composition (%) of P.hysterophorus essential oils from two different locations.
| S. No. | Compounds
|
RI
|
Dehradun | Haridwar |
| 1. | Citronellal (OM) | 1159 | 0.14 | – |
| 2. | Silane,octyl- (O) | 1084 | 1.33 | – |
| 3. | 3-cis-(1,1-dimethylethyl)-4,cis-methoxycyclohexanol(O) | 1084 | 2.35 | 0.67 |
| 4. | Nonanal (H) | 1438 | 1.95 | 0.39 |
| 5. | 2,3-Bornanediol(H) | 1494 | 6.13 | 1.73 |
| 6. | Hydroxycitronellal (OM) | 1269 | 2.89 | – |
| 7. | p-Methane-1,2-diol (OM) | 1394 | 0.37 | – |
| 8. | p-Menth-3-ene (H) | 993 | 2.38 | 0.7 |
| 9. | 1-Isopropenyl-4-methyl-1,2-cyclohexanediol (OM) | 1167 | 3.7 | 3 |
| 10. | Isolongifolol (OS) | 1712 | 2.73 | 1.32 |
| 11. | Bicyclo[8.2.0]dodecan-11-one,12,12-dichloro-(1R*,10S*)- (O) | 1299 | 2.96 | – |
| 12. | Undec-10-ynoic acid, tridec-2-yn-1-yl ester (FA) | 2637 | 3.46 | 0.53 |
| 13. | Longifolenaldehyde (OS) | 1668 | 4.03 | 0.51 |
| 14. | Saussurea lactone (OD) | 1806 | 7.81 | 0.76 |
| 15. | Incensol oxide (OD) | 2241 | 1.75 | – |
| 16. | Sclareol (OD) | 2220 | 6.78 | 0.07 |
| 17. | (-)- Isolongifolol, methyl ether(H) | 1671 | 2.24 | – |
| 18. | 10-epi-γ-Eudesmol (OS) | 1660 | 6.68 | – |
| 19. | Spiro[4.5]decan-7-one, 1,8-dimethyl-
8,9-epoxy-4-isopropyl-(O) |
1681 | 1.26 | – |
| 20. | α-terpineol (OM) | 1189 | 2.51 | 0.21 |
| 21. | β-Cyperone (SH) | 1771 | 12.16 | 5.11 |
| 22. | 3-Hydroxy-5,6-epoxy-β-ionone (OM) | 1692 | 1.15 | – |
| 23. | Neoclovene oxide (OS) | 1754 | 1.48 | 0.97 |
| 24. | Longiborneol (OS) | 1592 | 1.12 | – |
| 25. | 5H-Benzo[b]pyran-8-ol, 2,3,5,5,8a pentamethyl-6,7,8,8a-tetrahydro-(H) | 1279 | 1.5 | – |
| 26. | Eudesma-4,11-dien-2-ol (OS) | 1690 | 2.32 | – |
| 27. | Fenchol (OM) | 1119 | – | 0.27 |
| 28. | α,α-Terpineol(OM) | 1189 | – | 0.57 |
| 29. | 1,4-Cyclododecanedione (O) | 1975 | – | 0.35 |
| 30. | 4-Hydroxymenthol (OM) | 1395 | – | 3.00 |
| 31. | Epicholestanol (FA) | 3285 | – | 0.18 |
| 32. | 4-Hydroxy-β-ionone (OM) | 1628 | – | 1.79 |
| 33. | β-Yatirenene (SH) | 1554 | – | 0.16 |
| 34. | Eremophilone (SH) | 1756 | – | 0.13 |
| 35. | 6-(1-Hydroxymethylvinyl)-4,8adimethyl-3,5,6,7,8,8a-hexahydro-1H naphthalen-2-one (O) | 1909 | – | 7.98 |
| 36. | Nootkaton-11,12-epoxide (OS) | 1823 | – | 8.38 |
| 37. | Valerenic acid (OS) | 1876 | – | 3.26 |
| 38. | 7-(1,3-Dimethylbuta-1,3-dienyl)-1,6,6-
trimethyl-3,8-dioxatricyclo [5.1.0.0(2,4)] octane (SH) |
1302 | – | 1.94 |
| 39. | Curcumenol (OS) | 1734 | – | 6.06 |
| 40. | Ambrosin(SH) | – | 1.78 | |
| 41. | Carvacrol (OM) | 1298 | – | 1.59 |
| 42. | 2,7-Octanedione, 4,4-dimethyl-3-[2-(1-hydroxy-1-methylethyl)-3-methyl-3-butenylidene)- (H) | 1290 | – | 1.66 |
| 43. | Salsoline (O) | 1803 | – | 1.33 |
| 44. | 8-Acetyl-5,5-dimethyl-nona-2,3,8-trienoic acid, methyl ester (H) | 2085 | – | 5.3 |
| 45. | α,α-Di-t-butyl-omethoxybenzyl alcohol (O) | 1393 | – | 7.17 |
| 46. | Curcumenone (OS) | 1844 | – | 1.28 |
| 47. | 2,5-Dimethoxyterephthalic acid(O) | 1431 | – | 1.25 |
| 48. | 5-Phosphatricyclo [6.1.1.0(2,6)]dec- 2(6)-ene, 5,9,9-trimethyl- (H) | 937 | – | 3.49 |
| Chemical Classes | Dehradun | Haridwar |
| Sesquiterpene Hydrocarbons (SH) | 12.16 | 9.12 |
| Oxygenated Sesquiterpene (OS) | 18.36 | 21.78 |
| Oxygenated Monoterpenes (OM) | 10.76 | 10.43 |
| Oxygenated Diterpenes (OD) | 16.34 | 0.83 |
| Fatty acids (FA) | 3.46 | 0.71 |
| Hydrocarbons (H) | 14.2 | 13.27 |
| Others (O) | 7.9 | 18.75 |
| Total | 83.18 | 74.89 |
RI= retention index
Biological Activities
Allelopathic activity
The mean percentage and IC50 values (Fig. 4) for the inhibition of seed germination, root length, and shoot length by P. hysterophorus essential oils (from Dehradun and Haridwar) at various concentrations (50, 100, 150, and 200 μL/mL) indicated that the essential oils exhibit moderate to strong allelopathic activity in a dose-dependent manner.
Inhibition of seed germination
The inhibition of seed germination by the tested EOs at the maximum concentration (200 μL/mL) is presented in Fig. 1. The percent inhibition of seed germination follows this order: Dehradun (92.33%) > Haridwar (91.66%). The IC50 values for seed germination inhibition are ranked as follows: Haridwar (104.6 ± 0.75 μL/mL) > Dehradun (108.66 ± 0.32 μL/mL).
Inhibition of root length
The percentage inhibition of root length at the highest concentration (200 μL/mL), as shown in Fig. 2, is as follows: Haridwar (97.77%) > Dehradun (97.40%). The IC50 values were calculated when the control group reached 100% growth. The IC50 values are ranked as follows: Dehradun (102.26 ± 0.32 μL/mL) > Haridwar (103.30 ± 0.75 μL/mL).
Inhibition of shoot length
At the highest concentration of essential oils (200 μL/mL), the inhibition of shoot length is illustrated in Fig. 3 as follows: Dehradun (95.18%) > Haridwar (90.00%), and the IC50 values are Dehradun (102.66 ± 0.41 μL/mL) and Haridwar (113.36 ± 1.11 μL/mL), respectively.
The significant allelopathic activity observed in the tested samples is likely attributed to their high content of sesquiterpene hydrocarbons, especially saussurea lactone, a type of sesquiterpene lactone. This compound has been previously reported to exhibit high toxicity against weed seeds.18 Sesquiterpene lactones, such as Parthenin, are known for their diverse biological activities, including cytotoxic, anti-tumor, allergenic, antimicrobial, antifeedant, phytotoxic, and insecticidal properties. In a study conducted by Datta and Saxena, 2001,8 both pure Parthenin and extracts from different parts of P. hysterophorus demonstrated allelopathic effects on various aquatic and terrestrial weeds.19,20 Among the sesquiterpene lactones, Parthenin has received significant attention for its potential allelopathic properties and highly effectiveness in inhibiting the germination and seedling growth of Cassia tora L.21
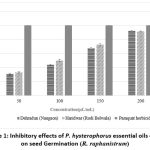 |
Figure 1: Inhibitory effects of P. hysterophorus essential oils doses on seed Germination (R. raphanistrum). |
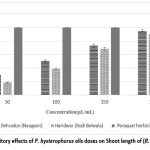 |
Figure 2: Inhibitory effects of P. hysterophorus oils doses on Shoot length of (R. raphanistrum) |
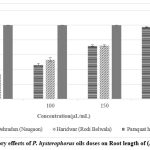 |
Figure 3: Inhibitory effects of P. hysterophorus oils doses on Root length of (R. raphanistrum) |
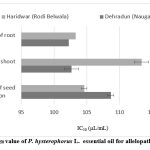 |
Figure 4: IC50 value of P. hysterophorus L. essential oil for allelopathic activity |
Principal component Analysis (PCA)
After determining the components and biological activity of each oil, an analysis of the allelopathic activity variability was conducted. Principal Component Analysis (PCA) was utilized to evaluate the allelopathic activity variability between two essential oils. The analysis focused on three parameters: percentage inhibition of seed germination, percentage inhibition of root length, and percentage inhibition of shoot length (Fig. 5). These parameters were assessed in relation to the different locations of collection of plants from which the EOs were gained. The joint contribution rate of change from the first two principal components (PC1 and PC2) found through PCA was 100%, capturing the full extent of the allelopathic activity differences. This indicates that PC1 and PC2 together described all the variability in the allelopathic activities of the essential oils. PC1 reported for 95.4% of the total variance and was positively correlated with shoot inhibition. Meanwhile, PC2 contributed 4.3% to the variance and was positively correlated with seed germination inhibition and root inhibition activity.
 |
Figure 5: PCA of P. hysterophorus Dehradun and Haridwar essential oil |
Antifungal activity
The antifungal activity of essential oils from Dehradun and Haridwar was investigated against two plant pathogenic fungi, Colletotrichum lindemuthianum & Curvularia lunata, across a range of concentrations from 50 to 200 µL/mL. As detailed in the (Fig.6 and Fig. 7), the essential oils demonstrated moderate capabilities in suppressing the mycelial growth of these pathogenic fungi. Notably, Dehradun sample exhibited the highest antifungal effectiveness against both C. lindemuthianum and C. lunata, achieving inhibition rates of 99.25% and 92.22% respectively at the highest tested concentration of 200 µL/mL.
The fungal pathogens are sensitive to sesquiterpene lactones, which act as active agents in the Asteraceae family plant species- P. hysterophorus.22 Another study confirmed that carvacrol, a phytochemical found in plants of the Asteraceae family, is effective against pathogenic fungi such as Colletotrichum acutatum, Colletotrichum fragariae, and Colletotrichum gloeosporioides.23 Similarly, it has been reported that the antifungal activity can be attributed to components such as carvacrol. Maximum fungi of the genus Fusarium can cause fusariosis in turf, particularly in the species Lolium perenne (ray grass) on golf courses. Carvacrol, a natural agent derivative from plants like Thymus vulgaris and Origanum vulgare, was used against three Fusarium species responsible for ray grass fusarium head blight using the microdilution method. The minimum inhibitory concentration (MIC) values obtained confirmed that the compound tested has high activity against these three Fusarium species.24 Previous studies have also investigated the antifungal activity of α-terpineol, present in the leaves of Alpinia malaccensis. At different concentrations, it shows significant antifungal activity against Rhizoctonia solani, Sclerotinia sclerotiorum, and Sclerotium rolfsii.25
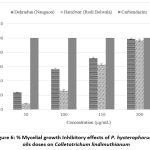 |
Figure 6: % Mycelial growth Inhibitory effects of P. hysterophorus L. oils doses on Colletotrichum lindimuthianum |
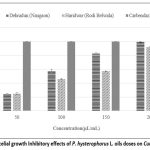 |
Figure 7: % Mycelial growth Inhibitory effects of P. hysterophorus L. oils doses on Curvularia lunata |
Conclusion
This study compared essential oils from P. hysterophorus collected from Dehradun and Haridwar districts of Uttarakhand, India, using GC-MS analysis. Both oils contained oxygenated sesquiterpenes, with β-Cyperone prominent in both but differing significantly in other compounds. Dehradun had higher Saussurea lactone and 2,3-Bornanediol, while Haridwar sample was richer in Nootkatone-11,12-epoxide and Curcumenol. Both oils showed significant allelopathic activity on R. raphanistrum subsp. sativus, with Dehradun sample slightly more allelopathic activity due to its higher sesquiterpene lactone content. In antifungal tests, Dehradun sample exhibited greater efficacy against Colletotrichum lindemuthianum and Curvularia lunata. These findings highlight that essential oil composition and biological activity in P. hysterophorus can vary between locations, emphasizing the influence of geographic origin on potential agricultural applications.
Acknowledgment
The authors acknowledge the Dev Bhoomi Uttarakhand University (Dehradun), Uttarakhand, India, for providing academic support and Sophisticated Industrial Materials Analytic Labs Pvt. Ltd. in Haridwar, for providing facility for GC-MS analysis.
Funding Sources
The authors received no financial support for the research, authorship, or publication of this article.
Conflict of Interest
The authors do not have any conflict of interest
Data Availability Statement
This statement does not apply to this article
Ethics Statement
This research did not involve human participants, animal subjects, or any material that requires ethical approval.
Informed Consent Statement
This study did not involve human participants, and therefore, informed
Author Contributions
Kamini Yadav: Conceptualization, Formal analysis, Writing original draft
Sushila Arya: Super vision, Writing– review & editing
Sandhya Kumari: Formal analysis, Methodology, and Writing– original draft
Pratiksha Khadka: Analyse the data and statistical analysis.
References
- Fazal HINA, Ahmad N, Ullah I, Inayat H, Khan L, Abbasi BH. Antibacterial potential in Parthenium hysterophorus, Stevia rebaudiana and Ginkgo biloba. Pakistan Journal of Botany. 2011; 43(2): 1307-1313.
CrossRef - Arya S, Kumar R, Prakash O, Kumar S, Mahawer SK, Chamoli S, and de Oliveira MS. Chemical composition and biological activities of Hedychium coccineum– Ham. ex Sm. essential oils from Kumaun hills of Uttarakhand. Molecules. 2022; 27(15): 4833.
- Adkins SW, Navie SC, and McFadyen RE. Control of parthenium weed (Parthenium hysterophorus): A Centre for Tropical Pest Management team effort. Seed. 1996; 2: 1000.
- Sharma S, Gupta N. Antimicrobial potential of Partherium hysterphorus: An In-vivo International Journal of Pharmaceutical Research and Development. 2012; 4: 112-118.
- Jha U, Chhajed PM, Shelke TT, Oswal RJ, and Adkar PP. CNS activity of methanol extract of Parthenium hysterophorus in experimental animals. Der Pharmacia Letter. 2011; 3(4): 335-341.
- Roy DC, and Shaik MM. Toxicology, phytochemistry, bioactive compounds and pharmacology of Parthenium hysterophorus. Journal of Medicinal Plant Studies. 2013; 1(3): 126-141.
- Amir H, Butt BZ, Vehra SE. Evaluation of larvicidal activity of Parthenium hysterophorus against Aedes aegypti. International Journal of Mosquito Research. 2017; 4(2): 1-4.
- Datta S, Saxena DB. Pesticidal properties of parthenin (from Parthenium hysterophorus) and related compounds. Pest Management Science. 2001;57(1): 95-101.
CrossRef - Jain NK, and Kulkarni SK. Antinociceptive and anti-inflammatory effects of Tanacetum parthenium extract in mice and rats. Journal of Ethnopharmacology. 1999; 68(1-3):251-259.
CrossRef - de Miranda CAS, Cardoso MDG, de Carvalho MLM, Figueiredo ACS, Nelson DL, de Oliveira CM, and de Albuquerque LRM. Chemical composition and allelopathic activity of Parthenium hysterophorus and Ambrosia polystachya weeds essential oils. American Journal of Plant Sciences. 2014; 5(9):10
CrossRef - Clevenger JF. Apparatus for the determination of volatile oil. The Journal of the American Pharmaceutical Association. 1928; 17(4): 345-349.
CrossRef - Adams RP. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry, Allured business media publishing corporation, Carol Stream, IL, USA. 2007.
- Sahu A, and Devkota A. Allelopathic effects of aqueous extract of leaves of Mikania micrantha HBK on seed germination and seedling growth of Oryza sativa and Raphanus sativus L. Scientific World. 2013; 11(11): 90-93.
CrossRef - Watanabe T. Pictorial atlas of soil and seed fungi: morphologies of cultured fungi and key to species. CRC press. 2002.
CrossRef - Dugan FM. The identification of fungi: an illustrated introduction with keys, glossary, and guide to literature. 2006; 176.
- McKinney HH. Influence of soil temperature and moisture on infection of wheat seedlings by Helminthosporium sativu. Journal of Agriculture Research. 1923; 26: 195-218.
- Bouzid HA, Oubannin S, Ibourki M, Bijla L, Hamdouch A, Harhar H, and Gharby S. Comparative evaluation of chemical composition, antioxidant capacity, and some contaminants in six Moroccan medicinal and aromatic plants. Biocatalysis and Agricultural Biotechnology. 2023; 47: 102569.
CrossRef - Kushwaha VB, and Maurya S. Biological utilities of Parthenium hysterophorus. Journal of Applied and Natural Science. 2012; 4(1): 137-143.
CrossRef - Acharya SS, and Rahman A. Allelopathic effect of Parthenium hysterophorus On seed germination and seedling growth of Cassia tora Linn., Journal of Environment and Ecology.1997; 15: 335-337.
- Pandey D.K. Phytotoxicity of sesquiterpene lactone parthenin on aquatic weeds. Journal of Chemical Ecology. 1996; 22: 151-160.
CrossRef - Duke SO, Wedge CE, Cerdeira AL, and Matallo MB. Herbicide effects on plant disease. Outlooks on Pest Management. 2007; 18: 36-40.
CrossRef - Rai MK, Acharya D, and Wadegaonkar P. Plant derived-antimycotics: potential of Asteraceous plants. In: Plant-Derived Antmycotics; Current Trends and Future Prospets, Haworth Press, New York. 2003; 165-185.
CrossRef - Altintas A, Tabanca N, Tyihák E, Ott PG, Móricz ÁM, Mincsovics E, and Wedge DE. Characterization of volatile constituents from Origanum onites and their antifungal and antibacterial activity. Journal of AOAC International. 2013; 96(6):1200-1208.
CrossRef - Saghrouchni H, El Barnossi A, Chefchaou H, Mzabi A, Tanghort M, Remmal A, and Fouzia C. Study the effect of carvacrol, eugenol and thymol on Fusariums responsible for Lolium perenne fusariosis.Ecology, Environment and Conservation. 2020; 26(3): 1059-1067.
- Sethi S, Prakash O, and Pant AK. Phytochemical analysis, antioxidant assay and antifungal activity of essential oil and various extracts of Alpinia malaccensis ( f.) Roscoe leaves. Cogent Chemistry. 2016; 2(1): 1223781.
CrossRef
Abbreviations
%: Percentage
°C: Degree Celsius
ANOVA: Analysis of variance
DMRT: Duncan’s Multiple Range Test
EOs: Essential oils
GC-MS: Gas Chromatography-Mass Spectrometry
i.d. : Internal diameter
MIC: Minimum inhibitory concentration
Na₂SO₄: Sodium sulfate
NaOCl: Sodium hypochlorite
NIST: National Institute of Standards and Technology
PCA: Principal Component Analysis
PDA: Potato Dextrose Agar
RI: Retention index
SRPLOT: Science and Research online plot.
μL: Micro liter
Views: 29
