Introduction
Cotton (Gossypium hirsutum L.), a member of the Malvaceae family, stands as one of India’s primary crops, serving as a crucial source of fiber and revenue. It is cultivated across an extensive area of 133 lakh hectares, resulting in a production of 360 lakh bales. However, in the state of Odisha, its cultivation is primarily concentrated in the districts of Koraput, Balangir, Rayagada, and Kalahandi, where the soil and climate conditions are conducive. In 2019, cotton cultivation in Odisha encompassed an area of 169.56 thousand hectares, yielding a production of 578.50 thousand bales, with a productivity rate of 580.0 kg/ha.1
Seed vigor holds significant importance in determining seed quality. Seeds with high vigor demonstrate swift and consistent germination, emerging effectively, particularly in challenging environmental circumstances.2 Employing high-quality planting seeds can enhance growers’ profitability by ensuring consistent stand establishment across diverse weather conditions. This, in turn, reduces the need for replanting, lowers planting rates, and ultimately contributes to optimal yields. The high vigor of a seed denotes its capacity to sprout and generate plants under favorable conditions, despite the crop experiencing elevated yield losses. Diseases such as wilt (caused by Fusarium oxysporum f. sp. vasinfectum and Verticillium dahlia), root rot (Rhizoctonia bataticola), and collar rot (Rhizoctonia bataticola), as well as India anthracnose triggered by (Colletotrichum capsici and C. gossypii), black arm (Xanthomonas campestris pv. malvacearum), grey mildew (Ramularia areola), powdery mildew (Leveillula taurica), Ascochyta blight (Ascochyta gossypii), leaf spot (Helminthosporum gossypii or Phakospora gossypii), and various viral and mycoplasmal infections, notably limit cotton production.3
Significantly, many of these infections are transmitted through seeds, either internally or externally. Alternaria blight, caused by Alternaria macrospora, and bacterial blight (Xanthomonas axonopodis pv. malvacearum) are both internal seed-borne diseases resulting in yield losses of up to 26% and 30% respectively.3 Research conducted at the Central Institute for Cotton Research (CICR) in Nagpur from 1983 to 1998 indicates that the leaf and boll spot pathogen A. macrospora and the anthracnose pathogen Colletatricum capsici can become deeply embedded (embryo-borne) and transmitted through seeds in diploid cotton varieties and hybrids, specifically Gossypium arboretum and G. herbaceum. The most notable losses are associated with severe defoliation and reduced boll development, particularly when the peduncle is affected.
Materials and Methods
Location
College of Agriculture, Bhawanipatna, OUAT, where the present investigations were undertaken is located on 19.9187 north latitude and 83.1586 east longitude and has an elevation of 25.5 meters above the mean sea level.
Climate
Bhawanipatna experiences a tropical climate characterized by distinct wet and dry seasons, maintaining moderate temperatures year-round. However, from March to June, the region encounters exceptionally high temperatures.
Collection of Cotton seed Sample
In the fiscal year 2022-24, a total of seven cotton seed genotypes, each weighing 250 grams, were procured from the AICRP on Cotton RRTTS in Bhawanipatna, Kalahandi, OUAT, Odisha (Table-1). These seeds were collected for the purpose of evaluating seed quality, assessing mycoflora, and conducting seed biopriming trials. To facilitate seed quality and health evaluations, the collected seed samples underwent shade drying and were subsequently stored in containers resistant to moisture, maintaining ambient storage temperatures of 28.2°C.
Table 1: Seed bank, variety name and accession no.
| Germplasm | Seed bank | Variety Name | Accession no. |
| G1 | RRTTS, Bhawanipatna | Br. 06a
(N) 410 |
OUAT-RRTTS-CO1 |
| G2 | RRTTS, Bhawanipatna | Br. 06a
(N) 409 |
OUAT-RRTTS-CO2 |
| G3 | RRTTS, Bhawanipatna | 4a (Z)
2034 |
OUAT-RRTTS-CO3 |
| G4 | RRTTS, Bhawanipatna | 4a (Z)
2035 |
OUAT-RRTTS-CO4 |
| G5 | RRTTS, Bhawanipatna | CZ 6a
(Z) 2051 |
OUAT-RRTTS-CO5 |
| G6 | RRTTS, Bhawanipatna | Br .06a
(N) 411 |
OUAT-RRTTS-CO6 |
| G7 | RRTTS, Bhawanipatna | 4a (Z)
2032 |
OUAT-RRTTS-CO7 |
Seed Quality Parameters
Physical Purity Analysis (%)
Each specimen underwent a thorough examination employing a magnifying lens connected to the physical purity board. Subsequently, the samples were categorized into distinct groups: pure seeds, other seeds (including crop seeds and weed seeds), and inert material. The pure seeds and inert materials were individually weighed using a digital scale. Concurrently, additional seeds, such as other crop seeds and weed seeds, were enumerated and expressed in numbers per kilogram. Various inert substances such as soil, sand, stones, plant debris, and broken seeds were quantified in terms of percentages.
The weight of the physically pure seed was determined, and the results were presented as percentages rounded to one decimal place, as calculated using the following formulas:
Weight of pure seed (g) / Total sample weight (g) \times
100 = Physical pure seed percentage
Weight of pure seed (g) / Total sample weight (g) \times100 = Physical pure seed percentage
Number of other crop seeds / Weight of seeds examined in grams \times
1000 = Number of other crop seeds per kilogram
Number of other crop seeds / Weight of seeds examined in grams \times1000 = Number of other crop seeds per kilogram
Number of weed seeds / Weight of seeds examined in grams \times
1000 = Number of weed seeds per kilogram
Number of weed seeds / Weight of seeds examined in grams \times1000 = Number of weed seeds per kilogram
These procedures facilitated accurate assessment, and the obtained data were meticulously reported in a precise and standardized manner.
Seed Moisture Content
The moisture content of cotton seeds was assessed following the ISTA (International Seed Testing Association) guidelines from 2008.4 Five grams of seeds were weighed and placed in aluminum cups, which were then subjected to a 17-hour drying process in a hot air oven set at 103°C. The moisture content was calculated on a dry weight basis using the formula (2−3)/ (2−1) ×100(W2−W3)/(W2−W1) ×100, where 1W1 represents the weight of an empty container with its lid (g), 2W2 is the weight of the container with its cover and seeds before drying, and 3W3 is the weight after drying.
Germination Percentage (%)
For germination assessment, four sets of 100 seeds each were randomly selected from the seed samples. These seeds were placed on germination paper with uniform spacing, rolled, and positioned vertically in a seed germinator cabinet maintained at a constant temperature of 25°C and a relative humidity of 95.0%. On the seventh day, the germination percentage was calculated, incorporating normal seedlings, aberrant seedlings, hard seeds, fresh ungerminated seeds, and dead seeds. The germination rate was expressed as a percentage.
Root and Shoot Length
On the twelfth day, ten randomly chosen normal seedlings from each germination test replication were used to measure root and shoot length. The shoot length was measured from the cotyledonary node to the tip of the apical bud, while the primary root length was measured from the cotyledonary node to the tip. Average measurements in millimeters were recorded.
Seedlings Dry Weight
After measuring root and shoot lengths, ten representative seedlings were wrapped in blotter paper and dried in an 80°C hot-air oven for 24 hours. Following a 30-minute cooling period in a desiccator, the seedlings’ dry weight was measured using an electronic balance and expressed in grams.
Seedling Vigour Index (SVI-I)
The rolled paper towel method was employed to conduct germination tests on the seeds (Fig- 2). On the twelfth day, the germination percentage was determined. Seedling lengths in centimeters were measured for tens of thousands of seedlings per seed sample. The shoot lengths, measured from the cotyledonary node to the apical bud’s tip, and the primary root length, from the cotyledonary node to the tip, were recorded in millimeters on average. The average seedling lengths (total of root and shoot length) were then multiplied by the germination percentage for each collected seed.5
Seedling Vigour Index (SVI-II)
Following the measurement of root and shoot lengths, ten representative seedlings were wrapped in butter paper and dried in an 80°C hot air oven for 24 hours. After cooling for 30 minutes in desiccators, their dry weight was measured using an automated balance. The seedling vigour index (SVI-II) was determined by multiplying the germination percentage by the seedling dry weight.
1000 Seed Weight
Using a computerized electronic balance, ten random samples of 1000 seeds were selected from each source and location, and their weights were measured.
Electrical Conductivity (E.C.) of Seed Leachate (dSm-1)
The Electrical Conductivity (E.C.) of seed samples was assessed by soaking 200 seeds in 250 mL of de-ionized water for 24 hours at 25°C. A control beaker without seeds was used for comparison. The seed-soaked water was then swirled and poured into another beaker. The electrical conductivity of the water, containing the specific sample’s leachates, was calculated using Desi-Simon and expressed in Deci siemens per meter (dSm-1). This measurement provides insights into seed quality based on solute leakage, with lower conductivity indicating higher seed quality.6
Agar Method
In the agar method, 100 seeds were placed at equal intervals in 200 x 30 mm Petri plates containing 100 ml of pre-poured PDA. After incubation for seven days, fungal growth was examined under a binocular microscope. Both the blotter and agar methods were performed under aseptic conditions to prevent contamination. Each method used 100 seeds per plate with three replications, totaling 2100 seeds.7
Isolation Procedure Pathogen (Fungus and Bacteria)
The pathogen was obtained from seeds gathered at the AICRP on Cotton Bhawanipatna through the following steps. Initially, the samples underwent washing with distilled water to eliminate foreign materials. Cotton seeds were then subjected to a 0.1% mercuric chloride solution for 2 minutes, followed by three thorough washes with sterile water to remove any residual mercuric chloride. Subsequently, the aseptically handled cotton seeds were placed in the center of Petri plates containing either host extracts or potato dextrose agar medium. The Petri plates were incubated at room temperature (25°C) for 7-10 days, resulting in the emergence of a pure fungal culture within 3-4 days post-inoculation.
Purification and Identification of Fungi
To purify and identify fungi, each fungus isolates initially maintained on PDA slants was transferred to agar plates. Hyphae from the outer edges of young colonies were carefully examined and moved to PDA slants. This process was repeated 2-3 times until the specific fungus was confirmed to be free from other fungi and bacteria. Pure fungal cultures were obtained through both ‘single spore’ and ‘hyphal tip’ methods.8 Characteristics of the fungal colony on PDA and detailed morphological information were recorded for each isolate. Taxonomic identification was performed using existing cultures and literature.
Single Spore Isolation
In the process of single spore isolation, spores were suspended in sterile water and examined under a microscope. Culture tubes with sterile water agar were melted and cooled to 40°C. A loop of spore suspension was transferred to a second tube with melted agar, and dilutions were poured onto sterilized Petri plates. Incubation at 28±1°C followed. After 24 hours, germinating spores on Petri plates were marked and transferred to the middle of potato dextrose agar slants under aseptic conditions. Slants with single germinating spores were incubated at 28±1°C, and pure culture growth was observed in 2-3 days.9
Hyphal Tip Culture
In hyphal tip culture, the fungus was cultivated on sterile potato dextrose agar in a Petri dish. The isolated hyphal tip, identified and marked under a microscope, was carefully transferred to a potato dextrose agar slant at room temperature. Pure culture growth was observed in the culture tube after 2-3 days. The obtained pure culture was maintained on potato dextrose agar and sub-cultured every two weeks.
Inoculation and Incubation
For inoculation and incubation, aseptic conditions were maintained in the inoculation chamber. Inoculums, small pieces of medium with pure culture, were taken from slants using an inoculating needle and incubated at 25±1°C.
Culture Maintenance
Cultural maintenance involved keeping the pure fungal culture on Potato Dextrose Agar (PDA) slants, sub-culturing every two months, and storing at 25±1°C. Fifteen-day-old mycelial cultures were used in studies.
Identification of Pathogen
Identification of the pathogen involved microscopic examination based on spore morphology and colony characteristics. Confirmation was sought through the Indian Type Culture Collection (ITCC), Division of Plant Pathology, IARI, New Delhi-110012.
Morphological Characteristics Study
The study of morphological characteristics included staining and mounting isolated fungal colonies for microscopic examination. Cultural characteristics were observed after incubation at 28±1°C, noting colony shape and growth habits. Observations recorded included the frequency of seeds colonized by fungi and types of fungi associated with seeds. The culture was further purified using single spore isolation.
Result
Evaluation of Seed Quality Parameters of Cotton Genotypes
Seed Quality Studies
(%) Physical Purity Analysis
The physical purity of cotton seeds varied among different genotypes from RRTTS, Bhawanipatna (Table 1). V1 had the highest purity at 98.60%, followed by V2 and V7. The lowest purity was in V5 at 98.00%. Despite variations, all genotypes met or exceeded the minimum seed certification standard of 98.0%, ensuring the presence of physically pure seeds in each variety (Fig-1).
Evaluation of Seed Germination (%)
Seven cotton seed samples were assessed for germination using the paper method, revealing notable differences in normal seedling development among cotton genotypes from RRTTS, Bhawanipatna (Table 1). Germination percentages ranged from 52.33% to 77.00%. V1 exhibited the highest germination rate at 77.00%, followed by V7 (73.00%), V2 (70.00%), and V4 (69.00%). In contrast, V5 displayed the lowest germination at 52.33%, followed by V3 (67.66%) and V4 (69.00%).
Root Length (cm)
Differences in root length among various collected seeds were significant (Table 2). V4 displayed the highest root length at 8.82 cm, closely followed by V1 (8.80 cm) and V2 (8.72 cm), all statistically similar. Conversely, the lowest value was in V5 at 7.92 cm. The overall average for root length ranged from 7.92 cm to 8.82 cm, indicating substantial variability among cotton genotypes. Root length serves as a valuable indicator for seed vigor, potentially contributing to improved seedling establishment.
Shoot Length (cm)
Significant variations were observed in shoot length among different cotton genotypes’ seeds. The range of shoot length was 9.30 cm to 10.13 cm, with the highest observed in V7 (10.13 cm) (Table 2). The differences in shoot length among cotton genotypes from RRTTS, Bhawanipatna, may be attributed to variations in their genetic makeup. Both shoot and root lengths are crucial indicators for estimating seedling vigor, potentially influencing overall seedling growth and development.
Seedling Dry Weight (g)
Variations in seedling dry weight were significant among cotton genotypes, ranging from 0.062g to 0.080g (Table 5). The highest dry weight was in V4 (0.088g), followed by V7 and V2, while the lowest was in V6 (0.062g). Seedling dry weight is crucial for seedling vigor and influences seedling length, fresh weight, and Seedling Vigour Index-II.
Seedling Vigour Index-I (SVI-I)
Significant differences in SVI-I were recorded, ranging from 1059.33 to 1442.33 (Table 2). V1 exhibited the highest value (1442.33), indicating high seedling vigor. V5 recorded the lowest SVI-I (1059.33). The study found that all genotypes displayed high vigor, meeting or exceeding the minimum seed certification standard for cotton seeds (65%).
Seedling Vigour Index-II (SVI-II)
Significant differences in SVI-II were observed, with V1 having the highest value (6.167) followed by V7 and V2. V5 had the lowest SVI-II (3.267). SVI-II correlates with root and shoot length and seedling dry weight, ranging from 4.567 to 6.167. It serves as a crucial parameter for monitoring seedling survival and growth after germination.
Seed Moisture (%)
Moisture content varied significantly (9.3% to 10.7%), with V5 having the highest (10.7%) and V1 the lowest (9.3%). Only V1, V2, and V7 met or fell below the minimum seed certification standard of 10.0%. Moisture content variations are attributed to differences in seed drying and storage practices.
1000 Seed Weight
A significant difference in 1000 seed weight was observed, with V1 having the highest (90.45g) and V5 the lowest (67.12g). Variations in seed weight may result from plant nutrition, seed size, and genetic factors.
Electrical Conductivity (µS/cm.g)
Significant differences in electrical conductivity were noted, with V5 exhibiting the highest (30.30µS/cm.g) and V1 the lowest (16.46µS/cm.g). Higher electrical conductivity suggests poor seed quality and storage, indicating low seed vigor. High electrolyte leakage is associated with low seed vigor.
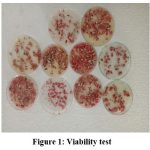 |
Figure 1: Viability test |
 |
Figure 2: Cotton seed germination |
 |
Table 2: Seed quality parameters of Cotton |
Analysis of Cotton Seed Mycoflora
The examination of cotton seed mycoflora, following ISTA guidelines through Blotter paper and PDA method, focused on assessing seed health.10 The recorded percentage of mycoflora infection varied among different cotton genotypes. The identification of specific seed-borne pathogens in cotton involved morphological and colony characteristic analyses.11
Identified Seed-Borne Pathogens
Cotton seed samples obtained from AICRP on Cotton revealed the presence of seven fungi and one bacterium. The identified pathogens include Fusarium oxysporum (Fig-5,6), Alternaria alternata (Fig-7), Curvularia lunata (Fpg-9), Aspergillus flavus, Aspergillus niger (Fig-10), Macrophomina phaseolina (Fig-11), and Xanthomonas spp. (Fig-8). These microorganisms exhibited varying degrees of infection in different cotton samples.
Description of Conidia/Spore Morphology and Colony Characteristics
The identification process involved detailed examinations of conidia/spore morphology and colony characteristics for each pathogen. This information contributes to understanding the diversity and nature of the seed-borne pathogens affecting cotton seeds.
PDA method
The assessment of seed mycoflora in cotton genotypes collected from AICRP on Cotton, RRTTS, Bhawanipatna, revealed significant differences using both PDA and Blotter paper methods, as outlined in Table 3. Notably, three fungi—Macrophomina phaseolina (Fig-11), Fusarium oxysporum (Fig-5,6), and Alternaria alternata (Fig-7) were exclusively identified through the PDA method (Fig- 3,4). The percentage of seed mycoflora, determined by the PDA method, ranged from 5.1% in V1 (Br.06a(N) 410) to 13.1% in V4 – 4a (Z) 2035. Fusarium oxysporum (Fig-5,6) emerged as the predominant fungus, with a prevalence of 23.5%, showcasing varying infection percentages (1.0-7.0%) across different genotypes. Macrophomina phaseolina (Fig-11) demonstrated consistent infection rates (1.2-4.0%) across all cotton genotypes. In the case of Aspergillus flavus (Fig-10), infection percentages varied from 2.0% to 5.2%, with significant differences observed in genotypes V1 and V5. Aspergillus niger (Fig-10) exhibited statistically similar infection percentages (2.2-5.0%) among several genotypes, with exceptions. Xanthomonas spp. (Fig-8) caused infections ranging from 1.2% to 5.5%, with all genotypes displaying statistical significance. In conclusion, this comprehensive analysis provides valuable insights into the diverse mycoflora present in cotton seeds, aiding in the development of strategies for enhanced seed health management and improved cotton cultivation practices.
 |
Figure 3: Cotton seeds in PDA plate |
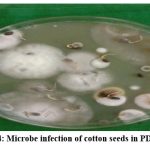 |
Figure 4: Microbe infection of cotton seeds in PDA plate |
Table 3: Seed microflora status of cotton by PDA method
| Sl. No | Name of the variety | Alt | Fusa | Mac | Xan spp. | Total
Infection ( %) |
| V1 | Br. 06 a (N) 410 | 1.00 | 1.6 | 1.3 | 1.2 | 5.1 |
| V2 | Br. 06 a (N) 409 | 3.23 | 4.3 | 2.8 | 2.5 | 12.7 |
| V3 | 4a (Z) 2034 | 3.00 | 4.1 | 3.0 | 4.1 | 14.2 |
| V4 | 4a (Z) 2035 | 4.23 | 3.3 | 2.8 | 2.9 | 13.1 |
| V5 | CZ 6 a (Z) 2051 | 5.00 | 5.2 | 2.3 | 5.3 | 17.8 |
| V6 | Br. 06 a (N) 411 | 4.00 | 2.9 | 3.0 | 3.2 | 13.1 |
| V7 | 4a (Z) 2032 | 2.00 | 2.1 | 2.3 | 2.2 | 8.5 |
| Total | 22.47 | 23.5 | 17.3 | 20.2 | ||
| Mean | 3.21 | 3.35 | 2.46 | 2.88 | ||
| CD (P=0.05) | 0.06 | 0.08 | 0.07 | 0.08 | ||
| SE(m)± | 0.19 | 0.24 | 0.21 | 0.23 | ||
| C.V. | 3.47 | 4.13 | 5.0 | 4.3 |
N.B.: Mac-Macrophomina phaseolina., Alt- Alternaria alternate, Fus-Fusarium oxysporum, Xan- Xanthomonas spp.
Standard Blotter Method
Microorganisms associated with cotton seed samples were collected from AICRP on Cotton, RRTTS, Bhawanipatna, utilizing the standard blotter method, as detailed in (Table 4). Significant differences in the occurrence of seed mycoflora among different genotypes were observed. The results revealed a total of four fungal species and one bacterial species, spanning five genera, detected across all evaluated seed samples.
Six fungi, namely Aspergillus niger (Fig-10), Aspergillus flavus (Fig-10), Fusarium oxysporum (Fig-5,6), Macrophomina phaseolina, and Alternaria alternata (Fig-7), were identified. Additionally, one bacterial species, Xanthomonas spp. (Fig-8) was found at a 3.6% level. Utilizing the blotter paper method, Fusarium emerged as the predominant fungus (3.8%), followed by Alternaria alternata (3.6%), Aspergillus niger (3.6%), Aspergillus flavus (3.5%), Curvularia lunata (Fig-9) (3.1%) and Macrophomina phaseolina (Fig-11) (2.7%). These fungi collectively contributed to an infection range of 11.6% in Br. 06a(N) 410 to 42.7% in CZ6a(Z) 2051. The overall percentage occurrence of seed mycoflora in different genotypes.
Table 4: Seed microbes ‘status of cotton seeds by blotter paper method
|
BLOTTER PAPER METHOD |
||||||||
| Name of the variety | Alt | Fusa | Mac | Xan spp. | Asp.fla. | Asp. niger | Cur | Infection (%) |
| Br. 06 a (N) 410 | 1.2 | 1 | 1.2 | 1.2 | 3.0 | 3 | 1.0 | 11.6 |
| Br. 06 a (N) 409 | 2.2 | 2.0 | 2.2 | 2.2 | 4.0 | 4 | 3.0 | 19.6 |
| 4a (Z) 2034 | 4.2 | 2 | 3.5 | 4.2 | 4.0 | 3 | 6.2 | 27.1 |
| 4a (Z) 2035 | 3.2 | 6.1 | 3.1 | 5.3 | 4.0 | 5 | 4.1 | 30.6 |
| CZ 6 a (Z) 2051 | 7.9 | 7.1 | 4.1 | 5.5 | 5.2 | 5.1 | 8.0 | 42.7 |
| Br . 06 a (N) 411 | 5.2 | 6.1 | 3.3 | 4.5 | 2.0 | 3.1 | 5.2 | 29.1 |
| 4a (Z) 2032 | 1.6 | 2.2 | 1.5 | 2.1 | 2.0 | 2.2 | 2.0 | 13.7 |
| Mean | 3.6 | 3.8 | 2.7 | 3.6 | 3.5 | 3.6 | 3.1 | 24.91 |
| C.D (0.05%) | 0.4 | 0.3 | 0.2 | 0.15 | 0.13 | 0.23 | 0.36 | |
| SE(m) | 0.1 | 0.1 | 0.06 | 0.05 | 0.04 | 0.07 | 0.11 | |
| C.V. | 5.3 | 4.6 | 4.3 | 2.3 | 2.2 | 3.5 | 4.7 | |
N.B.: Alt- Alternaria alternate, Fus-Fusarium oxysporum, Mac-Macrophomina phaseolina., Asp- Aspergillus flavus, Aspergillus niger. Cur- Curvularia lunata
 |
Figure 5: Microphotograph of Fusarium oxysporum showing conidia and mycelium |
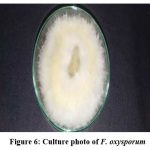 |
Figure 6: Culture photo of F. oxysporum |
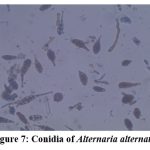 |
Figure 7: Conidia of Alternaria alternata |
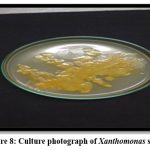 |
Figure 8: Culture photograph of Xanthomonas spp. |
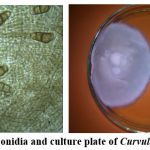 |
Figure 9: Conidia and culture plate of Curvularia lunata |
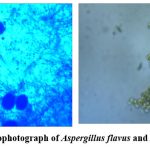 |
Figure 10: Microphotograph of Aspergillus flavus and Aspergillus niger |
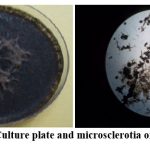 |
Figure 11: Culture plate and microsclerotia of M.phaseolina |
Estimation of mean infection (%)
The assessment of mean seed mycoflora infection percentage revealed notable disparities between the blotter paper method (24.91%) and the PDA method (12.07%), as outlined in Table 5. Among the cotton genotypes, CZ 6a(Z) 2051 displayed the highest mean seed mycoflora infection percentage at 30.25%, while the lowest infection percentage of 8.35% was recorded in BR-06a(N)410.
Table 5: Comparison study about infection (%) by both the methods.
| Sl. No | Name of the variety | Blotter method (%) | PDA method
(%) |
Mean Mycoflora
(%) |
| V1 | Br. 06 a (N) 410 | 11.6 | 5.1 | 8.35 |
| V2 | Br. 06 a (N) 409 | 19.6 | 12.7 | 16.15 |
| V3 | 4a (Z) 2034 | 27.1 | 14.2 | 20.65 |
| V4 | 4a (Z) 2035 | 30.6 | 13.1 | 21.85 |
| V5 | CZ 6 a (Z) 2051 | 42.7 | 17.8 | 30.25 |
| V6 | Br. 06 a (N) 411 | 29.1 | 13.1 | 21.1 |
| V7 | 4a (Z) 2032 | 13.7 | 8.5 | 11.1 |
| Mean infection (%) | 24.91 | 12.07 |
Conclusion on Method Comparison
In comparing the blotter paper and PDA methods, it is evident that the blotter paper method exhibited higher infection rates than the PDA method, primarily due to the absence of surface disinfection with 0.1% HgCl2. Despite methodological variations, the seed microbe infection percentage remained consistent, ranging from 8.35% in V1- Br. 06a (N) 410 to 30.25% in V5- CZ 6a (Z) 2051.
Results: Correlation between Seed Quality Parameters and Seed Mycoflora
The analysis of seed quality parameters in correlation with seed mycoflora reveals positive associations with moisture percentage and electrical conductivity (EC). Conversely, negative correlations are observed with germination percentage, seed viability, physical purity, 1000-grain weight, dry weight, SV1, and SV2. The correlation coefficients (r) indicate high significance for all parameters, excluding root length (RL) and shoot length (SL), which exhibit a non-significant correlation with mycoflora.
Discussion
Physical Purity analysis (%)
The percentage of physically pure seeds varied significantly among different Cotton genotypes collected from RRTTS, Bhawanipatna. The highest percentage of pure seeds was observed in V1 (98.60%), followed by V2 (98.46%) and V7 (98.46%), which were statistically similar. The lowest percentage of pure seeds was recorded in both V5 (98.00%) and V6 (98.26%), as well as V3 and V4 (98.4%). This suggests that the physical purity of seeds across all genotypes ranged from 98.00% to 98.6%, exceeding the minimum seed certification standards (98.0%), providing assurance of physical seed purity in all the seeds. This improvement may be attributed to proper harvesting, threshing, and cleaning processes, leading to higher seed quality in terms of physical purity. Variability in physical purity was analyzed for different okra seed genotypes, aligned with our findings.12
Evaluation of Seed germination (%)
Seven cotton seed samples were assessed for germination using the between-paper method. Notable variations in normal seedling development were identified among the cotton genotypes collected from RRTTS Bhawanipatna. Germination percentages ranged from 52.33% to 77.00%. The highest germination percentage was recorded in V1 (77.00%), followed by V7 (73.00%), V2 (70.00%), and V4 (69.00%). In contrast, lower germination rates were observed in V5 (52.33%), V3 (67.66%), and V4 (69.00%). Only V1 among the genotypes met the minimum seed certification standards (65.0%) specified for cotton seeds. The variation in germination rates may be attributed to the genetic makeup of each genotype and environmental effects during production, harvesting, packaging, and storage. Germination appears to be a biological process influenced by various factors, including the distinct behavior of different genotypes. A wide range of differences in standard germination percentage in okra seeds13, while 14 observed similar variations in cotton genotypes.
Root and shoot lengths (cm)
The maximum root length was observed in V4 (8.82 cm), followed closely by V1 (8.80 cm) and V2 (8.72 cm), which were statistically similar in terms of root length. In contrast, the minimum value for this characteristic was recorded in V5 (7.92 cm). The overall mean for root length across various genotypes ranged from 7.92 cm to 8.82 cm, indicating considerable variability in root length among different cotton genotypes. Root length serves as a valuable indicator for assessing seed vigor, potentially contributing to the enhanced establishment of seedlings. Similarly, the range of shoot length varied from 9.30 cm to 10.13 cm, with the highest shoot length observed in V7 (10.13 cm). Conversely, the lowest shoot length was noted in Variation in shoot length among different cotton genotypes from RRTTS, Bhawanipatna, possibly due to differences in genetic constitution. Both shoot and root lengths play crucial roles in estimating seedling vigor, contributing to the overall growth and development of seedlings. Similar trends in root and shoot characteristics were in wheat 15 and in cotton.13
Seedling Dry Weight (g)
The highest seedling dry weight was observed in V4-4a (Z)2035 (0.088g), followed by V7-4a (Z)2032 (0.078g), V2-Br. 06a (N)409 (0.069g), V4-4a (Z)2035, and V6-Br .06a (N)411 (0.068g), while the significantly lowest dry weight was noted in V6-Br .06a (N)411 (0.062g). The range of seedling dry weight varied from 0.062g to 0.080g. Maximum seedling length, seedling fresh weight, and seedling vigor index-II depend on the expression of seedling dry weight (g) found a similar range of seedling dry weight in cotton.16
Seedling Vigor Index-I (SVI-I, on seedling length basis)
Seedling vigor (SVI-I) ranged from 1059.33 to 1442.33 among the cotton seed samples. The highest SVI-I value was recorded for V1-Br. 06a (N)410 (1442.33), followed by V7-4a (Z)2032 (1388.00), V2-Br. 06a (N)409 (1319.0), V3-4a (Z)2034 (1299.00), and V6-Br .06a (N)411 (1293.33). The significantly lowest SVI-I value was recorded for V5-CZ 6a (Z)2051 (1059.33). These results align with the findings of (Salam, 2017) in cotton.17
Seedling Vigor Index-II (SVI-II, on dry weight basis)
The significantly highest SVI-II value was observed for V1 (6.167), followed by V7 (5.70), V2 (4.867), V4, and V6 (4.70). The lowest value was recorded for V5 (3.267). Similar trends and positions were observed for SVI-I and SVI-II for cotton seed, reflecting the effects of root and shoot length and seedling dry weight. The mean value of SVI-II ranged from 4.567 to 6.167. SVI-II is an essential parameter for monitoring and ensuring the survival and growth rate of seedlings after the germination period. These results are consistent with the findings (Salam ,2017) in cotton.17
Seed Moisture (%)
Seed moisture content ranged from 9.3% to 10.7%. The maximum moisture content was recorded in V5-CZ 6a (Z)2051 (10.7%), followed by V3-4a (Z)2034 and V6-Br .06a (N)411 (10.3%), and V4-4a (Z)2035 (10.2%). The minimum moisture content was in V1-Br. 06a (N)410 (9.3%), followed by V7-4a (Z)2032 (9.7%), and V2-Br. 06a (N)409 (9.9%). Only three genotypes, V1, V2, and V7, had moisture content equal to or below the minimum seed certification standard specified for cotton seeds (10.0%). Variation in moisture content among different genotypes may be due to differences in seed drying and packing for storage. However, higher seed moisture content before storage is not conducive to the health of stored seeds and their normal seedling percentage. Only 32% of cotton genotypes had moisture content equal to or below the standard. This result is consistent with in wheat seeds.18
1000 Seed Weight
V1-Br. 06a (N)410 recorded the highest 1000 seed weight (90.45g), followed by V7-4a (Z)2032 (86.92g), V2-Br. 06a (N)409 (75.05g), and V4-4a (Z)2035 (74.46g). The lowest 1000 seed weight was recorded in V5-CZ 6a (Z)2051 (67.12g), followed by V6-Br .06a (N)411 (71.67g), V3-4a (Z)2034 (72.44g), and V4-4a (Z)2035 (74.46g). Differences in 1000 seed weight in seed samples may be due to improper plant nutrition during crop production and seed size, influenced by the unique genetic constitution. Lettuce seeds differ depending on seed weight. Cotton seed weight affects germination time, seedling growth, and dry matter accumulation, according to the findings of (Rezapour, 2013) in soybean.19
Electrical Conductivity (µS/cm.g)
Among these seed collections, the highest electrical conductivity (E.C.) value was recorded in V5-CZ 6a (Z)2051 (30.30µS/cm.g), followed by V3-4a (Z)2034 (29.50µS/cm.g), V6-Br .06a (N)411, and V4-4a (Z)2035 (23.80µS/cm.g), while the lowest value was recorded in V1-Br. 06a (N)410 (16.46µS/cm.g), followed by V7-4a (Z)2032 (16.48µS/cm.g) and V2-Br. 06a (N)409 (223.03µS/cm.g). A higher value for electrical conductivity indicates that the collected genotypes had poor seed quality and storage. Similar findings in okra. Cotton seeds with increased electrical conductivity of solute leakage were associated with decreased seed germination and viability according to Cotton seed stated by (Rezapour, 2013).19
Identification and Detection of Cotton Seed Mycoflora Status
The current study aimed to investigate the presence of various microbes in cotton seeds collected from different regions of Odisha. The presence of external and internal pathogens was examined and documented during this investigation, with supporting evidence from relevant literature. A total of five fungi and one bacterium were identified using both PDA and blotter paper methods. Three fungi—Macrophomina phaseolina, Fusarium oxysporum, and Alternaria alternata—and one bacterium (Xanthomonas spp.) were identified using the PDA method. Aspergillus flavus and Aspergillus niger were predominantly observed in the blotter paper method due to the absence of surface sterilization by 0.1% HgCl2. Similar findings were reported by (Shamsi and Nahar,2019) in their studies on seed mycoflora in cotton.20
Individual Infection (%) and Association of Different Mycoflora
PDA Methods
In the PDA method, the percentage of seed mycoflora ranged from 5.1% in the V1 (Br.06a(N) 410) genotype to 13.1% in V4-4a (Z) 2035. Fusarium oxysporum was the predominant fungal species (23.5%), followed by Alternaria alternata (22.47%), Macrophomina phaseolina (17.3%), and Xanthomonas spp. (20.2%). The findings highlight the importance of studying the association of seed mycoflora, directly influencing seed quality parameters. It has been estimated that Macrophomina phaseolina is a seed-borne pathogen in cotton, causing infection ranging from 1.3-3.0 % among the genotypes.
Blotter Paper Methods
In the blotter paper method, Aspergillus niger’s infection ranged from 2.2-5.1% in genotypes V7-4a (Z) 2032 and V5-CZ 6a (Z) 2051. Xanthomonas spp. infection was recorded at 3.6%. Six fungi—Aspergillus niger, Aspergillus flavus, Fusarium oxysporum, Macrophomina phaseolina, Alternaria alternata, and Curvularia lunata were identified, contributing to 11.6% to 42.7% infection. These findings align with (Tomar in 2012), (El-Samawaty in 2012) emphasizing the diversity of mycoflora associated with cotton seeds.21, 22
Correlation of Cotton Seed Mycoflora on Seed Quality Parameters
The correlation analysis revealed a positive correlation of seed quality parameters with moisture percentage. Additionally, seed microbe infection (%) showed a negative correlation with germination %, dry weight, SVI-I, and SVII-II. The correlation coefficient (r) values indicated a high significance for all parameters concerning mycoflora. These findings are consistent with the results of seed inoculation with certain fungi inhibits seed germination, vigor, and seedling growth. Rhizoctonia solani, Fusarium semitectum, Fusarium moniliforme, and Fusarium solani significantly inhibited seed germination and seedling growth. Similar correlations were reported by (Tomar D. S. in 2012), emphasizing the impact of mycoflora on seed quality parameters.21 The correlation study highlighted the significance of moisture content, showing that lower moisture percentages result in higher germination percentages. This finding is supported by (Anil Kumar Kushwaha in 2016), who reported that the storage conditions of cotton seeds at different moisture levels affected fungal species diversity. Optimal conditions for the highest fungal species occurrence were found to be 86% relative humidity and 30°C temperature.23
Conclusion
Upon meticulous examination and critical evaluation of the results obtained in this study, the following key inferences can be summarized briefly:
Cotton genotype V1 (Br.06a(N) 410) exhibited superiority in physical seed purity (%), germination (%), viability (%), seedling vigor indices (SVI-I and SVI-II), and 1000-seed weight, along with the lowest level of seed infection. Notably, V1 (Br.06a(N) 410) demonstrated the lowest seed infection (11.6%), while the highest infection (42.7%) was observed in V5 – CZ 6a (Z) 2051, particularly using the blotter paper method. In the PDA method, V5 – CZ 6a (Z) 2051 still resulted in the highest seed infection (17.8%), while V1 (Br.06a(N) 410) exhibited the lowest infection (5.1%).
In the PDA method, infection (%) ranged from 5.1% to 17.8%, whereas in the blotter paper method, it varied from 11.6% to 42.7%. Notably, Macrophomina phaseolina, Fusarium oxysporum, and Alternaria alternata were identified as internal seed-borne pathogens found in both methods, with a higher number of seed microflora recorded in the blotter paper method compared to the PDA method. Six fungi (Aspergillus niger, Aspergillus flavus, Fusarium oxysporum, Curvularia lunata, Macrophomina phaseolina, and Alternaria alternata) and one bacterium (Xanthomonas sp.) were identified in different cotton genotypes using both methods. In the blotter paper method, Fusarium oxysporum predominated (3.8%), followed by Alternaria alternate (3.6%) and Aspergillus niger (3.6%). These fungi, along with others, contributed to an infection range from 11.6% in Br. 06a(N) 410 to 42.7% in CZ6a(Z) 2051. However, in the PDA method, Fusarium oxysporum was predominant, causing an infection rate of 3.35%.
Correlation analysis between seed quality parameters and seed mycoflora revealed a positive correlation with moisture percentage and a negative correlation with germination percentage, dry weight, SVI-I, and SVII-II. The correlation coefficient (r) values indicated high significance for all parameters. The highest germination (77.0%) was recorded in variety V1-Br.06a(N)410, attributed to the lowest seed moisture (9.30%) and the lowest seed microflora infection (11.6%).
Acknowledgement
Authors solemnly thankful to ICAR, Vice Chancellor and Dean for providing infrastructure facility for this research.
Funding Sources
The author(s) received no financial support for the research, authorship, and/or publication of this article.
Conflict of Interest
The authors do not have any conflict of interest.
Data Availability Statement
The authors confirm that the data supporting finding of this study are available within the article and its supplementary material. Raw data that support the finding of this study are available from the authors team and corresponding author, upon reasonable request.
Ethical Statement
This research did not involve human participants, animal subjects, or any material that requires ethical approval.
Authors contribution
Kumar Avinash Biswal – Data collection and analysis, manuscript writing, Nirakar Ranasingh – Framing Research, Rajeeb Lochan Moharana- Infrastructure support, Siddhartha Das – Data interpretation, framing the manuscript and overall analysis.
References
- Odisha Agriculture Statistics.2020. p. 68.
- Tao Q, Chen D, Bai M, Zhang Y, Zhang R, Chen X, Sun X, Niu T, Nie Y, Zhong S, Sun J. Hydrotime Model Parameters Estimate Seed Vigor and Predict Seedling Emergence Performance of Astragalus sinicus under Various Environmental Conditions. Plants (Basel). 2023; 12(9): 1876. doi: 10.3390/plants12091876.
CrossRef - Chaudhari RJ, Kelaiya DS, Vyas UM, Parmar SK. Impact of spray schedule on Alternaria leaf blight in cotton. The Pharma Innovation J. 2022; 11(7): 4215-4218.
- ISTA. Grinding In ISTA Moisture Testing. 2008. 50: 8303.
- Abdul-Baki A, Anderson JD. Vigor Determination in Soybean Seed by Multiple Criteria. Crop Sci. 1973; 13: 630-633. http://dx.doi.org/10.2135/cropsci1973.0011183X001300060013x.
CrossRef - Matthews S, Whitbread R. An association between seed exudates and the incidence of pre-emergence mortality in wrinkle-seeded peas. Plant Path. 1968; 17: 11-17. DOI: 10.1111/j.1365-3059. 1968.tb00407.x
CrossRef - Qessaoui, R., Bouharroud, R., & Furze, J.N. Applications of New Rhizobacteria Pseudomonas Isolates in Agroecology via Fundamental Processes Complementing Plant Growth. Science. 9, 12832. DOI: https://doi.org/10.1038/s41598-019-49216-8
CrossRef - Sailaja RJ, Reddi KM, Khayum AS, Koteswara RSR, Ravindra RB, Sarada JR. Cultural and Morphological Characterization of Streptomyces and Interaction Study with Sclerotium rolfsii by SEM. J Sci Res and Rep. 2024; 30(5): 176-92. DOI: https://doi.org/10.1038/s41598-019-49216-8
CrossRef - Purushotham P, Rakholiya KB, Vanani KD. The Pathogenicity Test, Cultural and Morphological Characterization of Anthracnose (Colletotrichum lindemuthianum) of Green Gram (Vigna radiata L. Wilczek). J of Adv in Biol & Biotech. 2024; 27(1): 94-103. DOI: https://doi.org/10.1038/s41598-019-49216-8
CrossRef - ISTA. International Rules for Seed Testing. Seed Sci and Tech. 1996; 13: 299-513.
- Snehal S, Zanjare AV, Suryawanshi SR, Zanjare, Karjule AP. Evaluation of different seed health testing methods for detection of seed borne leaf spot causing organism in safflower. The Pharma Innov J. 2023; 12(3): 5757-5760.
- Sarkar DD, Choudhury MSM, Akhtar N, Bhuiyan MZR, Nisha HAC. Health status of okra seeds collected from different location of Bangladesh. World J of AgriL Sci. 2015; 11(6): 371-379. DOI: 10.5829/idosi.wjas.2015.11.6.1889
- Yakkala SS, Arghya M. Studies on Seed Quality Parameters of Okra (Abelmoschus esculentus L). J of Agril sci and Technol. 2015; 2: 79-8.
- Pinki SS, Stwach RS, Sangwan, Sombir SVS, Mor SM, Rohila N. Correlation between Seed Germination (%) and Other Seed Quality Different Environments in Upland Cotton (Gossypium hirsutum L.), Int J of Curr Microbiol and Appl Sci. 2018; 7(05): 688-696.
CrossRef - Kumar VRC, Poonia CK. Assessment of the Seed Vigour Potential in Different Varieties of Wheat. Int J of Curr Microbiol and Appl Sci. 2018; 7(7): 354-361.
CrossRef - Sudharani M, Padmasri A. Assessment of Seed Vigour Tests for Relative Storability and Field Performance in Cotton. IOSR, Journal of Agri and Vet Sci. 2014; 7(9): 59-62. DOI: 10.9790/2380-07915962
CrossRef - Salam AM, Moynul HMM, Obaidul IMd, Nasir UM, Nazmul HMd. Genotypic variation in physiological quality of fresh cotton seed. Int J Pln & Soil Sci. 2017; 14(4): 1-8. DOI: https://doi.org/10.9734/IJPSS/2017/31205
CrossRef - Rahman MH, Sattar MA, Salim MMR, Quddus MA, Ali MM. Study on quality of Okra (Abelmoschus esculentus L.) Seed collected from different sources and locations of Bangladesh. American J of Plant Biol. 2017; 2(4): 129-135. DOI: 10.11648/j.ajpb.20170204.13
- Rezapour R, Hamdollah K, Mehrdad Y, Parisa ZM. Effect of seed size on germination and seed vigour of two soybean (Glycin max L.) Cultivar Int Res J of Appl and Basic Sci. 2013; 4(11): 3396- 3401.
- Shamsi S, Nahar MN. Pathogenic potentiality of fungi isolated from seeds of three hill cotton varieties (Gossypium arboreum L.). Dhaka University J Biol Sci. 2019; 28(2): 187–193.
CrossRef - Tomar DS, Shastry PP, Nayak M, Sikarwar P. Effect of seed borne mycoflora on cotton seed (JK 4) and their control. J of Cotton Res and Devel. 2012; 26 (1): 105-108.
- El-Samawaty AMA, Omar, MR, El-Wakil DA, Mohamed NT. Qualitative and quantitative estimation of seeds mycoflora and their influence on cotton seedling damping-off J of Plant protec and Pathol. 2012; 2(6): 583-595. DOI: 10.21608/jppp.2011.86504
CrossRef - Anil KK. Effect of Different Moisture and Storage Temperature on Seed Borne Mycoflora of Cotton (Gossypium arboreum L.). UK J of Pharma and Bio Sc. 2016; 4(2): 37-50. DOI: https://doi.org/10.20510/ukjpb/4/i2/99643
CrossRef

