Introduction
Weeds constitute the most significant biotic limitations on both developing and developed nations for agricultural production. Weeds have the tendency to compete with cultivated and native plants for moisture, light, nutrients, and space.1 They can also possess a serious threat by hosting the pathogens that cause diseases in cultivated plants2 that could reduce the yield of the crops by 20–50%.3 As a result, impact of weed poses a serious concern for crop productivity, and contemporary agriculture so it must be managed effectively to prevent yield losses and guarantee food security.4
Alien invasive species have the tendency to suppress germination, emergence and growth of other plants by secreting allelopathic compounds which resulted into promotion of the invasion success of alien plants via regulating the sprouting, proliferation and developing stages of the native plant species.5 When allelopathic substances enter into the soil they retard the growth and development of native species, affecting their occurrence and thus biodiversity released by different vegetative and floral parts of plants.6 Allelochemicals released are the different types of secondary metabolites like phenolics, terpenoids, alkaloids and their derivatives by which alien invasive plants communicate. Therefore, allelopathy is important plant interaction mechanism for the successful establishment of invasive exotic weeds. According to7 allelopathy may have the ability to reduce the potential of native plants by 25% and thereby play key role in contribution for invasion of alien plants. Himalayan forests are facing high risk of invasion; previous studies reported that there is allelopathic effect of invasive alien species on agriculturally used traditional crops and weeds.8-9One of them is L. camara which is worldwide known invasive plant and perennial aromatic shrub belongs to Verbenaceae family.
In 1809, L. camara was brought to India for its ornamental role at National Botanical Garden but now this plant has spread across all open areas along roadsides, railway tracks, edges of crop fields and open forests all over the country. L. camara is an alien invasive species hence allelopathy has a significant impact in its invasion and establishment by releasing differential amount of allelochemicals into the soil beneath its canopy cover. These secondary metabolites i.e., allelochemicals are released into surrounding environment and biogeochemical interface of plants by precipitation leachates, decomposition of different plant parts, root derived nutrients and volatilization which may results into the reduction of germination and early seedling length of native plant species.10-12 According to Sharma and Raghubanshi,13 L. camara interferes with the growth of surrounding vegetation by outcompeting for soil nutrients and altering microenvironment (e.g., light and temperature) by forming dense thickets. L. camara as an invasive plant releases certain amount of chemicals to discourage the growth of native plants, the phenomenon called as allelopathy.14 Aqueous extract of L. camara shows effect on germination and growth of five crops (Chinese cabbage, spinach, rapeseed, cucumber and chili) in laboratory, greenhouse, and field conditions.15 L. camara is one of the invasive species of Kumaun Himalaya that infest rice-based agroecosystems.16 Rice is one of the staple food crops of India which is negatively affected by this invasive species.17
In Himalayan belt the rice seeds are directly sown which then started developing together with weeds and compete for nutrients as well as space. Allelochemicals released by these weeds also contribute towards this competition. Himalayan economy is flourished by agriculture and related activities which plays a significant role in the lives of this region.18 Mostly the agriculture and allied activities are centered between 1200 and 2000 m above sea level (mid–hill zone), which accounts for about 80% of the rural economy. Further, as a result of climate change, rising global temperatures and cropland damage, the invasive weeds are increasing and causing considerable decline to the agricultural productivity.17 Therefore, the objectives for present study were: i) To evaluate the comparative effect of leaves and stem of Lantana camara and ii) To evaluate the comparative effect of soaked and crushed extract of Lantana camara against one of the rice variety (Chandan-21).
Materials and Methods
Collection of Plant Material and Rice Seeds
Plant material (leaves and stem) are collected from fields located in Bhowali near Nainital in Kumaun Himalaya. Certified rice seeds of variety Chandan-21 were used in experiment. These seeds were then tested for viability, washed and sterilized thoroughly. Experiments regarding this present study were conducted in the Department of Botany, D.S.B. Campus, Nainital.
Preparation of Aqueous Extracts
Collected leaves and stem were separated from healthy plants and brought to the laboratory. 10 g of different plant material were crushed and soaked in 200 mL of distilled water for 24 h at room temperature. Plant material is crushed into mortar and pastel. The extract was then filtered through double layered muslin cloth followed by Whatman no. 1 filter paper and was considered as stock solution (100%). This extract was then diluted to prepare different concentrations of crushed and soaked treatments. Different concentrations i.e., 2.5% (C1), 5% (C2), 7.5% (C3) and 10% (C4 ) were prepared from crushed and soaked plant material using distilled water as dilution factor. Control (C0) was also used to compare the results. Fresh stock solutions were prepared for each crushed and soaked extract preparation after one week of time period to maintain the chemical nature of phytotoxins and their viability.
Experimental Design
Experiments for investigation of germination and seedling growth of rice were conducted on root trainer having the height of 4 cm and diameter of 5 cm each. Soil for the experiment was collected from L. camara free oak forest around D.S.B. Campus, Nainital. Soil was oven sterilized for 6-8 hrs prior to the experiment. 10 seeds were sown in each root trainer and replicated 10 times per concentration per treatment. 10 mL aqueous extract of each concentration (C1, C2, C3 and C4 ) were added to each of the replicates separately. Similarly, the control was treated with distilled water. Germination test was conducted under condition of 12 h light/dark cycle for 15 days with 15°C minimum and 25°C maximum temperature. A seed was considered germinated when radical was 2 mm long. The root and shoot length were measured on the first 15 days then further harvestings were done after 30 days and 45 days. Prior to each harvesting, seedlings were thinned to equal numbers in each pot to maintain the intraspecific competition.
Data Analysis
Seed germination was recorded and observed on a daily basis for data analysis. Percentage and rate of germination were calculated.19 The length of plumule and radicle was measured after final count and was taken through ruler (0.1 cm accuracy). Simultaneously, the fresh weight of plumule and radicle was taken with the help of electronic weighing machine (0.0001g accuracy). Shoot and root dry weights were recorded after oven drying at 60 °C for 48 h. The moisture content respective to the fresh weight was calculated.20 Seed Vigor Index (SVI) was measured by following Kumar and Garkoti.21 The Response Index was calculated as per the formula given by 22 for the magnitude of inhibition versus stimulation by imposed stress on seed germination and seedling growth.
Statistical Analysis
The measured parameters were analyzed by Analysis of Variance (ANOVA). Statistical analysis was performed by SPSS version 25.
Results and Discussion
Statistical analysis showed that L. camara plant parts (stem and leaves) and process (crushed and soaked) significantly affected germination and early seedling growth phases of rice in laboratory bioassay. Statistical analysis done by two-way ANOVA showed significant effects of leaf and stem extracts, extract concentrations and their interactive effects on root length, plant biomass and relative moisture content of rice seedlings (Table1). The studied plant traits at three different harvesting periods performed after 15 days of time interval showed an inconsistent pattern. However, the overall results depicted that soaked leaf extract was more suppressive as compared to crushed leaf extract and similar to these lower concentrations promoted the trait values and higher concentration suppressed the same. Similar to our results, 23 reported that soaked leaves of L. camara exhibited more inhibitory as well as fluctuating results on germination and early growth of three agricultural crops i.e., maize, finger millet and teff (William love grass). Allelochemicals present in plants can influence germination and early seedling growth of other plants in concentration dependent manner; and alteration of these chemicals are selective and can vary from species to species.24-28
Impact of Plant Part and Extract type on Seed Germination
When control (C0) was compared with other concentrations (C1, C2, C3 and C4 ) of crushed leaf extract, all of them showed increased seed germination by 6%, 4%, 3.9% and 3.7%, respectively. Soaked leaf treatment recorded inhibition of 6.6%, 1.2% and 0.3% in C2, C3 and C1 while, increment of 1.8% in germination percentage of C4 , respectively. The inhibitory effect on the germination of the rice was proportional to the concentration of the extract and the higher concentration had the strongest inhibitory effect for both crushed and soaked extracts.
When compared with Control (C0), C1, C2 and C3 levels of crushed stem treatment inhibited the seed germination by 0.1%, 3.3% and 5.4%, respectively, whereas, C4 promoted the seed by 2% (Table 2). In soaked stem treatment, all the concentrations inhibited seed germination in comparison to control i.e., the recorded inhibition was as: C1 (1.13%) < C3 (1.39%) < C2 (3.38%) < C4 (3.38%) [Fig.1]. The inhibitory effect on the germination was inversely proportional to the concentration of the extract and the higher concentration had the strongest inhibitory effect for both crushed and soaked extracts. Plants have the capacity to sense the environmental stimuli and respond to the environment either by altering its functional traits or via adaptation in response to its environmental factors.29 In conformity with our results, prior studies on allelopathic influence of L. camara also demonstrated its potential impact on seed germination and growth of many plant species including agricultural crops.30-31, 25
Effect of Plant Part and Extract Type on Seedling Growth
At 1st harvest, crushed leaf extract [Fig. 2] showed inhibition in root length and the recorded order was as: C2 (45%) > C1 (40%) > C3 (22%) > C4 (2%). Soaked leaf extract showed increment in the order: C2 (35%) > C1 (23%) > C3 (22%) > C4 (20.7%). Crushed leaf extract showed 11%, 7.9%, 7.1% and 6.2% inhibition in shoot length at C4 , C1, C3 and C2, respectively, whereas, in case of soaked leaf extract, recorded inhibition was 13%, 8.6%, 2.8% and 2.7% at C4, C3, C1 and C2, respectively. Total shoot length in crushed leaf extract was suppressed by 21.9%, 23.07%, 13.6% and 16.8% in C1, C2, C3 and C4 , respectively, while, soaked leaf extract showed 1.6%- 16.8% increment. Crushed leaf extract inhibited shoot diameter by 0.39% in both C4 and C1, 0.16% in C3; and increased by 3.03% in C2. The soaked leaf extract showed inhibition of 8.2% in C4 and 6% in C3 level and increment of 3% in C2 and 2.6% in C1 level, respectively. After 2nd harvesting in [Fig. 3] crushed leaf extract, root length showed 13% and 4%increment in C4 as well as C3 level and 5% inhibition in both C1 and C2 level. Soaked leaf extract imposed maximum inhibition of 37% in C2, 19% in C3; while C4 and C1 promoted the root length by 11% and 3.6%. Shoot length in crushed leaf extract was inhibited in the order: C4 (8.8%) > C1 (3.3%) > C2 (2.3%) > C3 (0.9%), while in soaked leaf extract, maximum inhibition was shown by C2 (8%) followed by C4 (5.1%) and increment was observed in C1 (2.3%) and C3 (1.6%). The crushed leaf extract increased the total shoot length by 11% and 0.7% in C4 and C2, and inhibition of 2.9% and 1.4% was recorded in C3 and C1, respectively. In the soaked leaf extract, C4 and C1 promoted the total shoot length by 11% and 4.8% and suppressed the same by 24% and 9.9% in C2 and C3, respectively. Shoot diameter in crushed leaf extract showed increment of 6%, 5.2%, 4.6% and 1.7% at C4, C3, C2 and C1, respectively, whereas, in soaked leaf extract inhibition of 13%, 5.5%, 1.4% and 0.8% were recorded at C4, C3, C2 and C1 concentrations, respectively. At 3rd harvest, root length [Fig. 4] in crushed leaf extract showed increment in C4, C1 and C2 of 16.9%, 11% and 7.7%, respectively and inhibition of 4% in C3, while in soaked leaf extract, C1 showed maximum inhibition of 40%, C2 10% whereas, C4 and C3 showed increment of 33.3% and 29%, respectively.
Shoot length in crushed leaf extract showed inhibition of 8.9%, 6% and 2.9% in C4, C3 and C2, respectively, and C1 showed increment of 0.5%, while in soaked leaf treatment, there was increment of 10.8% and 3.8% for C3 and C1 and inhibition of 4.1% and 3.7% in C2 and C4.Total shoot length of crushed leaf treatment showed inhibition in both C1 (23%) and C3 (4.7%) and increment in C4 (10%) and C2 (5.3%), while, for soaked leaf treatment, the order of increment was: C3 (27%) > C4 (22%) > C1 (20.9%) > C2 (2.1%). Crushed leaf extract showed increment in shoot diameter by 8%, 4%, 2.3% and 1.9%at C3, C4, C2 and C1, respectively, and in soaked leaf extract C2 showed inhibition of 5.3% and promotion at C3 (5.6%), C1 (3.1%) and C4(1.6%). At 1st harvest, the root length in crushed stem extract showed increment of 1.2% (C3) and 17% (C4 ). In contrast, soaked stem extract showed inhibition of 30% in root length for higher concentration (C4) [Fig. 5]. However, crushed (14.8%) as well as soaked (11.63%) aqueous extract of L. camara stem suppressed shoot length of rice seedling at higher concentrations. Total plant length was stimulated by crushed stem extract and inhibited by soaked stem extract. An increasing pattern of inhibition in shoot diameter was recorded in crushed stem extract with the increasing extract concentrations: 3.18% at C1, 4.67% at C2, 5.38% at C3 and 6.2% at C4 and soaked stem extract showed increment in shoot diameter at lower concentration (C1 by 1.43%) and inhibition was recorded at higher concentrations: C2(0.03%), C3 (3.32%) and C4 (9.4%). As compared to 1st harvest, at 2nd harvest [Fig. 6] the application of crushed stem extract showed considerable inhibition in root length i.e., C1 (8.7%), C2 (17.1%), C3 (5.4%) and C4 (24.7%), whereas, soaked stem extract showed increment at C1 by 9.27%, C3 by 0.86%, C4 by 1.28% and suppression at C2 by 11.44%.
Shoot length was increased at lower concentration i.e., C1 (5.05%) of crushed stem while inhibited towards increasing concentrations: C2 (1.48%) < C3 (2.29%) < C4 (11.19%). Soaked stem extract also recorded increment in shoot length at C1 (6.36%), C2 (5.29%), C3 (3.9%) and inhibition at C4 (11.63%). Crushed stem extract suppressed total plant length at higher concentration (19.32%), whereas, soaked stem treatment promoted total plant length (12.02%) in C4 concentration. Shoot diameter showed an inconsistent pattern, it was promoted at C1 (2.85%) and C3 (0.74%) and inhibited at C2 (3.18%) and C4 (6.02%) in crushed stem extract. Similarly, it showed increment at C1 (1.71%) and C3 (3.95%) while, inhibition at C2 (2.83%) and C4 (0.33%) in soaked stem extract. At third harvest, crushed stem [Fig. 7] extract increased root length at lower concentrations i.e. C1 (22.63%) and C2 (11.6%) and inhibited at higher concentrations: C3 (22.44%) and C4 (2.63%). Soaked stem treatment showed inhibition in root length at all concentrations: C1 (13.9%), C2 (4.99%), C3 (20.58%) and C4 (21.46%). The shoot length showed inhibition at C2 (7.3%), C3 (8.69%), C4 (15.18%) and increment at C1 (2.5%) in crushed stem treatment, whereas, soaked stem extract showed inhibition at C2 (1.07%) and C4 (7.92%) and increment at C1 (10.62%) and C3 (1.07%). Total plant length showed inconsistent pattern along the concentration gradient; in crushed stem treatment, it increased at lower concentrations i.e. C1 (17.74%) and C2 (6.67%) and decreased at higher concentrations: C3 (14.48%) and C4 (5.48%) whereas, in soaked stem treatment it decreased at all concentrations: C1 (8.9%), C2 (2.8%), C3 (12.58%) and C4 (17.16%). Crushed stem treatment inhibited shoot diameter at C1 (1.47%) and C4 (4.04%) level and promoted at C2 (0.1%) and C3 (0.72%), in contrast, soaked stem treatment increased shoot diameter at C1 (0.96%) and C4 (0.37%) and decreased at C2 (2.81%) and C4 (83.34%).
Effect of Plant Part and Extract Type on Dry Biomass
During 1st harvest, dry root weight in crushed leaf extract showed inhibition and the order was: C4 (63.5%) > C1 (44.6%) > C2 (26%) > C3 (10%), whereas, in soaked leaf extract, order of increment was: C2 (24.1%) > C1 (23%) > C3 (20.5%) > C4 (20.4%). Dry shoot weight in crushed leaf extract showed increment in C3 (14.8%), C1 (4.4%), and C4 (2.6%), whereas, inhibition of 2.6% was recorded in C2. In soaked leaf extract, the increment of 10%, 6.2% and 3.8 % was shown by C1, C3 and C4 and inhibition of 4.1% by C2. Total dry weight in crushed leaf treatment was increased in C3, C1, and C2 by 15.4%, 12.8%, and 0.09%, respectively, and inhibition of 4.1% in C2 was observed. In soaked leaf extract, the increment was observed in C3, C2, C4 and C1 by 9.7%, 9.6%, 8.1% and 7.9%, respectively. After 2nd harvesting, dry root weight of crushed leaf extract showed inhibition of 65%, 46% and 7.4% in C1, C2 and C3 and increment of 7.6% in C4,while in soaked leaf extract, increment was observed in C4 (31.7%), C3 (8.6%) and C1 (4.3%), however, inhibition of 32% was recorded at C2. Dry shoot weight of crushed leaf extract had inhibition of 65%, 46% and 7% in C1, C2 and C3, respectively, while promotion of 7.6% in C4, whereas, in soaked leaf extract, increment of 31%, 8.6% and 4.3% at C4, C3, and C1, respectively and inhibition of 32% at C2 was observed. Total dry weight of crushed leaf extract showed increment in the order: C2 (14.7%) > C3 (13.4%) > C4 (8.8%) > C1 (3.5%), while in soaked leaf treatment, C3 and C4 showed increment by 7.5% and 3.1% and inhibition by 4.5 and 2.5% in C1 and C2, respectively.
At 3rd harvesting, promotion of 3.8%, 3% and 2.5% in C1, C2 and C3 concentration in dry root weight of crushed leaf extract and inhibition of 8.7% in C4 was reported, whereas, in soaked leaf extract, the increment was observed to be: C3 (35%), C1 (31%) and C4 (22%) and inhibition of 17.6% in C2. Dry shoot weight of crushed leaf extract showed increment in order: C4 (13.3%) > C3 (12.4%) > C2 (9.4%) > C1 (4.5%), whereas, in soaked leaf extract inhibition of 21% and 4% in C2 and C4 was observed and increment of 17.2% and 5.2% in C3 and C1.Total dry weight in crushed leaf extract showed increment in order of 23.9%, 15.3%, 9.4% and 4.5% for C2, C3, C4 and C1, respectively, while in soaked leaf extract, the increment was recorded to be 21%, 12% and 1.4% in C3, C1 and C4 , respectively, and inhibition was 20.6% in C2. During 1st harvest, crushed stem extract showed increase in dry root weight at all concentrations: 12.15% at C1, 18.3% at C2, 27.6% at C3and 24.9% at C4 , whereas, soaked stem treatment showed inhibition at all concentrations: by 22.7% at C1, 7.5% at C2, 27.8% at C3 and 17.5% at C4. Dry shoot weight was suppressed at C1 (0.09%), C2 (0.01%) and C4 (0.03%) and promoted it at C3 (0.02%) in crushed stem extract, whereas, soaked stem extract showed inhibition at C1 (1.5%) and C4 (1.8%) and increment in C2 (6.3%) and C3 (1.99%). Total dry weight was repressed at lower concentration: C1(7.02%) and enhanced at higher concentrations: C2 (2.11%), C3 (7.29%) and C4 (1.86%) in case of crushed stem treatment, whereas, soaked stem treatment inhibited the total dry weight by 4.16% at C1, 2.3% at C3 and 4% at C4 and promoted at C2 by 3.09%. At 2nd harvest, crushed stem extract showed inhibitory effect on dry root weight at C1 (5.22%) and C4 (6.73%) and promoted it at C2 (2.5%) and C3 (0.41%), whereas, soaked stem extract showed inhibition at C2 (15.63%) and C3 (6.85%) and increment at C1 (1.99%) and C4 (2.12%). Dry shoot weight was promoted by crushed stem extract at C1 by 17.58%, C3 by 2.09% and C4 by 2.16% and inhibited at C2 by 5.43%, however, soaked stem treatment showed considerable inhibition at all concentration: C1 (15.75%), C2 (0.08%), C3 (13.76%) and C4 (12.7%)
 |
Table 1: Two-way ANOVA showing the impact of L. camara stem extracts on the seed germination and early seedling growth of rice. |
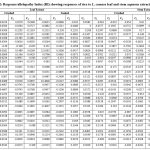 |
Table 2: Response/allelopathy Index (RI) showing responses of rice to L. camara leaf and stem aqueous extracts. |
 |
Figure 1: Effect of crushed and soaked leaf and stem extract on seed germination of rice. |
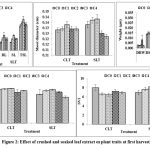 |
Figure 2: Effect of crushed and soaked leaf extract on plant traits at first harvest. |
 |
Figure 3: Effect of crushed and soaked leaf extract on plant traits at second harvest. |
 |
Figure 4: Effect of crushed and soaked leaf extract on plant traits at third harvest. |
 |
Figure 5: Effect of crushed and soaked stem extract on plant traits at first harvest. |
 |
Figure 6: Effect of crushed and soaked stem extract on plant traits at second harvest. |
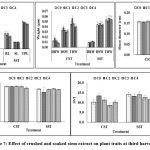 |
Figure 7: Effect of crushed and soaked stem extract on plant traits at third harvest. |
Crushed stem extract showed increment in total dry weight at C1 (14.3%), C3 (5.37%) and C4 (3.09%) and inhibition at C2 (1.9%), whereas, soaked stem extract suppressed total dry weight at all concentrations: C1 (10.6%), C2 (2.11%), C3 (10.81%) and C4 (8.87%). At 3rd harvesting, the dry root weight was observed to be promoted at lower concentration in crushed stem extract i.e. C1 (15.57%) and inhibited at higher concentrations: C2 (9.63%), C3 (28.72%) and C4 (31.6%), whereas, soaked stem treatment showed inhibition at C1 (7.73%) and C4 (4.64%) and increment at C2 (2.34%) and C3(9.47%) at 3rd harvest. Dry shoot weight was inhibited at higher concentrations: C2 (0.33%), C3 (12.5%) and C4 (5.9%) and promoted at lower concentration: C1 (10.21%) in crushed stem extract, whereas, soaked stem extract showed inhibition at C1 (5.1%), C3 (6.57%) and C4 (3.84%) and increment at C2 (1.98%).
Same trend was observed in case of total dry weight as crushed stem treatment showed inhibition in at C2 (0.92%), C3 (14.58%) and C4 (11.87%) and increment at C1 (15.78%), whereas, soaked stem extract inhibited the total dry weight at C1 (5.31%), C3 (2.67%) and C4 (3.51%) and promoted at C2 (2.32%). Roots showed stronger and sensitive responses towards L. camara leaf extracts than shoots. This could be due to the close contact of roots with the extract solution which was added to the soil.15 Individual plant roots grow through soil following beneficial mechanical and moisture gradients in order to obtain water and nutrients.32-33 By applying localized suction around the root-soil interface, plant roots pull water towards them.34 Cell growth is a biomechanically irreversible process in which wall stress relaxation, water uptake, wall expansion and turgor restoration are closely connected.35 There may be possible damage to plasma membrane due to seed pretreatment with the leaf extracts and leachates of L. camara can be demonstrated from the higher dissolution of amino acids and soluble carbohydrates from the water imbibed seeds.36 However, without any deposition of new materials into the existing wall during growth, the cell would eventually burst as a result of wall thinning and subsequent mechanical failure.35 Abiotic stress causes changes to cell wall structure 37-38, which can be sensed by cell wall integrity sensors. The reason behind the fluctuating results was the various phytochemicals like aromatic alkaloids and phenolic content released from soaked and crushed leaf extracts which may alter the physiochemical properties of soil that may have influenced the plant traits (root length, shoot length, fresh and dry biomass).39 The secondary metabolites present in L. camara such as phenolics, with umbelliferon, methyl coumarin and salicylic acid being the most toxic one.40
Some of them are: lantadene a and b, icterogenin, oleanoic acid, ursonicacid, 4-epihederagonic acid, 24-hydroxy-3-oxours-12-en-28-oic acid, lantanolic acid, lantanone, lantanilic acid, lantic acid, camarilic acid, camaracinic acid, camarinic acid, camaryolic acid,ursoxy acid, camarolide, linaroside, lantanoside, martynoside, α-phellandrene, dipentene, α-terpinol, geraniol, cineol, eugenol, citral, furfural, phellandrone, linalool.13,15 There are wide range of phenolic compounds which are often mentioned as assumed allelochemicals, in plants and soils. These phenolic compounds depicted inhibition in concentration dependent manner.18, 41 Previous researchers 27 assessed that plant phenolic acids occurrence and behavior in soil microenvironments results into its potential involvement in allelochemical interference interactions. Phenolic compounds have the ability to alter and influence several enzymatic activities and major physiological processes, such as plant hormone functions, nutrients uptake, water balance and stomatal functions, photosynthesis, respiration, and the metabolism of certain compounds and carbon flow.42-43 Aqueous leaf extract recorded maximum inhibitory effect followed by stem and root extract to selected crop species.44 Allelochemicals released into the soil can change soil properties, in turn affecting the composition and diversity of soil microbial community.45-46 They alter soil pH and change the microbial community activity, thereby can modify the native plant nutrient uptake.2,9
At 1st harvest, crushed stem extract showed decrease in relative moisture content in comparison to control and showed proportional relation in inhibition with increasing extract concentrations i.e., C1 (0.1%) < C2 (1.97%) < C3 (9.97%) < C4 (11.4%), in contrast, soaked stem extract promoted relative moisture content at C1(1.99%) and inhibited at C2, C3 and C4 by 1.22%, 2.34% and 8.9%, respectively. At 2nd harvest, relative moisture content was promoted by crushed stem extract at lower concentrations i.e., C1 (2.47%), C2 (0.65%), C3 (1.27%) and inhibited at the highest concentration: C4 (7.65%), whereas, soaked stem extract promoted relative moisture content at C1 (3.38%), C3 (5.11%), C4 (3.57%) and inhibited at C2 (0.39%). Crushed stem extract showed inhibition in relative moisture content at 3rd harvest at all concentrations- C1 (0.9 %), C2 (0.01%), C3 (1.13%) and C4 (5.08%), whereas, soaked stem extract promoted relative moisture content at C3 by 0.58% and inhibited at C1, C2 and C4 by 10.06%, 1.46% and 2.02%, respectively.
Effect of Plant Part and Extract Type on Seed Vigor Index
Seed vigor index in crushed leaf extract showed inhibition of 19.5%, 17.1%, 13.7% and 10.4% in C2, C1, C4 and C3, respectively.
The soaked leaf extract increased seed vigor index of rice by 10%, 8.3%, 3.2% and 3% in C2, C1, C4 and C3, respectively. When compared to 1st and 2nd harvest, crushed leaf extract showed increment in order of C4 (56.9%) > C3 (16.8%) > C2 (3.1%) > C1 (3%), while, soaked leaf extract showed inhibition at C2 (29%) and C3 (11.5%) and increment at C4 (10.8%) and C1 (3.6%) for seed vigor index. At 3rd harvest, crushed leaf extract showed inhibition in C2 (3.4%), C3 (2.7%) and C1 (0.0%) and increment in C4 (10.9%), while, in soaked leaf extract, C4 (20%), C3 (10.3%) and C2 (0.9%) showed increment while suppression of 5.2% was observed in C1 for seed vigor index. At 1st harvest, seed vigor index was promoted by crushed stem extract at C1 (9.17%) and C4 (2.24%) and inhibited at C2 (3.7%) and C3(5.8%), whereas, soaked stem extract inhibited seed vigor index at all the concentrations- C1 (19.21%), C2 (16.1%), C3 (22.5%) and C4 (17.5%). At 2nd harvest, crushed stem extract suppressed seed vigor index at all the concentrations: C1 (1.37%), C2 (10.46%), C3 (5%) and C4 (16.61%), whereas, soaked stem extract promoted seed vigor index in C1 (9.46%) and C3 (3.85%) and inhibited in C2 (5.59%) and C4 (1.44%). After 3rd harvesting, seed vigor index was observed to be promoted by crushed stem extract at lower concentrations i.e. C1 (17.05%) and C2 (3.4%) and inhibited it at higher concentrations: C3 (19.4%) and C4 (4.1%), however, soaked stem extract inhibited seed vigor index at all concentrations: C1 (9.64%), C2 (5.42%), C3 (12.83%) and C4 (18.44%).
The soaked leaf extract suppressed rice seed germination at higher (C4 ) concentration, while, in crushed leaf extract stimulated. If we compare the results according to harvesting time in leaf either the crushed or soaked extracts all the measured plant traits i.e., length, biomass, relative moisture content and seed vigor index exhibited differential pattern of inhibition or increment. As also reported by 47 all the plant traits such as length, biomass, moisture content and seed vigor index illustrated fluctuating pattern along the concentration gradient. At second and third harvest, relatively lower suppression was recorded in the majority of studied plant traits as compared to first harvesting. This could be due to the adaptation of seedlings towards different concentrations of both crushed as well as soaked leaf treatment after experiencing its environment during the initial developmental phases.48 On the other hand, soaked stem extract had more inhibitory effects on seed germination, seedling biomass and seed vigor index, whereas, crushed stem treatment was more suppressive on the traits like seedling growth and relative moisture content.
Soaked treatment resembles the allelopathic nature of plants in soil, since many allelochemicals are leached out of living plants or plant residue by rain or dew.49-50 The soaked stem extract demonstrated with maximum suppression in comparison to crushed stem extract could be due to the storage function of stem. As stem stores the nutrients and these nutrients would leach out during crushing, whereas, soaking would only release the phytochemicals which are present in either extracellular matrix or cell wall components without disrupting the cell organelles which store the photosynthetic and bioactive compounds.28 This study demonstrated that the aqueous soluble allelochemicals of L. camara decrease the initial growth of rice variety depicts that the effects are concentration-dependent.51, 52 According to three harvestings performed the last showed more inhibitory effects for plant traits, plant growth, biomass, moisture content and vigor index either for crushed or for soaked stem treatment. Previous experiments conducted indicate that invasive plants increase utilization of nutrients with allelopathic substances that also inhibit native plant growth and decrease the plant biomass.8, 53, 54
Conclusion
Experimental results indicated suppressive as well as promoting effects of aqueous stem extracts of L. camara on seed germination and seedling growth of rice plants. More pronounced inhibitory effects on measured traits were observed for soaked stem extract as compared to the crushed stem extract. In general, the lower aqueous extract concentration recorded increment in the studied traits while higher aqueous extract concentrations suppressed the germination and early seedling growth of selected rice variety. In this study with increasing time, the rice crop showed adaptation towards the allelochemical released from the aqueous extracts of leaves as indicated by insignificant effect at 2nd harvest. But in case of stem after second harvesting both crushed and soaked aqueous extract showed inhibition for every plant trait. Even though, laboratory experiments are important to demonstrate the allelopathic affects it necessitates in investigating the significance of these results under field conditions. Besides, isolation and identification of allelochemicals released by the L. camara plants may help in assessing the distinctive role of specific chemical on crop plant. Further field investigations are required to test the crops against the L. camara weed extracts considering other growth parameters and yield attributes. Ultimately this research will help to contribute the deeper understanding of the key factors influencing the allelochemical interactions and actual mechanisms involved in the differential effects of the stem allelochemicals on different agricultural fields.
Acknowledgement
We are thankful to the Head, Department of Botany, D.S.B. Campus, Nainital for providing necessary lab facilities.
Funding Sources
The author(s) received no financial support for the research, authorship, and/or publication of this article.
Conflict of Interest
The authors have no conflict of interest to disclose.
Data Availability
This statement does not apply to this article.
Ethics Statement
This research did not involve human participants, animal subjects, or any material that required ethical approval.
Clinical Trial Registration
This research does not involve any clinical trials.
Author contributions
Vartika Joshi: Methodology, Writing – Original Draft, Data Collection, Analysis, Writing – Review & Editing.
Charu Joshi: Methodology, Writing – Original Draft, Data Collection, Analysis, Writing – Review & Editing.
Archana Fartyal: Writing – Review & Editing, Analysis.
Kiran Bargali: Analysis, Visualization and Supervision.
Surendra Singh Bargali: Resources, Analysis, Visualization and Supervision.
References
- Khatri K., Negi B., Bargali K., Bargali S. S. Trait variability in co-occurring invasive and native plant species in road side population of Kumaun Himalaya. Braz. J. Bot.2022; 45(3): 1099-1110.
CrossRef - Ofosu R., Agyemang E. D., Marton A., Pasztor G., Taller J., Kazinczi G. Herbicide resistance: Managing weeds in a changing world. Agronomy. 2023; 13(6): 1-16. https://doi.org/10.3390/agronomy 13061595.
CrossRef - Kaur P., Kaur P., Bhullar M. S. Environmental aspects of herbicide use under intensive agriculture scenario of Punjab. In: Sondhia S, Choudhury P, Sharma A, eds. Herbicide Residue Research in India. Singapore: Springer; 2019: 1-13. doi:10.1007/978-981-13-1038-6_3.
CrossRef - Bajwa A. A., Mahajan G., Chauhan B. S. Nonconventional weed management strategies for modern agriculture. Weed Sci. 2015; 63(4): 723-747. https://doi.org/10.1614/WS-D-15-00064.1.
CrossRef - Khatri K., Negi B., Bargali K., Bargali S. S. Effects of different concentrations of leaf residues of Ageratina adenophora on seed germination and growth behavior of two native tree species of Kumaun Himalaya, India. Waste Biomass Valorization. 2024; 15(2): 923-943. https://doi.org/10.1007/s12649-023-02213-5
CrossRef - Khatri K., Negi B., Bargali K., Bargali S. S. Toxicological assessment of invasive Ageratina adenophora on germination and growth efficiency of native tree and crop species of Kumaun Himalaya. Ecotoxicology. 2024; 1-12.
CrossRef - Zhang Z., Liu Y., Yuan L., Weber E., van Kleunen M. Effect of allelopathy on plant performance: a meta‐analysis. Ecology Letters. 2021; 24(2): 348-362.
CrossRef - Khatri K., Negi B., Bargali K., Bargali S. S. Trait plasticity: A key attribute in the invasion success of Ageratina adenophora in different forest types of Kumaun Himalaya, India. Environ. Dev. Sustain.2024; 26(8): 21281-21302.
CrossRef - Negi B., Khatri K., Bargali K., Fartyal A., Chaturvedi R. Impact of invasive Ageratina adenophora on relative performance of woody vegetation in different forest ecosystems of Kumaun Himalaya, India. J. Mt. Sci. 2023; 20(9): 2557-2579.
CrossRef - Rice E. L. Allelopathy. 2nd ed. Orlando, FL: Academic Press; 1984.
- Joshi V., Joshi C., Bargali S. S., Bargali K. Effects of aqueous extracts from above ground parts of Lantana camara on seed germination, growth and yield of Wheat crop. Ecol. Front. 2024. http://dx.doi.org/10.1016/j.ecofro.2024.07.006
CrossRef - Ntaila Y. W., Mbeya R. A. Allelopathic effects of Lantana Camara extract on weeds and cultivated crops: A systematic review. J. Agric. Food Environ. Anim. Sci. 2023; 4(2): 243-258.
- Sharma G. P., Raghubanshi A. S. Effect of Lantana camara L. cover on local depletion of tree population in the Vindhyan tropical dry deciduous forest of India. Appl. Ecol. Environ. Res. 2007; 5(1): 109-121.
CrossRef - Heirro J. L., Callaway R. M. Allelopathy and exotic plant invasion. Plant Soil. 2003; 256(1): 29-39.
CrossRef - Kato-Noguchi H., Kurniadie D. Allelopathy of Lantana camara as an invasive plant. Plants.2021; 10(5): 1028.
CrossRef - Negi G. C. S., Sharma S., Vishvakarma S. C. R., Samant S. S., Maikhuri R. K., Prasad R. C., Palni L. M. S. Ecology and use of Lantana camara in India. The Bot Review.2019; 85(2): 109-130.
CrossRef - Negi B., Khatri K., Bargali S. S., Bargali K. Invasive Ageratina adenophora (Asteraceae) in agroecosystems of Kumaun Himalaya, India: a threat to plant diversity and sustainable crop yield. Sustainability. 2023; 15(14): 10748. https://doi.org/10.3390/su151410748.
CrossRef - Pathak D. K., Bargali K., Bargali S. S., Fartyal A. Seed germination and early seedling growth behavior of critically endangered Catamixis baccharoides to variation in soil type. Vegetos. 2024; 1-10.
CrossRef - Lalitha K. R., Tank R. V., Chawla S. L., Jena S. Effect of chemicals on seed germination and seedling growth of Aonla (Emblica officinalis Gaertn.). J. Pharm. Innov. 2020; 9(12): 239-243.
- Sumithra K., Jutur P. P., Carmel B. D., Reddy A. R. Salinity-induced changes in two cultivars of Vigna radiata: responses of antioxidative and proline metabolism. Plant Growth Regul. 2006; 50(1): 11-22.
CrossRef - Kumar M., Garkoti S. C. Allelopathy effects of invasive alien Ageratina adenophora on native shrub species of chir pine forest in the central Himalaya, India. J. For. Res. 2022; 27(1): 53-62.
CrossRef - Ma J., Feng X., Yang X., Cao Y., Zhao W., Sun L. The leaf extract of crofton weed (Eupatorium adenophorum) inhibits primary root growth by inducing cell death in maize root border cells. Plant Diver. 2020; 42(3): 174-180.
CrossRef - Tadele D. Allelopathic effects of Lantana (Lantana camara L.) leaf extracts on the germination and early growth of three agricultural crops in Ethiopia. Momona Ethiopian J. Sci. 2014; 6(1):111-119. https://doi.org/10.4314/mejs.v6i1.102419.
CrossRef - Morita S., Ito M., Harada J. Screening of an allelopathic potential in arbor species. Weed Biol. Manag. 2005; 5(1): 26-30.
CrossRef - Hossain M. K., Alam N. M. D. Allelopathic effects of Lantana camara leaf extract on germination and growth behavior of some agricultural and forest crops in Bangladesh. Pak. J. Weed Sci. Res. 2010; 16(2): 217-226.
- Negi B., Bargali S. S., Bargali K. Allelopathic interference of Ageratum conyzoides L. against rice varieties. Curr. Agric. Res. J. 2020; 8(2): 69-76.
CrossRef - Mishra A., Tripathi S. Lantana camara: a magical weed for weed control. Int. J. Multidiscip. Educ. Res. 2021; 12(4): 97-99. https://doi.org/10.2139/ssrn.3873581.
- Khatri K., Bargali K., Bargali S. S., Negi B. Effects of leaf residues from Ageratina adenophora on germination, growth, and productivity of two rabi crops. Acta Ecol. Sin. 2022; 43(2): 363-374. https://doi.org/10.1016/j.chnaes.2022.05.001.
CrossRef - Thellier M. Plant sensitivity to stimuli. In: Thellier M. Plant Responses to Environmental Stimuli. Dordrecht, France: Springer; 2017: 7-22.
CrossRef - Sharma G. P., Singh J. S., Raghubanshi A. S. Plant invasions: Emerging trends and future implications. Curr. Sci. 2005; 88(5): 726-734.
- Ahmed R., Uddin M. B., Khan M. A., Mukul S. A., Hossain M. K. Allelopathic effects of Lantana camara on germination and growth behavior of some agricultural crops in Bangladesh. J. For. Res. 2007; 18(4): 301-304.
CrossRef - Eapen D., Barroso M. L., Ponce G., Campos M. E., Cassab G. I. Hydrotropism: root growth responses to water. Trends Plant Sci. 2005; 10(1): 44-50.
CrossRef - Colombi T., Braun S., Keller T., Walter A. Artificial macropores attract crop roots and enhance plant productivity on compacted soils. Sci. Total Environ. 2017; 574: 1283-1293.
CrossRef - Duncan S., Daly K., Sweeney P., Roose T. Mathematical modelling of water and solute movement in ridged versus flat planting systems. Eur. J. Soil Sci. 2018; 69(6): 967-979.
CrossRef - Cosgrove D. J. Plant cell growth and elongation. In: eLS. Chichester, UK: John Wiley & Sons, Ltd; 2014.
CrossRef - Mishra A. Allelopathic properties of Lantana camara. Int. Res. J. Basic Clin. Stud. 2015; 3(1): 13-28.
- Tenhaken R. Cell wall remodeling under abiotic stress. Front. Plant Sci. 2014; 5: 771.
CrossRef - Novakovic L., Guo T., Bacic A., Sampathkumar A., Johnson K. L. Hitting the wall – sensing and signaling pathways involved in plant cell wall remodeling in response to abiotic stress. Plants. 2018; 7(4): 89.
CrossRef - El-Kenany Eman T., Salama M. Suppression effects of Lantana camara L. aqueous extracts on germination efficiency of Phalaris minor Retz. and Sorghum bicolor L. (Moench). J. Taibah Univ. Sci. 2013; 7(2): 64-71.
CrossRef - Kumar B., Verma S. K., Ram G., Singh H. P. Temperature relations for seed germination potential and seedling vigor in Palmarosa (Cymbopogon martinii). J. Crop Improv. 2012; 26(6): 791-801. https://doi.org/10.1080/15427528.2012.689799.
CrossRef - Gindri D. M., Coelho C. M. M., Uarrota V. G., Rebelo A. M. Herbicidal bioactivity of natural compounds from Lantana camara on the germination and seedling growth of Bidens pilosa. Pesqui Agropecu Trop. 2020; 50: 1-10. https://doi.org/10.1590/1983-40632020v5057746.
CrossRef - Einhellig F. A. Interactions involving allelopathy in cropping systems. Agron. J. 1996; 88(6): 886-893.
CrossRef - Acharya K., Poudel S., Acharya D. Effect of leaf extracts of Lantana camara L. on germination and growth of some crops species. Saptagandaki J. 2022; 13(1): 32-47. https://doi.org/10.3126/sj. v13i1.54945.
CrossRef - Chaudhary B. L., Vyas V. Regeneration of Funaria hygrometrica in half knop’s liquid culture medium. Panjab Univ. Res. J (Sci). 2004; 54(1-4): 143-146.
- Liu Xin Gang L. X., Tian Fa Jun T. F., Tian Ying Ying T. Y., Wu Yan Bing W. Y., Dong Feng Shou D. F., Xu Jun X. J. Zheng Yong Quan Z. Y. Isolation and identification of potential allelochemicals from aerial parts of Avena fatua L. and their allelopathic effect on wheat. J. Agric. Food Chem. 2016; 64(18): 3492-3500
CrossRef - Puig C. G., Goncalves R. F., Valentao P., Andrade P. B., Reigosa M. J. Pedrol N. The consistency between phytotoxic effects and the dynamics of allelochemicals release from Eucalyptus globulus leaves used as bioherbicide green manure. J. Chem. Ecol.2018; 44: 658-670.
CrossRef - Khatri K., Negi B., Bargali K., Bargali S. S. Effects of elevation and habitat on leaf and reproductive traits of Ageratina adenophora (Sprengel) King & Robinson. S. Afri. J. Bot.2022; 147, 859-870.
CrossRef - Talhi F., Gherraf N., Zellagui A. Allelopathic effect of the aqueous extract of Lantana camara L. on the germination and development of four vegetable species. Int. J. Chem. Biochem. Sci. 2020; 18: 116-121. https://doi.org/10.21744/ijcbss.v18n1.1329.
- Kumar J., Devashree Y. The phytotoxicity of Lantana camara leaf extract on the germination and growth of Vigna radiata. Int. J. Res. Anal. Rev. 2018; 5(4): 316-319. https://doi.org/10.2139/ssrn.3239028.
- Julio A., Carven T. W., Daniel T. H., Franzine V. Y., Yanesa Z., Tare V. R. J. K. Allelopathic effect of Lantana camara and Chromolaena odorata leaf extracts on plant germination. Asian J. Agric. Biol. 2019; 7(2): 190-196. https://doi.org/10.3935/ajab.2019.01.02.
- Khatri K., Bargali K., Negi B. Germination and early seedling growth of two rice varieties as affected by invasive Ageratina adenophora. Curr. Agric. Res. J. 2020; 8(2): 108-117.
CrossRef - Zhang F., Guo J., Chen F., Liu W., Wan F. Identification of volatile compounds released by leaves of the invasive plant crofton weed (Ageratina adenophora, Compositae), and their inhibition of rice seedling growth. Weed Sci. 2012; 60(2): 205-211.
CrossRef - Negi B., Khatri K., Bargali S. S., Bargali K. Factors determining the invasion pattern of Ageratina adenophora Spreng. in Kumaun Himalaya India. Environ. Exp. Bot. 2024; 106027. https://doi.org/10.1016/j.envexpbot.2024.106027.
CrossRef - Khatri K., Bargali K., Negi B., Fartyal A., Bargali S. S. Evidences of phenotypic plasticity, and lack of local adaptation and clinal differentiation in invasive Ageratina adenophora from Uttarakhand Himalaya. Environ. Dev. Sustain.2024; (Accepted).

