Introduction
On Earth, all living being experiences gravitational acceleration of 9.8 m/s2, termed as one Earth’s gravity or simply 1 g. If this value is reduced to 10-3 to 10-6 times 1 g then it is called as microgravity or low gravity or simply µg. To achieve the condition of microgravity one has to either travel into deep space (real microgravity) or it can be simulated on Earth by using different platforms such as parabolic flights, sounding rockets, drop towers or more commonly used instruments by plant biologists, clinostat (simulated microgravity or SMG). Recent studies revealed that gravity has had a profound impact on the form, structure and function of plants (Gilles Clement, 2005). The instrument called clinostat developed by Von Sachs (1879) has been widely used to simulate microgravity condition on ground (Jack Van Loon, 2007). Previous reports show that microgravity (real or simulated) affects growth, chlorophyll content and photosynthetic performance in different plants. However, variation in results was obtained in studies carried out by using clinostat.
For instance, increase in shoot length in rice seeds (Sasanishiki) under clinorotation was also observed by Takakura et al. (1996). The enhancement in growth as measured by root, shoot length and weight are consistent with similar observations on soybean (Hilaire et al., 1996), azuki bean (Hoson et al., 1992) as well as pea and maize (Shimazu et al., 2001) using 3-D clinostat. Jagtap et al. showed similar increase in root shoot length and weight as well as chlorophyll content in rice seedlings when rotated at 2 rpm in 1-D clinostat (Jagtap et al., 2010). The photosynthesis process might also affected as observed in 4 – 10 days old garden cress seedlings when exposed to 3-D clinostat rotated at lower rpm (Yamada et al., 1993). The proton permeability in pea chloroplasts grown under slow clinorotation (2 rpm) was increased (Mikhaylenko et al., 2001). The rate of CO2 assimilation was found to be greater in Pisum sativum grown on a horizontal axis clinostat as compared to control (Brown et al., 1996).
Though the enhancement in growth, chlorophyll content and photosynthetic performance was observed in different plants under microgravity, some of the previous reports showed no change or decrease in plant growth and development (Briarty et al., 1995; Kiss et al., 1998; Levine et al., 2001), chlorophyll content (Laurinavichius et al., 1986, Moleshko et al., 1991; Mikhaylenko et al., 2001) and photosynthetic performance (Tripathy et al., 1996, Chen et al., 2013) in space and clinorotation.
Thus, these results show variation and do not show consistency. The discrepancies in the results may be due to the difference in methodologies, different environmental conditions and use of different species (Aarrouf et al., 1999). These inconsistent results could also be due to the duration of exposure to clinorotation (Jagtap et al., 2011). Besides these factors, it would also be interesting to study whether clinorotation has similar effects on monocotyledonous and dicotyledonous plants. Therefore, in the present work, the attempt has been made to study and compare the seed germination, growth and especially the chlorophyll a fluorescence (O-J-I-P) transient and other important photosynthetic parameters in rice and mungbean seedlings grown under slow rotating (2 rpm) clinostat under similar environmental conditions. The response against simulated microgravity was compared in two plants viz., monocotyledonous rice and dicotyledonous mungbean.
Materials and Methods
Seed selection
Healthy seeds of monocotyledonous rice (Oryza sativa ‘PRH-10’) and dicotyledonous mungbean [Vigna radiata (L.) Wilczeck] were selected for the experiment. Seeds were surface sterilized by using 0.5 % fungicide (Uthane M-45) and thoroughly washed with distilled water (D/W) to remove the traces of fungicide. Seeds were then soaked in D/W for 24 hours at room temperature. After 24 hours, these seeds were mounted on 0.8 % agar gel in Perspex beakers of volume 150 ml. Total 8 seeds per beaker were mounted on a periphery of a circle of radius 1.5 cm.
Simulated microgravity (Clinorotation) treatment
Beakers were covered with polythene bags containing five holes at upper side for ventilation and placed on a clinostat with one rotation axis developed in-house. Seeds were rotated continuously for 5 days at a constant speed of 2 rpm (simulated microgravity value approx. 7 x 10-5 g). Most of the previous studies on various plant seeds and seedlings were carried out at 2 rpm.
Measurements
Percentage seed germination and Growth
Percentage seed germination and growth parameters were measured on 5th day from sowing. Orientations of root and shoot were continuously monitored.
Chlorophyll extraction
Chlorophyll was extracted by using DMF (N, N- dimethyl formamide) as a solvent (Porra et al., 1989). Shoots from 5 days old seedlings were immersed in DMF solution and stored at 8 – 10 °C for overnight. On the next day, the solution was filtered using Whatman® filter paper. Filtered chlorophyll solution was used for spectroscopic measurement. Absorption spectra of chlorophyll were recorded for both control and clinorotated samples by using UV-Visible spectrometer (Perkin Elmer Lambda 950). Chlorophyll- a, chlorophyll – b and total chlorophyll content were calculated from absorption spectra by using Arnon formula (Arnon, 1949).
Chlorophyll fluorescence measurements
The fifth day expanded leaf was used to measure chlorophyll fluorescence with portable Handy PEA (Hansatech Instruments, Kings Lynn, UK). After dark adaptation of leaves for ten minutes, a single strong 1 s-light pulse (3500 μmol/m2/s) was applied on them with the help of three light-emitting diodes (650 nm). The fast fluorescence kinetics (Fo to Fm) of control and simulated microgravity treated seedlings was recorded during 10 μs to 1 s. The chlorophyll fluorescence parameters were analyzed on the basis of data extracted from the O-J-I-P transient (Strasser et al., 2000).
Results
Growth
The results of the present study showed no significant difference in the percentage seed germination in rice and mungbean. Figure 1 shows photographs of control and clinorotated rice seedlings recorded on 2nd day from sowing. As seen from the figure, control seedlings clearly follows normal gravitropism behaviour i.e. shoots were grown upwards and roots grown downwards (towards the direction of gravity). However, in clinorotated seedlings, direction of shoots and roots was random i.e. shoots were oriented outward (towards the open end of the beaker). Similar trend for control and clinorotated samples was observed in case of mungbean seedlings.
 |
Figure 1: Representative photograph showing behaviour of 2 days old rice seedlings for A) control and B) clinorotated samples. |
 |
Figure 2: Representative photograph showing growth of 5 days old rice and mungbean seedlings for A) control and B) clinorotated samples. |
Figure 2 shows representative photograph of growth of rice and mungbean seedlings on 5th day from sowing. A comparison between root lengths and shoot lengths in rice and mungbean seedlings is given in figure 3. Figure 4 represents a comparison between leaf area in rice and mungbean seedlings. As shown in figure 3, enhancement in root and shoot length was observed in rice and mungbean in clinorotated samples than control samples.
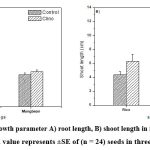 |
Figure 3: Changes in growth parameter A) root length, B) shoot length in rice and mungbean after five days sowing. Each value represents ±SE of (n = 24) seeds in three repeated experiments. |
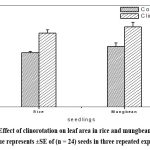 |
Figure 4: Effect of clinorotation on leaf area in rice and mungbean seedlings. Each value represents ±SE of (n = 24) seeds in three repeated experiments. |
Interestingly, the percentage increase in root length in mungbean was found to be less (9 %) as compared with that in rice (17 %). Similarly, percentage increase in shoot length was also less in mungbean (12 %) as compared with that in rice (30 %). It is seen from figure 4 that the leaf area was found to be increased in clinorotated samples than control samples in rice and mungbean. The percentage increase in leaf area was also marginally less in mungbean (25 %) as compared with that of rice (27%).
Chlorophyll content
Results of chlorophyll content showed significant increase in chlorophyll contents (chlorophyll a, chlorophyll b and total chlorophyll) in clinorotated samples than control samples in rice as well as mungbean. Increase in chlorophyll content in clinorotated samples was also observed by Abilov (1986), Aliyev (1987) and Jagtap (2011). However, chlorophyll a/b ratio was found to be decreased in clinorotated samples than control samples in all three seedlings. Table 1 shows the percentage of increase or decrease in chlorophyll a, chlorophyll b and total chlorophyll contents and chlorophyll a/b ratio in clinorotated samples with respect to the controls. As shown in table 1, percentage increase in chlorophyll contents in clinorotated samples in rice was more (Chl a-52 %; Chl b-59 % and total chl-53 %) as compared with that in mungbean (Chl a-19 %; Chl b-35 % and total chl-22 %). However, percentage decrease in chlorophyll a/b ratio in rice is less (14 %) as compared with mungbean (21 %).
Table 1: Percentage increase or decrease (indicated by – sign) in chlorophyll a, chlorophyll b, total chlorophyll and chlorophyll a/b ratio in clinorotated samples with respect to those of the controls. This table is a representative of 3 experiments.
|
Percentage increase or decrease
|
||
|
Rice |
Mungbean |
|
|
Chlorophyll-a |
51.56 % |
18.89 % |
|
Chlorophyll-b |
58.60 % |
34.68 % |
|
Total chlorophyll |
52.91 % |
21.82 % |
|
Chlorophyll a/b |
-14.36 % |
-21.09 % |
Chlorophyll fluorescence parameters
Chlorophyll a fluorescence measurement is useful non invasive probe for monitoring the PS II behavior in plants under different environmental conditions (Strasser BJ and Strasser RJ, 1995; Strasser et al., 2000). The fluorescence transient curve during illumination is labeled as O-J-I-P (Strasser BJ and Strasser RJ, 1995; Strauss et al., 2003). The analysis of fluorescence parameters based on theory of fluxes indicates the changes of absorbed, dissipative, trapping and electron fluxes named JIP test. The fluorescence curve begins with initial fluorescence Fo (O) and achieve maximum fluorescence Fm (P) via two intermediate steps J and I with respect to time. The entire curve is divided into three stages O-J, J-I and I-P. The OJIP test can be used to study the behavior of PSII when plants are exposed to different environmental conditions The (Fv/Fm) is more sensitive to the reaction centre side. The ratio of Fv/Fm in healthy leaves is about 0.78 – 0.83 regardless of plant species (Abbaspoor and Streibig, 2007).
Table 2: A – Effects of Clinorotation on chlorophyll fluorescence parameters in rice and mungbean on fifth day. Values are means ± SE (n=10).* and ** indicates the significant differences after treatments (P< 0.05 and P<0.01 respectively). Values indicated in parenthesis are percentage increase in clinorotated samples with respect to the control.
|
Chlorophyll fluorescence Parameters |
Rice
Control Clino |
Mungbean
Control Clino |
|
Fv/Fm |
0.76 ± 0.02 0.81* ± 0.007 (6%) |
0.81 ± 0.005 0.83* ± 0.003 (1.8%) |
|
Fm/Fo |
4.31 ± 0.28 5.30* ± 0.12 (18.59%) |
5.53± 0.46 5.92± 0.32 (9.87%) |
|
Fv/Fo |
3.34± 0.28 4.29* ± 0.12 (22%) |
4.45± 0.16 4.91* ± 0.11 (9.37%) |
|
PI (abs) |
0.55 ± 0.03 0.77**± 0.02 (27%) |
2.53 ± 0.01 2.91* ± 0.16 (12.91%) |
|
PI total |
0.76± 0.05 1.05**± 0.03 (24%) |
1.90 ± 0.02 2.48* ±0.26 (23%) |
Table 2: B – Effects of Clinorotation on chlorophyll fluorescence parameters in rice and mungbean on fifth day. Values are means ± SE (n=10).* indicate the significant differences after treatments (P< 0.05). Values indicated in parenthesis are percentage decrease in clinorotated samples with respect to the control.
|
Parameters |
Rice
Control Clino. |
Mungbean
Control Clino. |
|
δRo |
1.44± 0.19 1.37 0.63 (Negligible or no change) |
0.43± 0.002 0.46± 0.02 (6.65%) |
|
φRo |
0.41 ± 0.02 0.84 ± 0.26 (50%) |
0.21 ± 0.01 0.23± 0.001 (7.55%) |
|
ρRo |
0.54 ± 0.04 0.95 ± 0.37 (45%) |
0.25 ± 0.02 0.27 ± 0.01 (5.69%) |
|
φDo |
0.23 ± 0.01 0.19*± 0.04 (-19.23%) |
0.19 ± 0.005 0.17* ± 0.03 (-9.84%) |
|
DIo/RC |
0.87± 0.008 0.54* ± 0.14 (-38.54%) |
0.48 ± 0.06 0.42* ± 0.04 (-12.33%) |
Table 2: C – Effects of Clinorotation on chlorophyll fluorescence parameters in rice and mungbean on fifth day. Values are means ± SE (n=10).
|
Parameters |
Rice
Control Clino. |
Mungbean
Control Clino. |
|
Vj |
0.62± 0.02 0.59 ± 0.008 |
0.40 ± 0.04 0.40 ± 0.02 |
|
Vi |
0.44 ± 0.03 0.45± 0.003 |
0.74 ± 0.05 0.73± 0.05 |
|
φEo |
0.29 ± 0.03 0.33 ± 0.10 |
0.48 ± 0.01 0.49 ± 0.02 |
|
ΨEo |
0.39 ± 0.05 0.41 ± 0.01 |
0.60 ± 0.02 0.60 ± 0.004 |
|
ABS/RC |
3.75± 0.34 3.72± 0.08 |
2.54± 0.13 2.44 ± 0.18 |
|
TRo/RC |
2.87 ± 0.31 3.01 ± 0.06 |
2.04 ± 0.10 2.01 ± 0.15 |
An increase was observed in Fv/Fm, Fm/Fo, Fv/Fo ratios. Performance index (PI abs) and PI total were also significantly increased (Table 2 A). There was also increase in photosynthetic yields or flux ratios such as δRo, φRo, and ρRo (Table 2 B). Density of reaction centers was also increased. However, no significant change (Table 2 C) was observed in relative variable fluorescence at Vj and Vi φEo, Ψeo. ABS/RC,TRo/RC. There was decrease (Table 2 B) in dissipation energy yield like DIo/Rc and φDo under clinorotation as compared to the control. This may have happened, due to the reoxidation of QA which was increased by the electron transfer from PSII to PSI.
Discussion
In SMG seedlings, direction of shoots and roots was found to be random in rice as well as mungbean seedlings. Such random growth behavior of root and shoots in clinorotated samples was also observed by (Shimazu et al., 2001) in etiolated pea (Pisum sativum L. cv. Alaska) and maize (Zea mays L. cv. Golden Cross Bantam) seedlings grown under 3-dimensional clinostat.
The enhancement in growth is consistent with those obtained by Aarrouf where, Brassica napus seedlings grown for 5 days on the horizontal clinostat exhibited a faster development than in the controls. The biomass (fresh weight) of the root system, and also that of the shoot, was greater on the horizontal clinostat than in the controls (Aarroufet al., 1999). Similar results were obtained by Levine (Levine et al., 1990) and Jagtap (Jagtap et al., 2011) on rice seedlings.
As far as chlorophyll fluorescence parameters are concerned, the increase in Fv/Fm ratio could be associated with decrease in energy dissipation φDo and Dio/Rc in the antennae of PSII. However, PSII activity was unchanged under random positioning machine (RPM) condition as evident from chlorophyll fluorescence and thermoluminescence studies (Chen et al., 2013) as compared to 1 g control. Aluminium toxicity decreased Fv/Fm indicated that photoinhibition occurs in leaves exposed to Al (Jiang et al., 2008). Our findings lead to the hypothesis that, no photoinhibition occurs under clinorotation. No change in φEo in rice and mungbean plants also supports this hypothesis.
Fv/Fo represents the maximum primary yield of PSII. It also indicates the activity of water splitting complex at the donor side of PSII (Jafarinia and Shariati, 2012). An increase was observed in FV/Fo under clinorotation in mungbean and rice. Our results are consistent with that of strawberry and carnation (Zhao et al., 2002). In rice and mungbean, the ratio Fv/Fo increased by 22 % and 9.37% (p < 0.05), respectively during clinorotation as compared to the control (Table 2 A). It indicates that clinorotation plays significant role in increasing the activity of water splitting complex. The decrease in the ratio Fm/Fo indicates the damage to the photochemical apparatus (Lacuesta et al.,1992). In the present study, the ratio of Fm/Fo in mungbean increased by 10% under clinorotation with respect to the control. These findings suggest that clinorotation does not cause damage to the photosynthetic apparatus. Fluorescence rise at I step was observed when treated with different concentration of aluminium (Al) in Citrus leaves, indicating that Aluminium (Al) inhibits on the acceptor side of PSII (Jiang et al., 2008). An increase of Vj and Vi were used as probe for the inhibition of the electron transport at the acceptor side of the PSII (Lu 1999). On the basis of obtained results no change in Vj and Vi suggests that no photoinhibition occurred under clinorotation.Thus it can be concluded that clinorotation does not affect the acceptor side of PSII. The parameter performance index (PI abs) is used to determine the plant vitality with respect to different stress (Strasser et al., 2004; Zheng et al., 2009) An increase in performance index (PI abs) in response to clinorotation was mainly due to increase in the photochemical efficiency and photosynthetic electron transport. It is also accompanied by decreased φDo – quantum yield for energy dissipation. Increase in performance index (PI) in rice is about 27 % (p < 0.05) higher in clinorotated samples than control. It is also accompanied by decreased φDo – quantum yield (19.23 %; p<0.05) for energy dissipation, DIo/RC – dissipation energy (38.54 %; p<0.05) per reaction centre. Increase in performance index (PI) in mungbean is about 12.91% (p < 0.05) higher in clinorotated samples than control. It is also accompanied by decreased φDo – quantum yield (9.84 %; p<0.05) for energy dissipation, DIo/RC – dissipation energy (12.33%; p<0.05) per reaction centre.
Conclusion
The study revealed that simulated microgravity (SMG) led to increased growth, chlorophyll content, and chlorophyll fluorescence in both monocotyledonous rice and dicotyledonous mungbean seedlings, compared to control samples. However, the chlorophyll a/b ratio decreased significantly in SMG samples. Monocotyledonous rice showed a more pronounced improvement in growth and chlorophyll content compared to dicotyledonous mungbean. This pattern of response aligns with observations in other stress conditions. Monocotyledonous rice displayed greater adaptability to SMG than dicotyledonous mungbean. Further research at the cellular and molecular levels is needed to understand these adaptive mechanisms and potentially identify marker proteins or growth hormones associated with environmental stresses, including gravity.
Acknowledgement
The authors gratefully acknowledge Board of College and University Development (BCUD), SPPU, Pune and Indian Space Research Organization (ISRO) under scheme GOI- A-597 for providing financial support.
Funding Sources
Indian Space Research Organization (GOI-A-597)
Conflict of Interest
The Authors declare no Competing Financial or Non-Financial Interests.
Data Availability Statement
This statement does not apply to this article.
Ethics Approval Statement
This research did not involve human participants, animal, subjects, or any material that requires The ethical approval.
Authors’ Contribution
Sagar S. Jagtap: Design, optimization and conduction of experiments, result analysis, manuscript writing and editing
S M Kamble: Conduction of experiments, result analysis,
Jyotsana Dixit: Result analysis, manuscript writing and editing,
Pandit Vidyasagar: Experimental planning, manuscript editing and revision, supervisor.
References
- Gilles Clement. Fundamentals of Space Medicine. Space Technology Library, 2005, 17, 1-46
CrossRef - Jack J.W.A. van Loon Some history and use of the random positioning machine, RPM, in gravity related research Adv. Space Res.2007; 39: 1161–1165
- Takakura T., Goto E. and Tanaka M. The effect of gravity on plant germination. Space Res. 1996; 18, 41.5, pp. (4/5)255-(4/5)258,
CrossRef - Hilaire E., Peterson B. V., Giekema J. A., Brown C. S. Clinorotation affects morphology and ethylene production in Soybean seedlings. Plant Cell Physiol., 1996; 37 (7), 929-934
CrossRef - Hoson T., Kamisaka S., Masuda, Yamashita M. Changes in plant growth processes under microgravity conditions simulated by a three-dimensional clinostat. Mag. Tokyo, 1992; 105, 53-70
CrossRef - Shimazu T., Yuda T., Miyamota K., Yuda T., Miyamoto K., Yamashita M., Ueda J. Growth and development in higher plants under simulated microgravity conditions on a 3- dimensional clinostat. Space Res. 2001; 27 (5): 995 -1000
CrossRef - Jagtap S.S., Awhad R.B., Santosh B., Vidyasagar P.B. Effects of clinorotation on growth and chlorophyll content of rice seeds. Microgravity Sci.Technol. 2010; 23:1 41-48
CrossRef - Yamada M., Takeuchi Y., Kasahara H., Murakami S., Yamashita M. Plant growth under clinostat– microgravity condition. Sci. Space. 1993; 7(2): 116-119
CrossRef - Mikhaylenko N.F., Sytnik S.K., Zolotareva E.K. Effects of slow clinorotation on lipid contents and proton permeability of thylakoid membranes of pea chloroplasts. Space Res. 2001; 27(5): 2007-1010
CrossRef - Brown C.S., Tripathy B.C., Stutte G.W. Photosynthesis and carbohydrate metabolism in space workshop. IN: Suge, H.: (eds) Plants in space biology. Institute of Genetic Ecology. Tohoku University, Sendai, Japan 1996; pp 127-134.
- Briarty L.G., Maher E.P., Iversen T.H. Growth, differentiation and development of Arabidopsis thaliana under microgravity conditions. In:Biorack on Spacelab IML-I. European Space Agency. Edited by Mattok, C. ESA Publications Division, Estec, Noordwijk, The Netherlands. 1995; pp. 141-154.
- Kiss J.Z., Katembe W.J., Edelmann R.E. Gravitropism and development of wild-type and starch-deficient mutants of Arabidopsis during spaceflight. Plant. 1998; 102: 493-502
CrossRef - Levine H. , A. Gerard H., Levine H. G. , Joon-Weon Choi, Laurence B. D., Krikorian A. D. , Lewis N. G. Cell-wall architecture and lignin composition of wheat developed in a microgravity environment. Photochemistry, 2001, 57:(6), 835-846
CrossRef - Laurinavichius R.A., Yaroshyus A.V., Marchyukaytis A., Shvyaghdene D.V., Mashinskiy A.L. Metabolism of pea plants grown under space flight conditions. USSR Space Sci. Digest. 1986; 4: 23-25
- Moleshko G.I., Antonyan A.A., Sycheyev V.N., Solontsova I.P., Shetlik I., Doukha Y. The effects of space flight factors on the pigment system of one-celled algae. USSR Space Life Sci. Digest. 1991;31: 43-45
- Tripathy B.C., Brown C.S., Levine H.G., Krikorian D. Growth and photosynthetic responses of wheat plants grown in space. Plant Physiol. 1996; 110: 801-806
CrossRef - Chen B., Zhang A., Lu Q., Kuang T., Lu C., Wen X. Characterization of photosystem I in rice (Oryza sativa) seedlings upon exposure to random positioning machine Photosynth Res. 2013; 116: 93–105
CrossRef - Aarrouf J., Darbelley N., Demandre C., Razafindramboa N., Perbal G. – Effect of Horizontal Clinorotation on the Root System Development and on Lipid Breakdown in Rapeseed (Brassica napus) Plant Cell. Physiol. 1999;40(4): 396-405.
CrossRef - Jagtap S.S., Dhumal K.N. and Vidyasagar P.B. Effects of Slow Clinorotation on Growth and Yield in Field Grown Rice. Gravitational and Space Biology 2011; 25 (1):48-50
CrossRef - Porra R.J., Thompson W.A., Kriedmann P.A. Determination of accurate extinction coefficients and simultaneous equations for assaying chlorophylls a and bextracted with four different solvents: verification of the concentration of chlorophyll standards by atomic absorption spectroscopy. Bioenergetics 1989; 975: 384–394
CrossRef - Arnon D.I. Copper enzymes in isolated chloroplasts Polyphenoloxidase in Beta Plant Physiol. 1949; 24(1): 1-15
CrossRef - Strasser R.J., Srivastava A., Tsimilli-Michael M. The fluorescence transient as a tool to characterize and screen photosynthetic samples. In probing photosynthesis: Mechanics, Regulation and adoption Edited by: Yunus M, Pathre U, Mohanty P, (Taylor & Francis; London) 2000; pp. 443-480.
- Abilov Z.K., Alekperov U.K., Mashinskiy A.L., Aliyev A.A. The morphological and functional state of the photosynthetic system of plant cells grown for varying periods under space flight conditions. USSR Space Life Sci. Digest. 1986; 8: 15-18
- Aliyev A.A., Abilov Z.K., Mashinskiy A.L., Ganiyeva R.A., Ragimova G.K. The ultrastructure and physiological characteristics of the photosynthesis system of shoots of garden pea grown for 29 days on the ‘Salyut-7’ space station. USSR Space Life Sci. Digest. 1987; 10: 15-16
- Strasser B.J., Strasser R.J. Measuring fast fluorescence transients to address environmental question: the JIP test. In: Mathis P (ed.). Photosynthesis: from light to biosphere, (The Netherlands: Kluwer Academic Publisher) 1995; pp. 977-980.
CrossRef - Strauss A.J., Kruger G.H.J., Strasser R.J., Van Heerden PDR Ranking of dark chilling tolerance in soyabean genotypes probed by the chlorophyll a fluorescence transient O-J-I-P. Exp. Bot.2003;60: 438-466
- Abbaspoor M., Streibig J.C. Monitoring the efficacy and metabolism of phenylcarbamates in sugar beet and black nightshade by chlorophyll fluorescence parameters. Pest manag Sci. 2007;63:576-585.
CrossRef - Levine H.G., Kann R.P., Krikorian A.D. Plant development in space: observations on root formation and growth. InProceedings of the 4th European Symposium on Life Sciences Research in Space. Edited by David, V. Trieste (Italy) 28 May-1 June. Noordwijk, The Netherlands, ESA SP-307. 1990; pp. 503-508.
- Jiang Huan-X., Cgen Li- S., Zheng Jin-G., Hang S., Tan N., Smith B. R.S. Aluminium-induced effects on photosystem II photochemistry in Citrus leaves assessed by the chlorophyll a fluorescence transient. Tree Physiology. 2008; 28: 1863-1871
CrossRef - Jafarinia , Shariati M. Effects of salt stress on photosystem II of canola plant (Brassica napus, L.) probing by chlorophyll a fluorescence measurements . Iranian Jr. of Sci. Tech. 2012; A1: 71-76
- Zhao Qi, L. I. Jun, LIU Min Effects of simulated microgravity on characteristics of photosynthesis in plant seedlings. Space medicine and medical engineering 2002;15(2): 79-82
- Lacuesta M.A., Muzoz-Rueda C., Gonzalez-Murua M.N. Effects of phosphinothriein (glufosinate) on photosynthesis and chlorophyll fluorescence emission by barley leaves illuminated under photorespiratory and non-photorespiratory conditions. Exp Bot. 1992; 43:159-165
CrossRef - Strasser R.J., Tsimilli-Michael, Srivastava A. Analysis of the chlorophyll a fluorescence transient, In chlorophyll a fluorescence: A signature of photosynthesis (Springer; Netherland), 2004; pp. 321-362.
CrossRef - Zheng-He L., Li-Song C., Rong-Bing C., Fang-Zhou Z., Huan-Xin J., Ning T. CO2 assimilation, ribulose -1,5-bisphosphate carboxylase/oxygenas, carbohydrates and photosynthetic electron transport probed by the JIP test, of tea leaves in response to phosphorus supply BMC Plant Biology 2009; 9: 43

