Introduction
Salinity, which is persuaded by a greater amount of salt in the soil and affects the considerable amount of the plant and is an major issue for most plants particularly in areas with hot, dry conditions, and is one of the most severe issues inhibiting crop plant germination and productivity.1,2 The contrary impacts of high soil salinity levels due to the combination effects of large osmotic potential ,ion toxicity, and capacity of plant production, lead to destroying seed germination, seedling growth, and developmental phases.3,4 The germination percentage, physiological parameters were all remarkably abnormal, and this is due to the higher salt concentration.1 Plants suffer from two direct problems in saline environments, there is quite a lot of salt in the soil which shrinkages the osmotic potential of the soil solution, which further causes less water to be absorbed by plants and a water deficit. Moreover, increased Na + and Cl– ion absorption renders plants toxic by preventing them from gripping vital nutrients.5,6
Phytohormones are endogenously produced organic substances essential for regulating plant growth and productivity. This hypotheses states that the gibberellins acid seems to be critical modules of complex gesturing networks and have been integrated into current models of stress response.9 Hence, they play an important role in prompting plant tolerance to various stress conditionsGA3 plant growth regulators are highly active for encouraging and optimising the plant development, and photosynthetic activity because they are also notorious to trigger numerous physiological responses in plants.7
In saline conditions, patterns of growth reduction can occur; this can be decreased by the advanced effectiveness of exogenous GA3 application on different activities of the plants. The use of GA3 helps diminish saline stress, and its adeptness is more vigorous in salt resistant cultivars.8 By improving vigour, anti-oxidation enzyme activity, and the accretion of osmolytes, gibberellic acid slightly alleviates the deleterious effects of salinity. Additionally, GA3 treatment promotes the production of the hydrolytic enzyme, which is required to break down endospermic starch when seeds renew growth during seed germination.9,10
Consequently, the goal of the present study was to appreciate the germination activity and seedling growth through priming with GA3 in seeds of Acacia auriculiformis A.Cunn. ex Benth. (Ear-pod Wattle), Delonix regia (Bojer ex Hook.) R af.(Royal Poinciana), Cassia fistula L. (golden shower tree), and Samanea saman (Jacq.) Merr (Rain Tree). In addition, the seeds were also treated with NaCl to symbolise their tolerance capacity during germination and the early seedling growth stages.
Materials and Methods
Seed collection
The mature seeds of Acacia auriculiformis, Delonix regia, Cassia fistula, Samanea samand an (Fabaceae) were collected during September to December 2020 from the Western Ghats, Sadivayal area in Coimbatore district (11.0168° N, 76.9558° E). The collection site has a mean temperature of 27°C ranging from 32°C in September to 19°C in December. Several hundred seeds were collected from 10 randomly chosen trees located within a 20-km radius by gently shaking the mature trees. Seeds were transported to the lab via road on the same day, visually inspected, and any unhealthy seeds and debris were removed. The seed surface was sterilized with 2% sodium hypochlorite solution for 10 minutes and thoroughly washed with distilled water 6to 8 times. The seeds were then bench dried for a day and subsequently stored in air-tight glass bottles under room temperature(27°C) and humidity (30to 50%)until used in the experiment.
Hormonal priming
The seeds were soaked with 150 ppm GA3 solution for 12 hours at 27°C in darkness, achieved by covering the petri dishes with two layers of aluminium foil paper. After GA3 treatment, seeds were removed and washed in running tap water and then rinsed three times with distilled water. Seeds were dried by placing them between filter papers. After drying, the seeds were kept in petri dishes containing moist no.1 Whatman filter paper in room condition at 27°C. Control seeds (no GA3 treatment) are soaked in water for 12 hours, re-dried, and then placed in petri dishes containing moist no.1 Whatman filter paper in room condition at 27°C.
A fully randomized factorial experiment consisting of three replicates of 25 seeds each from control and primed groups were moistened with equal volumes of NaCl solutions at two different concentrations: 50 mM and 100 mM. Physiological factors were calculated by formulae given below. The shoot and root lengths of all seedlings were measured using a transparent ruler. Seedlings fresh weights were measured using a weighing balance. Seedlings were dried in hot air oven at 50°C for 2 days, and then dry weights were documented.11,12
Measurements of germination efficiency
Total germination (TG) were calculated every day and terminated at end of day 15 after sowing.11 It was computed as:
![]()
Where n is the total germinated seeds, and N is the total seeds were sown.
Mean germination time (MGT) was designed according to13
![]()
where n = number of seeds germinated on every day,
d = number of days from the start of the germination, and
N = total number of seeds germinated at the 15 days
The Vigour index (VI) were measured using the formula of 14
VI= TG% × Seedling Length (cm)/100
Statistical analysis
All data collected from the experiments were exposed to analysis of variance (Two way ANOVA) using the Statistical Package of the SPSS, version 20 software and the difference between means were calculated by Fisher’s Post Hoc Least Significance Difference Test at P < 0.05.
Results
GA3 treated and control seeds of Acacia auriculiformis A.Cunn. ex Benth., Delonix regia (Bojer ex Hook.) Raf. Cassia fistula L. and Samanea saman (Jacq.) Merr were spread and moistened with different concentrations of Nacl solutions, and the significances of the study were composed by significant various physiological parameters.
Germination Rate and Germination Mean Time of the four Fabaceae Seeds
By variance of analysis presented that both Nacl and treated with GA3 pointedly affected the germination rate and mean germination time of Acacia auriculiformis, Delonix regia, Cassia fistula, and Samanea saman seeds (P < 0.05) (Table 1). At 50 mM Nacl salinity level, a greater germination percentage (44.67–38.12%) was achieved in Samanea saman, followed by Acacia auriculiformis (42.10–36.80%), Delonix regia (39.15–26.35%), and the least percentage (24.78–20.22%) in Cassia fistula. Germination were significantly good up to the 50 mM Nacl salinity level in the primed seeds when compared to 100 mM Nacl.
Table: 1 Analysis of variance of growth parameters in Fabaceae seeds of treated with gibberellic acid and germinated using various concentration of NaCl solutions.
| Source of variation | Total Germination Percentage | Mean Germination Time (days) | Seedling vigour | Shoot Length(cm) | Root Length(cm) | Seedling Fresh Weight(g) | Seedlings Dry Weight(g) |
| Treatment | 6502.33* | 60.94* | 264* | 17.08* | 5.02* | 3.35* | 2.3* |
| seed type | 1056.78* | 34.40* | 34* | 4.75* | 4.75* | 4.38* | 1.1* |
| Salt conc. | 8097.71* | 50.70* | 24* | 4.43* | 4.3* | 1.23* | 1.3* |
| Treatment ×seed type | 454.73* | 10.09* | 4.3* | 1.73* | 3.4* | 5.23* | 5.52* |
| Treatment ×salt conc. | 994.97* | 0.50* | 5.5* | 2.13* | 5.7* | 6.11* | 4.35* |
| Seed variety × salt conc. Treatment ×seed variety ×salt conc | 263.09*
506.73* |
1.32*
1.68* |
3.2*
8.2* |
4.73*
5.27* |
8.1*
7.9* |
3.58*
2.11* |
3.72*
0.91* |
| Error | 14.73 | 0.8 | 2.82 | 3.72 | 0.054 | 0.0045 | 0.077 |
*Significantly different at P < 0.05
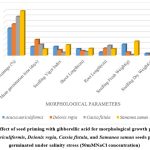 |
Figure1: Effect of seed priming with gibberellic acid for morphological growth parameters of Acacia auriculiformis, Delonix regia, Cassia fistula, and Samanea saman seeds primed after germinated under salinity stress (50mMNaCl concentration) |
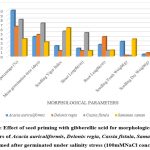 |
Figure 2: Effect of seed priming with gibberellic acid for morphological growth parameters of Acacia auriculiformis, Delonix regia, Cassia fistula, Samanea saman seeds primed after germinated under salinity stress (100mMNaCl concentration). |
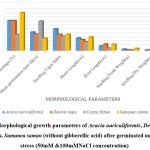 |
Figure 3: Morphological growth parameters of Acacia auriculiformis, Delonix regia, Cassia fistula, Samanea saman (without gibberellic acid) after germinated under salinity stress (50mM &100mMNaCl concentration) |
Effect of Salinity and GA3 Treated on the Physiological Growth Performance of the four Fabaceae seeds:
Higher Nacl concentrations exposed to seeds result in stress and a decline in all experimental physiological characteristics. The growth of the seedlings is indirectly interconnected with the salinity conditions. It was clearly shown that seedling development drastically lessened when saline levels increased. Nevertheless, treated with GA3 had a provoking effect on maximum physiological characters up to a definite salinity level of 50 mM NaCl. Relatively, a low number (14%) of seedling performances were recorded at control seedlings with saline water levels from 50 mM to 100 mM of Nacl for Cassia fistula, followed by Delonix regia (25%), Acacia auriculiformis (24%), and Samanea saman (39%). A considerably high number of seedling performances were documented at treated seedlings (65%) moistened with Nacl levels up to 50 mM Nacl for Samanea saman, followed by Acacia auriculiformis (54%), Delonix regia (47%), and Cassia fistula (33%).
Seedling growth of Acacia auriculiformis, Delonix regia, Cassia fistula, and Samanea saman was considerably compacted when grown in high saline conditions. Seedling vigor immeasurably declined from control (no salt) to the 50 mM NaCl concentration. Maximum seedling vigor index was observed at 50 mM Nacl (38.20 for primed and 17.20 for unprimed) and minimum seedling vigor index was at 100 mM Nacl (9.45 for primed and 7.20 for unprimed). Consequently, these declines in seedling performance were detected in all tested seedling physiological characters (Figures 1 to 3).
It is well predictable that the growth medium’s effect on salinity caused Fabaceae seeds to have fewer seedling characteristics as salinity levels increased and that GA3 was unable to inverse this effect when the stress reached its highest point (100 mM Nacl).
50 mM Nacl concentrations of primed seeds intensely stimulate shoot and root growth in Acacia auriculiformis, Delonix regia, and Samanea saman but reduce root growth in Cassia fistula at both 50 and 100 mM Nacl concentrations. 100 mM Nacl concentration expressively condensed the length of the shoot and root in four seeds. The greater decrease (for primed 8.14 % and unprimed 4.40 %) and (for primed 5.46 % and unprimed 2.32%) was pragmatic in Cassia fistula, and the lower reduction (for primed 25.22 % and unprimed 10.75 %) and (for primed 16.03% and unprimed 8.11%) in Samanea saman were documented respectively in the shoot and root length at 50 mM Nacl concentration.
In 50 mM Nacl, the highest regular of the total dry weights was observed. (Figures 1 to3), and less alteration was recorded for the total dry weight of the seedling at a higher salinity level of 100 mM Nacl. In 50 mM Nacl, primed seed of Samanea saman shows greater reduction (for primed4.32 and unprimed0.32) while Cassia fistula shows lesser reduction (for primed2.31 and unprimed0.24).GA3 application-induced growth stimulation of wheat under zinc oxide nanoparticle stress was attributed to improved nutritional status 22.
Discussion
Germination Rate and Germination Mean Time of the four Fabaceae Seeds:
Improving the salt tolerance of crops is an essential target of plant breeders, in aiming to meet the future food demands of coming generations.23 Pre-sowing priming with GA3is used to accelerate seed germination and standardized seedling emergence, to enhance further plant growth, and to establish the stress resistance of seedlings and adult plants, which ultimately increases crop production.24
The significant and crucial stages in the life sequence of plants is seed germination, which has an impact on plant establishment and productivity. Moreover, a number of abiotic stresses can obstruct seeds from germinating, and salt in particular has a variety of dissimilar consequences. But the recent work is in accordance with15 reported that plants use the build-up of some inorganic ions or organic compounds to balance the osmotic potential against the early problems of salinity, which prevents water uptake by the roots due to excessive ion content in the soil solution. When seeds were sown at different salt levels, the rate of germination and the amount of time required for germination were directly correlated. Seed priming with 150 ppm GA3 upgraded the effect of Nacl on seed germination. According to reports, GA3 increases the germination rate and counteracts the negative effects of saline stress on germination.16
As a general response to salt stress, plant growth is affected negatively by a variety of physiological changes, such as osmotic shock, ion toxicity, and nutritional imbalance.23 This study showed that, although stem length, fresh and dry weight, and water content decreased the adverse effect of NaCl by GA3 treatment, the impact of GA3 was reduced slightly with rising salt level. It has been known that, GA 3 enhances plant growth by promoting cell division and elongation24 under salinity, for instance in Arabidopsis,25 wheat26 and rice.8
The improvement of water absorption and cell wall flexibility, as well as the stimulation of cytological enzymes, all contribute to GA3‘s role in germination percentage.17 Comparable outcomes were observed on the germination of oats18, Hordeum vulgare19, Lathyrus sativus20, Ricinus communis,21 Satureja thymbra,22 Triticum aestivum,23 and Phaseolus mungo.24
Effect of Salinity and GA3 on the Physiological Growth Performance of the four Fabaceae seeds:
In spite of optimistic beyond-minced growth in the dwarf lines,25 testified that GA3 had adverse possessions on length of root in the Rht12 dwarf of wheat plants by reducing overall root length and root dry weight in higher concentration of Nacl. The destructive effect was more prominent in the root than the shoot because plants under salt stress have less water-absorbent roots that develop more gradually.26,27 projected that the possessions of exogenously administered GA3 on the removal of salt stress may be due to the activation of specific enzymes involved in RNA and protein synthesis28 found that GA3 treatment increased both the shoot and root development of soybean plants.
Consistently, several previous studies30,31 have also documented that salinity is highly detrimental to plant growth. Salt stress in Zea mays reduced the root and shoot length, dry and fresh biomass and leaves growth compared to the control. The decrease in plant biomass might be due to the higher Na+ ion concentration in roots and outside the plant cell.33 In the soil, higher salt concentration limits the absorption of water and nutrients by plant roots.
This could be due to the exogenous application of GA3, which has generally stimulated oat plant development parameters and reduced the oppressive effect of salt stress to some extent. GA3 can also improve the weights of seedlings, which also speeds up the step of photosynthetic growth.29 Similar findings were made by,24 who showed that saline lowered the weight of plants. The inhibitory effect of salt stress on Cucurbita pepo biomass was alleviated partially or entirely by all investigated hormonal treatments. In accordance with our results, other studies reported that treatment of plants with GA3 is efficient in relieving salinity stress effects at various stages of plant growth by enhancing plant height, root length, root diameter, and shoot fresh weight.30
Their findings recommended that the loss of fresh and dry biomass at higher concentrations may be due to inadequate water absorption from the growth medium as a result of physiological dryness.
It utilises several kinds of growth-promoting hormones to boost productivity in agriculture. Gibberellic acid (GA3), one of the main plant hormones, regulates when plants grow and develop as well as how it impacts blooming, leaf, stem, and seed germination. GA3 application has been shown to improve plant growth by altering the ratio between endogenous ABA and SA, reducing the quantity of polyamines, which are involved in the regulation of aging7 and GA3 can induce salt tolerance in plants by increasing CK and IAA, it seems that sorghum is able to survive under salinity with less damage through changes to CK and IAA levels.35 Investigate those gibberellins normalize numerous plant metabolic processes by interacting with other necessary hormones.30 Acacia auriculiformis, Delonix regia, Cassia fistula, and Samanea saman all had their early seedling growth negatively affected by NaCl salinity until GA3 was administered. The supply of GA3 plays an essential role in protein biosynthesis because it can increase the uptake of N from the soil.32 Accumulation of organic solutes, including soluble protein and phenolics could contribute to osmotic adjustment and stabilization of membranes in plants.28 It is well-known that organic solutes can enhance plant tolerance against different stresses, including salinity, through maintaining pressure potential and membrane integrity, protection of proteins, and scavenging of free radicals.34,35
Conclusion
In summary, salinity stress significantly inhibited the growth and development of seeds, however, the application of GA3 can effectively ameliorate the damage caused by salt stress. This research revealed important details on the salt-stressed seeds of Acacia auriculiformis, Delonix regia, Cassia fistula, and Samanea saman, as well as the effects of growth hormone pre-treatments (GA3). Primed and unprimed seeds of the listed species have different, although slightly distinct reactions to the various salinity environments. Seed priming with GA3 significantly improved the physiological parameters of Acacia auriculiformis, Delonix regia, Cassia fistula, and Samanea saman. Gibberellic acid amendment enhanced the plant growth, K+ ion concentration, while reduced the oxidative stress and Na+ ion accumulation under salinity. Up to 50 mM NaCl, priming the negative consequence of salt stress on seed germination and seedling growth of the analysed tree plants. The control seeds showed declined in physiological parameters with increasing salt stress than the primed seeds. At the highest salt levels in reductions in all seedling performances were strongly resisted. Furthermore, the roots of the studied tree plants were more affected than the shoots. The overall percentage of seeds that germinated after pre-treatment with GA3 improved the time of germination, and seedling growth performances increased. In order to lessen the negative properties of salinity stress on seed characters in vitro, this study revealed the efficiency of smearing seed priming strategies in salt-stressed conditions. However, more study is required to determine how the four primed (GA3) tree seeds perform in terms of vegetative growth and yield under field conditions.
Acknowledgment
The authors would like to acknowledge DST-FIST for providing the infrastructural facilities for successfully completion the work.
Conflict of interests
The authors declare that there is no competing interest.
Funding Sources
There is no funding sources for this article.
References
- Mahajan, S., and Tuteja, N. Cold, salinity and drought stresses: an overview. Archives of Biochemistry and Biophysics 2005:444:139-158.
CrossRef - Hasanuzzaman, M., Hossain, M.A., da Silva, J.A.T., and Fujita, M. Plant response and tolerance to abiotic oxidative stress: antioxidant defense is a key factor//Crop stress and its management: Perspectives and strategies. Springer, Dordrecht, The Netherlands.2012: p. 261-315
CrossRef - Bahrani, A., and Haghjoo, M. 2011. Response of some wheat (Triticum aestivum L.) genotypes to salinity at germination and early seedling growth stages. World Applied Sciences Journal 13:887-897.
- Ratanavathi, C.V., Dayakar, R.B., and Seet, H.N. Sweet sorghum: A new raw material for fuel alcohol, in a study report on technological aspects in manufacturing ethyl alcohol from cereal grains in Maharashtra. Part II. Department of Scientific & Industrial Research, Ministry of Science & Technology, Government of India, New Delhi and Mitcon Consultancy Services Limited, Pune, India2004.
- Cavusoglu, A., and Sulusoglu, M. Effects of gibberellic acid (GA3), indole-3-acetic acid (IAA) and water treatments on seed germination of Melia azedarach L. Scientific Papers. Series B, Horticulture 2015:59:319-326.
- Sun, T.P. Gibberellin metabolism, perception and signaling pathways in Arabidopsis. The Arabidopsis Book 6:e0103 2008.
CrossRef - Saberali, S.F., and Moradi, M. Effect of salinity on germination and seedling growth of Trigonella foenumgraecum, Dracocephalum moldavica, Satureja hortensis and Anethum graveolens. Journal of the Saudi Society of Agricultural Sciences (In Press) 2017.
- Almodares, A., Hadi, M.R., and Kharazian, Z.A. Sweet sorghum: salt tolerance and high biomass sugar crop. p. 441-460. In Matovic, M. (ed.) Biomass-detection, production and usage. InTech, Rijeka, Croatia. 2011 doi:10.5772/19044
CrossRef - Munns, R., and Tester, M. Mechanisms of salinity tolerance. Annual Review of Plant Biology 2008:59:651-681.
CrossRef - Bahrani, A., and Pourreza, J. Gibberellic acid and salicylic acid effects on seed germination and seedlings growth of wheat (Triticum aestivum L.) under salt stress condition. World Applied Sciences Journal 2012:18:633-641.
- Yadav, P. V., Khatri, D., & Nasim, M. Salt and PEG induced osmotic stress tolerance at germination and seedling stage in Camelina sativa: A potential biofuel crop. Seed Sci, 2017:10, 27-32.
CrossRef - Chauhan, A., AbuAmarah, B. A., Kumar, A., Verma, J. S., Ghramh, H. A., Khan, K. A., & Ansari, M. J. Influence of gibberellic acid and different salt concentrations on germination percentage and physiological parameters of oat cultivars. Saudi journal of biological sciences, 2019: 26(6), 1298-1304.
CrossRef - Fuller MP, Hamza JH, Rihan HZ, Al-Issawi M.. Germination of primed seed under NaCl stress in wheat. International Scholarly Research Notices, 2012 http://dx.doi.org/ 10.5402/2012/167804
CrossRef - Elouaer, M. A., & Hannachi, C. Seed priming to improve germination and seedling growth of safflower (Carthamus tinctorius) under salt stress. Eurasian Journal of BioSciences, 2012:6(1), 76-84.
CrossRef - Chauhan, B. S. Germination biology of Hibiscus tridactylites in Australia and the implications for weed management. Scientific reports, 2016: 6(1), 1-6.
CrossRef - Patel, G., Mankad, A.U. Effect of gibberellins on seed germination of Tithonia rotundifolia Black. Int. J. Innovative Res. Sci. 2014:3, 10680–10683.
- Padma, L., Basvaraju, G.V., Sarika, G., Amrutha, N. Effect of seed treatments to enhance seed quality of papaya (Carica papaya L.) cv. surya. Global J. Biol., Agric. Health 2013:2 (3), 221–225.
- Benliglu, , Ozkan, U.,. Determination of responses of some oat cultivars (Avena sativa L.) to salt and drought stress at the germination period. Cienciae Tecnica Vitivinicola 2016:31, 6–25.
- Ahmed Nimir, N. E., Lu, S., Zhou, G., Ma, B. L., Guo, W., & Wang, Y. (2014). Exogenous hormones alleviated salinity and temperature stresses on germination and early seedling growth of sweet sorghum. Agronomy Journal, 2014:106(6), 2305-2315.
CrossRef - Moghaddam, S. S., Rahimi, A., Pourakbar, L., & Jangjoo, F.Seed Priming with salicylic acid improves germination and growth of Lathyrus sativus L. under salinity stress. Yuzuncu Yıl University Journal of Agricultural Sciences, 2020: 30(1), 68-79.
CrossRef - Jiao, X., Zhi, W., Liu, G., Zhu, G., Feng, G., Eltyb Ahmed Nimir, N., … & Zhou, G. Responses of foreign GA3 application on seedling growth of castor bean (Ricinus communis L.) under salinity stress conditions. Agronomy, 2019:9(6), 274
CrossRef - Ummahan, Ö. Z. The effect of salinity stress on germination parameters in Satureja thymbra L.(Lamiaceae). International Journal of Secondary Metabolite, 2022:9(1), 74-90.
CrossRef - Yongkriat, K. O., Leksungnoen, N., Onwimon, D., & Doomnil, P. Germination and salinity tolerance of seeds of sixteen Fabaceae species in Thailand for reclamation of salt-affected lands. Biodiversitas Journal of Biological Diversity, 2020:21(5).
CrossRef - Dheeba, , Sampathkumar, P., Kannan, K. Fertilizers and mixed crop cultivation of chromium tolerant and sensitive plants under chromium toxicity. J. Toxicol. 2015:9 (1), 51–60.
CrossRef - Chen, , Hao, L., Condon, A.O., Hu, Y.G. Exogenous GA3 application can compensate the morphogenetic effects of the GA-responsive Dwarfing gene Rht12 in Bread wheat. PLoS One 9 2014 :(1).
CrossRef - Rajput, , Kumar, A., Chaudhry, A.K. Effects of NaCl stress on seed germination and early seedling growth of castor bean (Ricinus communis L.). Int. J. Sci. Res. 2015:11, 104–107.
- Bejaoui, M. 1985. Interaction between NaCl and some phytohormones on soybean J. Plant Physiol. 1985:120, 95–110.
CrossRef - Shaddad, M.A.K., Abd El-Samad, H.M., Mostafa, D.Role of gibberellic acid in improving salt stress tolerance of two wheat cultivars. J. Plant Physiol. Biochem. 2013:5, 50–57.
- Abdul-Baki, A., Anderson, J.D. Viability and leaching of sugars from germinating barley. Crop Sci. 1970:10, 31–34.
CrossRef - Ramezani, E., Sepanlou, M.G., Badi, H.A.N. The effect of salinity on the growth, morphology and physiology of Echium amoenum Fisch & Afric. J. Biotech. 2011: 10, 8765–8773.
CrossRef - Hoai, N.T.T., Shim, I.S., Kobayashi, K., and Kenji, U. Accumulation of some nitrogen compounds in response to salt stress and their relationships with salt tolerance in rice (Oryza sativa L.) seedlings. Plant Growth Regulation 2003:41:159-164.
CrossRef - Saberali, S.F., and Moradi, M. Effect of salinity on germination and seedling growth of Trigonella foenumgraecum, Dracocephalum moldavica, Satureja hortensis and Anethum graveolens. Journal of the Saudi Society of Agricultural Sciences 2017(In Press).
- Tsegay, B. A., & Andargie, M.. Seed Priming with Gibberellic Acid (GA 3) Alleviates Salinity Induced Inhibition of Germination and Seedling Growth of Zea mays L., Pisum sativum Var. abyssinicum A. Braun and Lathyrus sativus L. Journal of Crop Science and Biotechnology, 2018:21(3), 261-267
CrossRef - Zhao, C.; Zhang, H.; Song, C.; Zhu, J.-K.; Shabala, S. Mechanisms of plant responses and adaptation to soil salinity. Innovation 2020, 1, 100017.
CrossRef - Ali, M.B.; Singh, N.; Shohael, A.M.; Hahn, E.J.; Paek, K.-Y. Phenolics metabolism and lignin synthesis in root suspension cultures of Panax ginseng in response to copper stress. Plant Sci. 2006, 171, 147–154.
CrossRef

