Introduction
Among various vegetables, cole crops are significant crops widely cultivated in different climatic regions around the globe. The green leafy Brassicaceae vegetables have a wider array of micronutrients and sulphur-containing phytonutrients that promote health. They have a positive cardiovascular protective role against gastrointestinal tract cancer.22 However, the qualitative and quantitative value of Brassicae vegetables is affected by different insect pests and is a very serious menace to the profitable cultivation of cruciferous vegetables. These crops are vulnerable to feeding damage from several chewing and sucking insect pests. Some pests attack the vegetables at root stages (e.g., wireworms), some attack the young seedlings (e.g. cutworms) while as some attack stems and foliage (e.g., caterpillars and beetles). Aphids suck the juices from the plants which results in an overall loss of plant vigor. Common insect pests on Brassicae in India includes cabbage butterflies: Pieris brassicae Linnaeus (Lepidoptera: Pieridae), Pieris rapae Linnaeus (Lepidoptera: Pieridae), diamondback moth: Plutella xylostella Linnaeus (Lepidoptera: Plutellidae), head caterpillar: Crocidolomia binotalis Zeller (Lepidoptera: Pyraustidae), Cabbage webworm: Hellula undalis 83 species of Fabricius (Lepidoptera: Crambidae), aphids: Lipaphis erysimi Kaltenbach (Hemiptera: Aphididae), Brevicoryne brassicae Linnaeus (Hemiptera: Aphididae), and flea beetle: Phyllotreta brassicae Goeze (Coleoptera: Chrysomelidae).8 Among these pests, P. brassicae is the key oligophagous insect pest of cole crops intended for human consumption. It has been found that it infested 83 species of Brassicaceae or Cruciferae and has attained the status of a major pest in various parts of India due to changes in ecosystems and habitats resulting in 30-40% yield loss in vegetables.5, 21 It generates major losses compared to the expenses of man-made pesticides for its control.14 P. brassicae is dispersed all over the world where Brassicaceae crops are cultivated and is employed as a model species in the field of insect pest biology.27 In India, it spends winter in plains and migrates to mountainous regions during summer.11
The development of the insect, its survival, and its reproduction are affected by the type of host plant.13,16,17,19 During larval development, host plant quality is a key determinant of both fecundity and fertility of adults.3,4 The body size of herbivorous insects may vary depending on the host plant’s quality, which can then impact life-history traits like fecundity, longevity, and survival.3,25 Moreover, the growth and reproduction can be affected by the host plant and geographical sources.36 Therefore, the discrepancies highlight the need for caution when comparing findings across regions.
The larvae devour the whole leaves of cauliflower, cabbage, and flower buds of broccoli and cause prevalent damage at all developing stages of cole crops, such as seedling, vegetative, and flowering stages7, 34 thereby making the first line of defence- the pesticide application mandatory for cole crop production. Though pesticide application has revealed positive results by improving the quality and yield of food, their indiscriminate use causes serious consequences of bio-magnification and persistent nature.26 Therefore, to control the insect pests in an eco-friendly manner, strategies encouraging biological control within the context of integrated pest management (IPM) are currently demanded. Studies on the fauna of parasitic insects in different regions are of immense significance for successful pest control as they destroy their hosts.33 In the present study, we were concerned in investigating the consequences of diverse host plants on the fitness of P. brassicae in district Rajouri and enable farmers to utilise the most suitable control strategy including biocontrol towards crop management.
Materials and Methods
Study area and sampling
The Rajouri district is situated on the southerly foothills of the Pir Panjal Himalaya in the union territory of Jammu and Kashmir (India). It is located at 32°57′ to 33°34′ N latitude and 74°00′ to 74°48′ E longitude and has seven tehsils, which cover a total area of 2,630 km2, out of which about half of the area is occupied by forests (1,267 km2) . Its topography is predominately hilly, undulating, and is distinctive as it possesses three agro-climatic regions: subtropical, intermediate, and temperate, which favors the cultivation of some diverse crops at diverse and particular elevations. The zones have dissimilar land use patterns, cropping prototypes, and inhabitation. The areas for cole crop production were purposely and randomly selected in district Rajouri (J&K UT) (Fig. 1). The cole crops used were radish (Raphanus sativus), cabbage (Brassica oleracea var. capitata), and cauliflower (Brassica oleracea var. botrytis).
 |
Figure 1: Locations of different study areas in district Rajouri (J&K UT) India |
Laboratory investigation and insect rearing
Various Brassica crops were raised in the period 2021-22. The seeds were sown and watered on subsequent days. The seedlings were transplanted in a bed of size 5×5 m. The crop beds were monitored frequently in order to assess the infestation of the butterfly, P. brassicae. Before starting the experiment, field-collected insects were reared for a generation to culminate the effect of food reserves on the eggs and subsequent larvae. The leaf-laden eggs were carefully plucked from each Brassicaceae bed (stock culture) and taken to laboratory for mass rearing in the plastic jars (20×15 cm). Newly hatched larvae from their respective host plants were transferred to separate plastic jars (12×08 cm), covered with muslin cloth for continuous air supply, and fresh food was provided regularly until pupation. The adults emerged were given 10% sugar solution soaked in cotton balls. The progeny of laboratory-raised P. brassicae were used for developing a life table tailored to each level. The maximum temperature recorded during this experiment was 24.3 to 26.3 oC with a minimum temperature as 7.5 to 9.5 oC and the relative humidity recorded was between 78 to 84 % in the morning and 32 to 45 % in the evening.
Development, survival, and mortality
Leaf discs of 4 cm diameter were taken from cabbage, cauliflower, and radish and placed in three clean petri dishes (14×10 cm) covered with a moist filter paper. One freshly emerged larva was released on each leaf disc of the host plants, and each petri dish was chosen as a replicate. Larval food was changed each day and the duration of development of various instars of the larva, total developmental period of the larva, pupal period, and total developmental period of the insect were recorded. The data obtained were analyzed in MS Excel 2010 for calculation of average SD and SE.
Specific life tables of various phases were created in order to determine the effects of cole crops on P. brassicae’s endurance and mortality stages. The data recorded from life tables from egg hatching to adult emergence were employed for computing different parameters like apparent mortality, survival fraction, mortality, survival ratio, indispensable mortality, and k values. It was computed following the protocol given by Ali and Rizwi.2 The subsequent conventional bases were employed.
x = age of the insect
dx = insect died during the age interval, x
The parameters are briefly summarized as under
Apparent Mortality (100qx)
It gives the details of the numeral vanishing as a proportion of the number entering that phase and was calculated by the formula:
Apparent mortality (100qx) = (dx/Ix) × 100
Where x is the age of the insect in days,
dx is the number of deaths during the age range x out of 100
Ix is the number of survivors at the start of each period x out of 100
Survival fraction (Sx)
The survival fraction was used to calculate the stage-specific survival percentage (Sx) of each stage using data on apparent mortality. It is calculated by using the equation:
Sx of a particular stage = (lx of subsequent stage) / (lx of a particular stage)
Mortality survival ratio (MSR)
MSR is the population growth that would have occurred if the mortality in the relevant stage hadn’t occurred. It is calculated as follows:
MSR of a particular stage = (Mortality in a particular stage) / (lx of subsequent stage)
Indispensable mortality (IM)
IM = Number of adults emerged × MSR of a particular stage
K-values
It is the primary element that drives the growth or decline in population from one age group to the next. The difference between the consecutive “log-lx” values is used to calculate the K value. By adding the k values for the various insect developmental phases, the overall generation mortality, K was determined according to Southwood, 1978.31
K = ke +ki1 + ki2 + ki3 + ki4 + ki5 + kp
where ke, ki1, ki2, ki3, ki4, ki5, and kp are the k values at the egg stage, first, second, third, fourth, fifth instars and, pupal stages, respectively.
Biocontrol agent and insect culture
The initial larvae of P. brassicae were collected from unsprayed cultivated cole crops (cabbage, cauliflower, and radish) during 2021-22 and were kept separately in rectangular plastic jars (25×20×15 cm) for rearing and for the recovery of parasitoids, if any. The jars were individually covered by a white cloth attached by rubber bands and fed with a fresh stock of food on subsequent days until pupation or parasitoids emergence, if any.
Ten P. brassicae of the first and second instar larvae were collected from the insect culture and placed in the centre of the respective host plants in ventilated plastic jars (10×15×10 cm) at room temperature. One disc of leaves was kept in each jar for each instar, and five adult male and female parasitoids were released in each plastic jar. Caterpillars were considered parasitized following Poelman et al (2014).20 To record the pupal period, the newly formed cocoons were separated, placed on clean petri plates and observed every 12 hours for the emergence of the parasitoid. The number of days was recorded from parasitism beginning to adult appearance to compute the developmental time of the host caterpillars. The experiments were replicated five times. The data obtained were subjected to MS Excel 2010 software to calculate the average SE and SD.
Results
Life cycle/ Development of Pieris brassicae
Mating was usually observed in an end-to-end position. The female raised the wings by exposing its end abdomen, and the male mounted on the female’s back. The female butterfly laid eggs in masses, with the biggest mass comprising between 120 to 200 eggs, mostly on the lower surface of the leaves. A complete life cycle is shown in Fig. 2. The incubation period, larval period, and total developmental time in different host plants are shown in Table 1.
The developmental periods from egg hatching to pupa formation varied significantly amongst the host plants. The larvae reared on cabbage had a shorter total development time (28.6 days), while as those reared on cauliflower and radish have longer developmental time of 30.4 and 32.6 days respectively.
Distinct phases of P. brassicae survival and mortality (Table 2, Fig. 3)
Table 1: Overview of P. brassicae developmental period (days) using various host plants in a laboratory
| Stages | Cabbage
Mean SE |
Radish
Mean SE |
Cauliflower
Mean SE |
| Incubation period | 03.6 0.69 | 05.2 0.63 | 05.2 0.69 |
| Larval period | 19.2 1.22 | 25.2 1.07 | 22.3 0.67 |
| Pupal stage | 05.8 0.78 | 07.2 0.78 | 04.5 0.52 |
| Total development period | 28.6 1.17 | 32.6 0.84 | 30.4 1.42 |
 |
Figure 2: Life cycle of Pieris brassicae (L.) (Lepidoptera: Pieridae) (a, b) Male & Female (c) Eggs (d) Hatching (e) An instar larva (f) Pupa (g) Pre emergence (h) Emergence of adult |
Table 2: Survival and mortality of various developmental phases of P. brassicae on diverse host plants under laboratory situation.
| Cabbage | ||||||||||
| Stages (x) | No of surviving at each stage (lx) | No of dying at each stage (dx) | Apparent mortality (100qx) | Survival fraction
(sx) |
Mortality/ Survival Ratio
(MSR) |
Indispensable mortality
(MSR×no of adults emerged) |
log 10 | k value | ||
| Incubation | 100 | 04 | 4.00 | 0.96 | 0.04 | 2.84 | 2.00 | 0.02 | ||
| I Instar | 96 | 02 | 2.08 | 0.97 | 0.02 | 1.42 | 1.98 | 0.01 | ||
| II Instar | 94 | 05 | 5.31 | 0.94 | 0.05 | 3.55 | 1.97 | 0.02 | ||
| III Instar | 89 | 09 | 10.11 | 0.89 | 0.11 | 7.81 | 1.95 | 0.05 | ||
| IV Instar | 80 | 07 | 08.75 | 0.91 | 0.09 | 6.39 | 1.90 | 0.04 | ||
| V Instar | 73 | 01 | 01.36 | 0.98 | 0.01 | 0.71 | 1.86 | 0.00 | ||
| Pupa | 72 | 01 | 01.38 | 0.98 | 0.01 | 0.71 | 1.86 | 0.01 | ||
| Adult | 71 | – | ˗ | ˗ | ˗ | – | 1.85 | K=0.15 | ||
| Radish | ||||||||||
| Incubation | 100 | 10 | 10 | 0.9 | 0.11 | 3.63 | 2.00 | 0.05 | ||
| I Instar | 90 | 07 | 7.78 | 0.92 | 0.08 | 2.64 | 1.95 | 0.03 | ||
| II Instar | 83 | 05 | 6.02 | 0.94 | 0.06 | 1.98 | 1.92 | 0.03 | ||
| III Instar | 78 | 09 | 11.54 | 0.88 | 0.13 | 4.29 | 1.89 | 0.05 | ||
| IV Instar | 69 | 15 | 21.74 | 0.78 | 0.28 | 9.24 | 1.84 | 0.11 | ||
| V Instar | 54 | 10 | 18.52 | 0.81 | 0.23 | 7.59 | 1.73 | 0.09 | ||
| Pupa | 44 | 11 | 25 | 0.75 | 0.33 | 10.89 | 1.64 | 0.12 | ||
| Adult | 33 | – | – | – | – | – | 1.52 | K=0.48 | ||
| Cauliflower | ||||||||||
| Incubation | 100 | 13 | 13 | 0.87 | 0.15 | 8.25 | 2.00 | 0.07 | ||
| I Instar | 87 | 05 | 5.75 | 0.94 | 0.06 | 3.3 | 1.93 | 0.02 | ||
| II Instar | 82 | 10 | 12.19 | 0.87 | 0.13 | 7.15 | 1.91 | 0.05 | ||
| III Instar | 72 | 08 | 11.11 | 0.88 | 0.12 | 6.6 | 1.86 | 0.05 | ||
| IV Instar | 64 | 04 | 6.25 | 0.93 | 0.06 | 3.3 | 1.81 | 0.03 | ||
| V Instar | 60 | 02 | 3.33 | 0.96 | 0.03 | 1.65 | 1.78 | 0.02 | ||
| Pupa | 58 | 03 | 5.17 | 0.94 | 0.05 | 2.75 | 1.76 | 0.02 | ||
| Adult | 55 | – | – | – | – | – | 1.74 | K=0.26 | ||
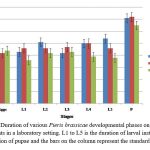 |
Figure 3: Duration of various Pieris brassicae developmental phases on various host plants in a laboratory setting. L1 to L5 is the duration of larval instars, P is duration of pupae and the bars on the column represent the standard error |
Apparent mortality
There was evidence of egg failure in all three host plants, with maximum egg mortality occurring on cauliflower (13%), followed by radish (10%) and cabbage (4%). However, larval mortality was observed highest in the second instar in radish (21.74%) and lowest in the fifth instar in cabbage (1.36%). Moreover, at the pupal stage, the highest mortality was observed in radish (25%) and the lowest in cabbage (1.38%).
Survival fraction
From the observation, the maximum survival fraction at egg stage was found on cabbage (0.97) in the first instar, followed by cauliflower (0.96) in the fifth instar, and the lowest on radish (0.75).
Mortality survival ratio
At the egg stage, the highest mortality survival ratio (MSR) was found on cauliflower (0.15), on cabbage (0.04). At various larval instars, radish had the highest MSR (0.28) in the fourth instar and the lowest MSR was found in cabbage (0.01) in the fifth instar. Moreover, at the pupal stage, the highest and lowest MSR was found on radish (0.33) and cabbage (0.01), respectively.
Indispensable mortality
The highest and lowest indispensable mortality (IM) of eggs was found on cauliflower (8.25) and cabbage (2.84), respectively. At the stage of pupal development, radish had the highest IM (10.89), while cabbage had the lowest IM (0.71).
K values
At the egg phase, cauliflower had the maximum k-value (0.07), while radish and cabbage had the K values of 0.05 and 0.02, respectively. Regarding the larval instars, the k-values were highest for radish (0.11) in the fourth instar and lowest for cabbage (0.00) in the fifth instar. Besides, the total generation mortality, K 31,35 of P. brassicae was recorded lowest on cabbage (0.15) and highest on radish (0.48), followed by cauliflower (0.26); thereby, cabbage resulted the most suitable host for P. brassicae development (Fig. 4). Moreover, the highest yield of adults was obtained from cabbage (71) in contrast to radish (33) and cauliflower (55).
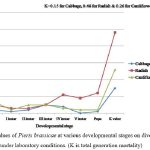 |
Figure 4: K-values of Pieris brassicae at various developmental stages on diverse host plants under laboratory conditions. (K is total generation mortality) |
Extent of damage
The caterpillars alone fed on leaves. The adult female butterflies only lay eggs and relied on visual cues to decide where to lay eggs. The plants used as oviposition sites typically contained glucosides. The caterpillars fed gregariously, and the initial caterpillars of the first instar stage just scraped the leaf surface and sometimes created circular irregular holes, but the grown-up caterpillars wandered from plant to plant and ate up the leaves in the middle of the leaf lamina and by the sides of the leaf margins, leaving intact the main veins, thereby resulting in heavy yield losses (Fig. 5, 6).
 |
Figure 5: (a) Hole formation, (b) scrapping of epidermal part of leaf, and (c) Leaf margin feeding by Pieris brassicae larva |
 |
Figure 6: Skeletization of cole crops by larvae of Pieris brassicae (a) Lab (b) Field |
Parasitization and behavior
Cultures of C. glomerata and P. brassicae were established from individuals collected from the agricultural fields. Laboratory rearing of field-collected larvae of P. brassicae revealed parasitism by a single wasp species, Cotesia glomerata Linnaeus (Hymenoptera: Braconidae), formerly known as Apanteles glomeratus Linnaeus. After 6-8 days, the signs of parasitism were quite evident. The first and second instars of white cabbage butterflies were recorded as the preferred hosts of wasps. The parasitoids completed their life cycle inside the host larva and emerged in an irregular mass of yellow-colored silken cocoons. They emerged from the cocoon after 7.3±1.3 days and were more than thirty-five individuals (38.6±3.05) in each mass of the cocoon (Fig. 7).
 |
Figure 7: Parasitoid of Pieris brassicae (a) Parasitoid emergence (b) Adult Cotesia glomerata emerging from cocoon (c) Mortality of parasitized larvae (d & e) Dorsal & ventral view of Cotesia glomerata |
Identification of Parasitoids
The parasitoids were preserved in 95% ethanol until further use. After emergence, the parasitoids were identified as Cotesia glomerata by Dr. Sarwan Kumar, Senior Entomologist and Associate Professor, Punjab Agriculture University, Ludhiana, Punjab, India.
Discussion
The parent-host plant accessibility and quality influenced pest population dynamics by affecting both immature and adult functions. The influence of host plant on the fitness was observed not only in P. brassicae but also in other insects like Helicoverpa armigera (Hubner) and Plutella xylostella (Linnaeus).24, 28, 30
In the present study, the developmental period of P. brassicae varied with diverse hosts. For example, it was longest on R. sativus and shortest on B. oleracea var. capitata. This difference was due to diverse food sources used by the parents during the larval period. A similar presumption had been reported for P. xylostella reared on diverse Brassica crops including cabbage.10 Besides, the host plants also influenced insect development rate.32 Our results showed that the longest and shortest development times of individuals from neonate to the end of the pupal stage were recorded on radish and cabbage, respectively. Hence, a shorter development time and lower rate of mortality on a host was inductive of that particular host. This was in accordance with the study conducted by Awmack and Leather, 2002.3 Therefore, it reinforced the hypothesis that cabbage is a more suitable host for the development of P. brassicae than cauliflower and radish. Besides, many factors that affected host suitability included its tissue texture and nutrient contents. In our study, the rate of early instar mortality was found to be higher on radish compared to cabbage and cauliflower due to the presence of hard tissue texture and trichome-like appendages in radish. This was supported by the studies of Gupta, 2002 and Ahmad et al. 2007.1,12 K value was a key factor primarily responsible for the increase or decrease in number from one generation to another. The total generation mortality, ‘K’ of P. brassicae was recorded maximum on radish (0.48) followed by cauliflower (0.26) and cabbage (0.15).
C. glomerata, an effective parasitoid of P. brassicae,was found in Palampur by Sood et al 2011.29 The mortality of larvae at the end of the fifth instar had also been reported by Laing and Levin, 1982 after the major damage to the crop.15, 18 In the field, C. glomerata had already been identified as a major natural enemy of P. brassicae and the host larvae expired 2 or 3 days later but did not feed throughout this time.9 In Iran, C. glomerata was observed as a parasitoid of P. brassicae, whereas in Kashmir Valley, it was also observed as a parasitoid of the cabbage butterfly.6,23 No pupal parasitism was recorded in the study.
Conclusion
In conclusion, this study provides information on the fitness of P. brassicae on diverse host plants. Understanding how the quality of the Brassica host plant affects the life table parameters of P. brassicae helps in understanding population dynamics and selecting appropriate management approaches for this pest. Radish can be employed as a trap crop when cabbage and cauliflower are the major vegetable cash crops. Besides, prolonged developmental time can enhance the exposure of an insect to its natural enemies. Many beneficial insects have been and continue to be raised for release as biological pest control strategies. Knowledge of biology and natural enemies is required for pest management strategies that are compatible with IPM (Integrated Pest Management), and a successful management plan requires information about a species biology including its diet and life cycle. Biocontrol is a promising solution for both environmental protection and pest problems in agriculture. C. glomerata is a successful biocontrol agent that can be used as a principal parasitoid against P. brassicae. Conservation of this wasp species and relocation of unhatched wasp cocoons to contaminated sites can help in the natural control of the pest. The parasitoid species, which completes its developmental cycle on caterpillars of other species from the genus Pieris and Pontia (P. rapae, P. protodice, P. napi, and Pontia daplidice) has the potential to reduce the harmfulness of the caterpillars from the genus Pieris. Further research, particularly concerning parasitism of the egg and pupal stages, is likely to reveal more parasitoid species in Rajouri.
Acknowledgment
We thank Dr. Sarwan Kumar, Associate Professor, Punjab Agricultural University, Ludhiana, Punjab for his help in the identification of the species. We also express our gratitude to Dr. Arif Tasleem Jan, Assistant Professor, Department of Botany, Baba Ghulam Shah Badshah University, Rajouri J&K for providing useful insights. We also thank the anonymous reviewers for their careful reading of our manuscript and their critical comments and suggestions during the final preparation of the manuscript.
Conflict of Interest
We have no conflicts of interest to disclose. All authors accept the previous version of the manuscript.
Funding sources
There are no funding sources.
References
- Ahmad H, Shankar U, Monobrullah M, Kaul V, Singh S (2007) Bionomics of cabbage butterfly, Pieris brassicae (Linn.) on cabbage. Ann Plant Protect Sci 15:47-52
- Ali A, Rizvi PQ (2008) Influence of aphid species on the development and predation of Menochilus sexmaculatus Fabricius (Coleoptera: Coccinellidae). J Eco-Friendly Agric 3(2):134–137
- Awmack CS, Leather SR (2002) Host plant quality and fecundity in herbivorous insects. Annu Rev Entomol 47: 817-844. https://doi.org/10.1146/annurev.ento.47.091201.145300
CrossRef - Bellows Jr TS, van Driesche RG, Elkinton JS (1992) Life table construction and analysis in the evaluation of natural enemies. Ann Rev Entomol 37:587-614. https://doi.org/1146/annurev.en.37.010192.003103
CrossRef - Bhandari K, Sood P, Mehta PK, Choudhary A, Prabhakar CS (2009) Effect of botanical extracts on the biological activity of granulosis virus against Pieris brassicae. Phytoparas 37:317–322. http://dx.doi.org/10.1007/s12600-009-0047-2
CrossRef - Bhat DM, Bhagat R C (2009) Natural parasitism of Pieris rapae (L.) and Pontia daplidice (L.) (Lepidoptera: Pieridae) on cruciferous crops in Kashmir Valley (India). Am Eurasian J Agric Environ Sci 5(4):590-591
- Boczek J, Lewandowski M (2016) Science about pests of crops. SGGW Publishing House 1-412
- Chaudhuri N, Ghosh S, Ghosh J, Senapati K (2001) Incidence of insect pests of cabbage in relation to prevailing climatic conditions of Terai region. Indian Journal of Entomology 63:421-428
- Feltwell J (1982) Large White Butterfly: The Biology, Biochemistry and Physiology of Pieris brassicae(Linnaeus). W. Junk Publishers, The Hague, Boston, London 535. http://doi.org/10.1007/978-94-009-8638-1
CrossRef - Golizadeh A, Kamali K, Fathipour Y, Abbasipour H (2009) Life table of the Diamondback moth, Plutella xylostella (L.) (Lepidoptera: Plutellidae) on five cultivated brassicaceous host plants. J Agric Sci Technol 11:115-124
- Gupta PP (1984) Bionomics of the cabbage butterfly, Pieris brassicae (Linn.) in the mid hill of Himachal Pardesh Him J Agril Res 10:49-54
- Gupta R (2002) Food preference of the 5th instar cabbage white butterfly, Pieris brassicae to cole crop. Pest Manag Econ Zool 10:205-207
- Hasan F, Ansari MS (2010a) Effect of different cole crops on the biological parameters of Pieris brassicae (L.) (Lepidoptera: Pieridae) under laboratory condition. J Crop Sci Biotechnol 3:195-202. https://doi.org/10.1007/s12892-010-0025-2
CrossRef - Kasi IK, Singh M, Waiba KM, Monika S, Waseem M, Archie D, Gilhotra H (2021) Bio-efficacy of entomopathogenic nematodes, Steinernema feltiae and Heterorhabditis bacteriophora against the Cabbage butterfly Pieris brassicae L under laboratory conditions. Egyptian J Biol Pest Control 31:1-7. https://doi.org/10.1186/s41938-021-00469-4
CrossRef - Laing JE, Levin DB (1982) A review of the biology and a bibliography of Apanteles glomeratus (L.) (Hymenoptera: Braconidae). Biocontrol News and Information 3(1):7-23
- Liu Z, Li D, Gong P, Wu K (2004) Life table studies of the cotton bollworm, Helicoverpa armigera (Hubner) (Lepidoptera: Noctuidae), on different host plants. Environment Entomology 33:1570-1576. https://doi.org/10.1603/0046-225X-33.6.1570
CrossRef - Morgan D, Walters KFA, Aegerter JN (2001) Effect of temperature and cultivar on pea aphid, Acyrthosiphon pisum (Hemiptera: Aphididae) life history. Bull Entomol Res 91:47-52. https://doi.org/10.1079/BER200062
CrossRef - Parker FD, Pinnell RE (1973) Effect of food consumption of the imported cabbageworm when parasitized by two species of Apantales. Environment Entomology 2:216-219
CrossRef - Pearl R, Parker SL (1921) Experimental studies on the duration of life. I. Introductory discussion of the duration of life in Drosophila. American Naturalists 55:481-509
CrossRef - Poelman EH, Gols R, Gumovsky AV, Cortesero AM, Dicke M Harvey JA (2014) Food plant and herbivore host species affect the outcome of intrinsic competition among parasitoid larvae. Ecological Entomology 39:693–702. https://doi.org/10.1111/een.12150
CrossRef - Rai AB, Halder J, Kodandaram MH (2014) Emerging insect pest problems in vegetable crops and their management in India: An appraisal. Pest Management Horticulture Ecosystem 20(2):113-122
- Raiola A, Errico A, Petruk G, Monti DM, Barone A, Rigano MM (2018) Bioactive compounds in brassicaceae vegetables with a role in the prevention of chronic diseases. Molecules 23(1):1–10. https://doi.org/10.3390/molecules23010015
CrossRef - Razmi M, Karimpour Y, Safaralizadeh MH Safavi SA (2011) Parasitoid complex of cabbage large white butterfly Pieris brassicae (L.) (Lepidoptera: Pieridae) in Urmia with new records from Iran. J Plant Prot Res 51(3):6248-52. https://doi.org/10.2478/V10045-011-0041-9
CrossRef - Sarfraz M, Dosdall LM, Keddie BA (2007) Resistance of some cultivated Brassicaceae to infestations by Plutella xylostella (Lepidoptera: Plutellidae). J Econ Entomol 100:215-224. https://doi.org/1603/0022-0493(2007)100[215:roscbt]2.0.co;2
CrossRef - Sequiera R, Dixon AFG (1996) Life history responses to host quality changes and competition in the Turkey-oak aphid, Myzocallis boerneri(Hemiptera: Sternorrhyncha: Callaphididae) Eur J Entomol 93(1):53-58
- Sharma A, Kumar V, Shahzad, B, Tanveer M, Sidhu GPS, Handa N, Kohli SK, Yadav P et al (2019) Worldwide pesticide usage and its impacts on ecosystem. SN Applied Science 1(11):1-6. https://doi.org/10.1007/s42452-019-1485-1
CrossRef - Sivapragasam A, Abdul Aziz AM (1990) Cabbage webworm on crucifers in Malaysia. In: Talekar NS (ed) Diamondback moth and other crucifer pests. Asian Vegetable Research and Development Center 75–80. 603
- Soleimannejad S, Fathipour Y, Moharramipour S, Zalucki MP (2010) Evaluation of potential resistance in seeds of different soybean cutivars to Helicoverpa armigera (Lepidoptera: Noctuidae) using demographic parameters and nutritional indices. J Econ Entomol 103:1420-1430. https://doi.org/10.1603/ec10022
CrossRef - Sood P, Prabhakar CS, Mehta PK (2011) Field evaluation of an indigenous granulosis virus isolate for Pieris brassicae (Linnaeus) management under north western Himalayan conditions. Journal of Biological Control 25:217-22. https://doi.org/10.18311/jbc/2011/3734
- Soufbaf M, Fathipour Y, Karimzadeh J, Zalucki MP (2010) Development and age specific mortality of diamondback moth on Brassica host plants: pattern and cause of mortality under laboratory conditions. Ann Entomol Soc Am 100:574-579. https://doi.org/10.1603/AN10010
CrossRef - Southwood TRE (1978) Ecological Methods with Pahcular Reference to Study of Insect Population. The English Language Book Society and Chapman and Hall, London 524
CrossRef - Talekar NS, Shelton AM (1993) Biology, ecology, and management of the diamondback moth. Annu Rev Entomol 38:275-301. https://doi.org/10.1146/annurev.en.38.010193.001423
CrossRef - Trdan S, Laznik Z, Bohinc T (2020) Thirty years of research and professional work in the field of biological control (predators, parasitoids, entomopathogenic and parasitic nematodes) in Slovenia: A review. Appl Sci 10(21):7468. http://dx.doi.org/10.3390/app10217468
CrossRef - Ullah MI, Arshad M, Ali S, Iftikhar Y, Mahmood SU, Arshad M (2016) Effect of thiamethoxam and some botanical extracts on Cotesia glomerata (Hymenoptera: Braconidae): an endoparasitoid of Pieris brassicae L. (Lepidoptera: Pieridae). Egypt J of Biol Pest Control 26(3):545-549
- Varley GC, GR Gradwell (1960) Key factor in population studied. J Anim Ecol 29:399-401
CrossRef - Younas M, Naeem M, Raquib A, Masud S (2004) Population dyanimcs of Pieris brassica on five cultivar of cauliflower at Peshawar. Asian J Plant Sci 3:391-393. https://doi.org/10.3923/ajps.2004.391.393
CrossRef
Abbreviations
IPM Integrated Pest Management
UT Union Territory
J&K Jammu and Kashmir
oC Degree Celsius
MS Microsoft
SD Standard Deviation
SE Standard Error

