Introduction
The weedy rice (Oryza sativa f. spontanea) is considered a crop wild relative (CWR) of cultivated rice Oryza sativa, with same genome type AA (2n = 24). Weedy rice is a common weed in paddy fields throughout the world mainly in temperate and subtropical areas.1 It is commonly known as ‘red rice’ due to red pericarp colour. Currently, research on the origin and evolution of weedy rice has made some progress; but still there is no definite conclusion about the origin of weedy rice,2,3 and it is a typical example of convergent evolution in rice crop. It was established that during the time of Neolithic period, approximately it took ancient farmers a few hundred/thousands years to convert wild plants to domesticated cultivars for better yield potentiality and quality food products.3 Very rarely, it was observed that the domesticated crop plants sporadically obtaining again (recapture) some of the habitat similar to wild-like traits at the time of natural evolution.4 This type of evolutionary event is considered as de-domestication phenomenon. This type of phenomenon is also termed as feralization and detected in both the animal and plant kingdoms specifically in livestock species and crop plants, for example found in wheat and rice.2,5 Crop domestication is an important event for transformation of wild species of crop plants into crop varieties through the process of artificial selection by the ancient people (Qiu et al. 2016). Domestication is a unique event through which fitness of a crop species has been enhanced under human cultivation but genetic diversity for the traits has been reduced tremendously in the natural ecological system.6 De-domestication process is a different kind of evolutionary phenomenon where losses of traits are detected (observed) that aggregated during domestication process, as a result domesticated species can be revolved as a self-sustainable ‘wild-like’ species with low genetic diversity commonly control by natural selection. Adaptability in the natural feral and harsh environmental conditions of the dedomesticated crop varieties remains unclear.4 Newly arisen additional novel mutations may play vital role in environmental adaptation during de-domestication. Wild rice gene(s) may play important role by contributing new mutation/variations which are introgressed into the domesticated crop varieties during the time of domestication and may play important roles for sustainable adaptation to the harsh environmental conditions during dedomestication event through balancing selection. Weedy rice (Oryza sativa f. spontanea) has been converted to an annual crop system commonly pervading the paddy fields are considered as mixed ancestral form of O. rufipogon/O. nivara and cultivated rice O. sativa.7
It was agreeing that weedy rice originated either through the de-domestication event independently from the rice cultivars which was supported by the advance technique of whole-genome sequencing analysis8,9 or other possibilities of the weedy rice origin may include the process of hybridization system or it can be a gene flow type mechanism occurred between cultivated rice and their wild relatives; whole process can be termed as inter-cultivar hybridization system. Two hypotheses were proposed regarding the origin of weedy rice. One group proposed hat evolution of weedy rice has been occurred through natural hybridization between rice cultivars and wild rice relatives, is called as exo-ferality,10 other group proposed that weedy rice has been originated from cultivated progenitors in straightway by the process of de-domestication and termed as endoferality.11 Whatever evolutionary phenomena (exoferalityor endoferality) are being taking place in this process of weedy rice evolution but recapturing of seed shattering characteristics is the crucial for the origin of Oryza sativa f. spontanea. Genome-wide SNPs variations and domestication-related genes likelihood mechanism support the views that weedy rice is a product of hybridization between cultivated rice and wild rice or it may simply a de-domestication process through which weedy rice has been originated.9
Mostly, weedy rice embraces intermediate type of morphological characteristics which are descent either from wild rice species (Oryza rufipogon) or cultivated rice (Oryza sativa L.).12 Following morphological characteristics were demonstrated in the red ricebiotypessuch astaller height, short growth period, hard and black hull, strong granulation and seed shattering, dormancy period lengthy, long awning, pericarp colour red, variation in panicle size, shorter grain-filling stage and early maturity.13,14 Ultrastructure of caryopsis in red rice has following layers, pericarp, seed coat, nucellus, aleurone layer, and endosperm from outside to inside respectively.15 Colour variation of the rice pericarp due to the accumulation of different pigments such as anthocyanins and proanthocyanidins mainly in the pericarp and seed coat layers. Pigmented rice grains are rich in protein, amino acids, vitamins, vegetable fats, and trace elements such as Ca, Fe, Zn, and Se. Coloured grains also content high amount of biologically active components including flavonoids that have antioxidant, hypoglycaemic properties, other medicinal properties like antitumor activity, free radical-scavenging characteristics16,17 and protective properties against several non-communicable diseases, cardiovascular diseases, diabetes mellitus, and metabolic syndrome including cancers. Therefore, consumers are preferring coloured rice for distinctive health benefits.18 Grain colour of weedy rice varies from purple, red, light-red, light green, to white.19,20
Weedy rice is well adapted in the natural harsh ecological niche with extreme versatile environmental situation because due to the presence of many novel gene(s) which are tolerant to several biotic/abiotic stresses and may be utilized in rice breeding to improve the released varieties with climate ready potentiality.12,21 Weedy rice genetic resources are considered as novel germplasm for the following characteristics such as tolerance to drought, cold, salinity and showing resistance against many diseases like bacterial blight and blast.12,14,21,22,23,24 It was reported that the resistance activity is genotype dependent and consequently indica type weedy rice shows better resistance in compare to genotype of japonica weedy rice.22 Tremendous phenotypic variation are prevailed in the weedy rice populations and may be the main reasons for high adaptability in the extreme environmental climatic conditions.
Huge tillering numbers with extremely expanded root surface makes weedy rice nitrogen use efficient plant and can easily adapted in low resourceful soil.24 Long seed dormancy and seed shattering phenotypic features donate to avoid unfavorable abiotic stresses, leading to escalation the species fitness in the harsh environment. Genetic diversity study in weedy rice revealed that nearly 18% of its genes are associated with tolerance to abiotic stresses including abundant adaptive characters which are not found in the cultivars. As a results they are showing high vigour and reproduction ability in order to make them added tolerant to many biotic/abiotic stresses.25
Thus, CWR has been considered a valuable reservoir of genetic diversity for any breeding program. Natural populations of weedy rice are in threatened conditions and leading to diminishing the genepool which needs utmost attention to conserve this virgin genetic resources for future use. Very few germplasm is conserved in the national/international genebank.7 Genetic basis and agro-morphological characteristics of naturally growing weedy rice are not fully studied to understand their eco-habitat.
Therefore, in this present investigation, a comprehensive phenotypic characterization of weedy rice (Oryza sativa f. spontanea) based on agromorphological traits and ultrastructure of caryopsis is investigated, which represent unique source of naturally occurring, highly admixed pre-breeding material for use in rice breeding. This CWR resource is currently available and can be extensively utilized in rice breeding program for varietal improvement which leads to sustainable food security.
Material and Methods
Plant material
Weedy rice at the seedling stage was (Oryza sativa f. spontanea) collected from the adjoining rice field of Mughalsarai (UP) and conserved (on farm) in the experimental rice field of the University of North Bengal for further research work. Wild rice (O. rufipogon) was growing in the ditches of Magurmari river flowing through the University campus near our Experimental rice field and used in this study. Seeds of cultivated rice (Banni, Sadaswarna, Yamuna, and Sadanunia) were collected from farmer’s field of Mekhliganj area,Coochbehar district, WB.
Agromophological characterization through DUS protocol
Agromorphological traits were characterized through DUS test protocol (Distinctiveness uniformity and stability test) of PPV&FRA Act (Govt. of India, 2001).
Study the Physicochemical properties of rice grains
Standard method26 was used to measure the alkali spreading value (ASV) (ranges from 1 to 7 scale basis) to judge grain quality. Result of ASV is low then it signifies the high GT value (gelatinization temperature), equally high ASV value designates a low GT. Aroma was detected using sensory based standard evaluation procedure27 which varies from 0 to 3 indicator index. Ten good quality seeds of each variety were taken and the husk of each rice seed was peeled with the help of a blade. Bran layer was removed from the grain and were dip in 10 ml solution of 1.7% KOH in a Petri plate. After 10 minutes in the KOH solution, aroma was tested with sensory feeling by us. The aroma is classified into three categories ranging as follows0, 1, 2, 3 index, non-scented to highly scented respectively. After 23 hours in the KOH solution for alkali spreading test, gelatinization temperature (GT) was measured according to DUS protocol.
Cooking and retrogradation
Cooking time and retrogradation was determined using common test procedure.28 In this test procedure, unpolished grain was boiled in a test tube and taken out at a definite time interval and pressed in between two glass plates to observe that there is no opaque region. After that the cooked rice was placed on glass plate for retrogradation naturally about 24hrs.
Scanning Electron Microscopy
Caryopsis was cut into round pieces with the help of fine scalpel and one round block was set into the SEM machine for in situ ultrastructure analysis. Histological ultra-structure was detected using a scanning electron microscope (SEM) of Jeol company, Japan (Jeol Model JSM-IT100). Study was conducted at various magnification level with an accelerating voltage of 10 kV.29,30,31 for uncovering the histo-anatomical ultrastructure of the different rice caryopses. Before observing under SEM machine, round piece of caryopsis should be coated with the thin film of gold using a Sputter coater of Jeol Company (Model no. Smart Coater PF 18001006-2). It takes only 2 minutes to finish the gold coating in this Sputter coater because it is used high vacuum evaporator condition to coat the sample.
Phenol colour test
Morphological characterization of rice grains is being performed based on phenol colour test (DUS test protocol, Govt. of India). It is being carried out to reveal the reactivity either positive or negative of the grains husk colour.32,33 Therefore, rice varieties can be classified into two main groups either phenol reaction positive (indica type) or negative group (japonica type).32,33 In this test, generally an aqueous (1-2%) phenol solution is used for colour reaction activity with the grain hull. After exposure to this phenol solution for 24 hours, colour variation is determined. In some cases, hull colour changed to dark black colouration but other do not show any change in the hull colour. Phenol positive tested rice varieties are mostly indica type and negative tested rice varieties generally grouped as japonica type. During this 24 hrs experimental time, phenol gets oxidized into dark melanin pigment. Colour conversion occurs due to the presence of polyphenol oxidase (PPO) activity (a positive response) in the seed coat.34,35 Wild Oryza species shows colour reaction if expose to phenol solution and changed to dark brown or black coloration. Indicating that the polyphenol oxidase (PPO) enzyme (tyrosinase protein family) activity is happened.34,35 Demonstrating that the Phr1 locus is functioning in the wild rice genotypes and synthesizes PPO enzyme, which ultimately catalyse the dark colour reaction obviously in indica type rice group. Due to loss-of-function mutations at this locus of Phr1, it is not expressing to synthesize PPO in the japonica rice varieties.35 It is showing genetically controlled reaction in the rice varieties. Thus, phenol test is vital test to be used to characterize the rice varieties for identification purposes.
Gel consistency test(GC)
Rice eating quality can be judged by the procedure of gel consistency.36 The 100 mg of milled rice powder were taken in test tubes and wetted by 0.2ml of 95% alcohol containing 0.025 % thymol blue. The 2 ml of 0.2 N KOH was added while shaking in Vortex Genie mixer. The tubes containing samples were transferred into water bath for 8 minutes. After removing the tubes from water bath, it was kept in room temperature for 5 minutes. The test tubes were now kept in ice water for 15 minutes for cooling. Following that the test tubes were placed in horizontally over the graph paper and gel spreading was measured after 30 minutes. Gel consistency are divided into three categories: soft (>60mm in length), medium (40-60 mm in length) and hard (30-40 mm in length).
Results and Discussion
Fifteen quantitative agromorpological traits of six different genotypes (two crop wild relatives and four farmer’s varieties) were evaluated based on DUS protocol, Govt. of India (PPV&FR Act 2001). Following traits were considered for evaluation- plant height (PH), flag leaf length (FLL), flag leaf breadth (FLB), panicle length (PnL), grain per panicle (Gr/Pn), grain length (GL), grain breadth (GB), 1000 grain weight (Gr/Wt), tillering (Till), heading date (HD), maturity time days (MT), yield per plant (YPP), kernel length (KL), kernel breadth and awn (Tables 1; Figs. 1-3). It suggested that there were inherent genetic differences among the six genotypes studied in the present investigation in respect to the morphological traits considered during the analysis. Plant height in weedy rice is on an average 94.40 cm, and in wild rice it is 120.19 cm. Flag leaf length is 33.69 cm in weedy rice, in case of wild rice it is 21.21cm. Thousand grain weight is 22.50 g in weedy rice whereas in wild rice it is only 13.50 g. Grain per panicle is high in weedy rice (117.10 grain/panicle) but very less in wild rice (39.80 grain/panicle) (Table 1). Detail morphological characteristics of thirty-nine traits of six genotypes were summarized (Table 2; Fig. 3) as described in DUS test protocol. Distinctive twenty-three traits of weedy rice were described (Table 3) to know the specific traits for weedyness (Fig.1). Weedy rice is showing distinguishing morpho-habitat features from that of wild rice (Fig. 1; Tables 1-2). Present characteristics of weedy rice are consistent with the earlier reports (Nadir et al., 2017; Pipatpongpinyo et al. 2019). Weedy rice panicles are showing variations in awns, hull color, and panicle size, which are supporting the view of previous analysis (Nadir et al., 2017).
Grain morphology and physicochemical properties of the six rice genotypes was studied (Table 4; Fig. 2) for comparative evaluation of the grain characteristics. Highest grain weight (1000 grain) was observed in local rice variety Banni (26.91 g) and lowest in wild rice (13.50 g), whereas weedy rice grain weight was moderate range (22.50 g) (Table 4). Grain length ranged from 8.20 mm, 7.92 mm, 9.21 mm, 7.06 mm, 7.77 mm, and 8.15 mm in weedy rice, wild rice, Banni, Sadaswarna, Yamuna and Sadanunia respectively. Sensory based aroma was detected in wild rice (index- 1) and in Sadanunia (index- 3), and GC illustrated as soft (ranged from 125 mm to 150 mm), cooked kernel elongation ration was 3.38, 3.60, 3.58, 2.93, 3.99 and 3.50 in weedy rice, wild rice, Banni, Sadaswarna, Yamuna and Sadanunia respectively (Table 4). Spikelet morphology and its different reproductive parts were dissected and characterized (Fig. 4). In the present investigation, it was observed that grain pericarp has diverse colours from red, light red, to white. Similar type of observation also demonstrated by some earlier researchers (Prathepha 2009; Han et al., 2022).
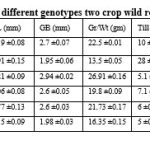 |
Table 1: Fifteen agromorphological traits were evaluated for six different genotypes (two crop wild relatives and four farmer’s varieties) according to DUS protocol. |
NA=not applicable
Table 2: Morphological characteristics of weedy rice, wild rice and four farmer’s varieties were summarized.
|
Sl no |
Characters |
Weedy rice |
Wild rice |
Banni |
Sadaswarna |
Yamuna |
Sadanunia |
|
1 |
Coleoptile colour |
Green |
Green |
Green |
Green |
Green |
Green |
|
2 |
Basal leaf Sheath |
Green |
Light purple |
Green |
Green |
Green |
Green |
|
3 |
Leaf: Intensity of green colour |
Medium |
Medium |
Dark |
Medium |
Medium |
Medium |
|
4 |
Leaf: Anthocyanin colouration |
Present |
Absent |
Absent |
Absent |
Absent |
Absent |
|
5 |
Leaf: Distribution of anthocyanin coloration |
Present |
Present |
Absent |
Absent |
Absent |
Absent |
|
6 |
Leaf Sheath anthocyanin colouration |
Absent |
Present |
Present |
Absent |
Absent |
Absent |
|
7 |
Leaf Sheath : Intensity of anthocyanin colouration |
Absent |
strong |
Low |
Absent |
Absent |
Absent |
|
8 |
Leaf: Pubescence of blade surface |
Present |
Present |
Present |
Present |
Present |
Present |
|
9 |
Leaf : Auricles |
Present |
Present |
Present |
Present |
Present |
Present |
|
10 |
Leaf :Anthocyanin of colouration of auricles |
Colourless |
Light purple |
Colourless |
Colourless |
Colourless |
Colourless |
|
11 |
Leaf : Collar |
Present |
Present |
Present |
Present |
Present |
Present |
|
12 |
Leaf :Anthocyanin colouration of collar |
Absent |
Absent |
Weak |
Absent |
Absent |
Absent |
|
13 |
Leaf : Ligule |
Present |
Present |
Present |
Present |
Present |
Present |
|
14 |
Leaf : Shape of ligule |
Split |
Split |
Split |
Split |
Split |
Split |
|
15 |
Leaf : Colour of ligule |
White |
Light purple |
White |
White |
White |
White |
|
16 |
Leaf : Length of blade |
30 cm |
31cm |
35cm |
27cm |
36cm |
39 cm |
|
17 |
Leaf : Width of blade |
1.15 |
0.8cm |
1.1cm |
1cm |
1cm |
0.9cm |
|
18 |
Culm: Attitude |
Spreading |
Spreading |
Erect |
Erect |
Erect |
Erect |
|
19 |
Flag leaf :Attitude of blade ( early observation ) |
Erect |
Semi erect |
Erect |
Erect |
Erect |
Erect |
|
20 |
Spikelet : Density of pubescence of lemma |
Strong |
strong |
Strong |
weak |
weak |
Medium |
|
21 |
Male Sterility |
No |
No |
No |
No |
No |
No |
|
22 |
Lemma : Anthocyanin colouration of keel |
Absent |
Strong |
Absent |
Absent |
Absent |
Absent |
|
23 |
Lemma : Anthocyanin colouration of area below apex |
No |
Strong |
Absent |
Absent |
Absent |
Absent |
|
24 |
Lemma: Anthocyanin colouration of apex |
Strong |
No |
Very strong |
Absent |
Absent |
Absent |
|
25 |
Spikelet : colour of stigma |
Purple |
Purple |
White |
White |
White |
White |
|
26 |
Stem : Anthocyanin colouration of nodes |
Present |
Present |
Absent |
Absent |
Absent |
Absent |
|
27 |
Stem : Intensity of Anthocyanin colouration of nodes |
weak |
Medium |
Absent |
Absent |
Absent |
Absent |
|
28 |
Stem : Anthocyanin colouration of internodes |
Present |
Present |
Present |
Absent |
Absent |
Absent |
|
29 |
Flag leaf :Attitude of blade ( Late observation) |
Semi erect |
Horizontal |
Erect |
Erect |
Erect |
Erect to semi erect |
|
30 |
Panicle: Curvature of main axis |
Straight |
Straight |
Deflex |
Deflex |
Deflex |
Deflex |
|
31 |
Spikelet : Colour of tip of lemma |
Purple |
Purple |
Purple |
Yellow |
Yellow |
straw |
|
32 |
Lemma and Palea : Colour |
Greenish week |
Black |
light purple |
Straw |
Straw |
straw white |
|
33 |
Panicle Awns |
Present |
Present |
present |
Absent |
Absent |
|
|
34 |
Panicle : Colour of awns ( late observation ) |
Blackish brown |
Radish brown |
Purple |
Absent |
Absent |
Straw white |
|
35 |
Panicle : Distribution of awns |
Tip only |
Tip only |
Tip only |
Absent |
Absent |
Tips only |
|
36 |
Panicle : Attitude of branches |
Semi erect |
Spreading |
Erect |
Erect |
Erect |
Erect to semi erect |
|
37 |
Panicle : Exertion |
Fully exerted |
Well exerted |
Well exerted |
Fully exerted |
Mostly exerted |
Fully exerted |
|
38 |
Leaf : Senescence |
Medium |
Late |
Medium |
Medium |
Medium |
medium |
|
39 |
Sterile lemma : Colour |
Straw |
Purple |
Straw |
straw |
Straw |
Straw |
Table 3: Agromorphometric traits were documented for weedy rice.
|
Weedy Rice (O. sativa f. spontanea) |
Trait range recorded |
|
Awn presence |
Awned |
|
Awn colour |
Black, Brown |
|
Awn distribution |
Tip only |
|
Awn length (mm) |
87-117 |
|
Hull coloration |
Black, Brown |
|
Pericarp colour |
Red |
|
1000 seeds weight (g) |
22.5 g |
|
Shattering nature |
Fully shattered |
|
Germination percentage |
30% |
|
Number of seeds per panicle |
87-162 |
|
Whole seed length (mm) |
7.91-8.7 |
|
Whole seed breadth (mm) |
2.08-2.87 |
|
Dehulled seed length (mm) |
5.84-6.55 |
|
Dehulled seed Breadth (mm) |
2.12-2.27 |
|
Plant height (excluding panicle) (cm) |
45-95 |
|
Flag leaf attitude of blade |
Erect , semi-erect |
|
Flag leaf length (cm) |
14.9-49.2 |
|
Flag leaf breadth (cm) |
1.1-1.6 |
|
Ligule length (cm) |
1.6-2.7 |
|
Ligule color |
Purple |
|
Anthocyanine coloration of auricles and nodes |
Whitish |
|
Panicle attitude in relation to stem |
Upright ,semi-upright |
|
Panicle length (cm) |
17-28 |
 |
Figure 1: Morphological characteristics of weedy rice, and wild rice were represented. A: Wild rice Oryza rufipogon growing in pot, B: Inflorescence of O. rufipogon with anthers and stigma |
Table 4: Summary of the grain morphology with physicochemical properties of the six genotypes was represented.
|
Grain quality traits |
Weedy rice |
Wild rice |
Banni |
Sadaswarna |
Yamuna |
Sadanunia |
|
1000 Gr/Wt(g) |
22.50 |
13.50 |
26.91 |
19.80 |
21.37 |
16.35 |
|
1000 Kernel/Wt(g) |
16.00 |
10.50 |
19.50 |
15.70 |
16.70 |
13.00 |
|
1000 Husk weight(g) |
6.50 |
3.00 |
7.41 |
4.10 |
4.67 |
3.35 |
|
Weight loss (g) |
6.50 |
3.00 |
7.41 |
4.10 |
4.67 |
3.35 |
|
Average grain length (mm) |
8.20 |
7.92 |
9.21 |
7.06 |
7.77 |
8.15 |
|
Average grain breadth(mm) |
2.70 |
1.95 |
2.94 |
2.60 |
2.60 |
1.98 |
|
Length and breadth ratio |
3.03 |
4.06 |
3.13 |
2.71 |
2.98 |
4.11 |
|
Cooked kernel length (mm) |
10.31 |
10.26 |
10.84 |
9.00 |
10.67 |
10.50 |
|
Cooked kernel breadth(mm) |
3.05 |
2.85 |
3.02 |
3.07 |
2.67 |
3.00 |
|
Elongation ratio |
3.38 |
3.60 |
3.58 |
2.93 |
3.99 |
3.50 |
|
ASV |
7 |
7 |
4 |
2 |
3 |
6 |
|
GT |
1 |
1 |
3 |
7 |
5 |
1 |
|
Aroma |
0 |
2 |
0 |
0 |
0 |
3 |
|
GC (mm) |
Soft (130) |
Soft (125) |
Soft (135) |
Soft (135) |
Soft (150) |
Soft (150) |
Only Sadanunia showed phenol negative reactivity but remaining five rice genotypes responded to phenol reaction (wild rice, weedy rice, Yamuna, Sadaswarna, Banni) and tested positive (Fig. 5). Based on phenol colour test, rice varieties may categorize into two groups. Phenol positive indica group and phenol negative japonica group.33 Phenol positive tested rice varieties are mostly indica type and negative tested rice varieties generally grouped as japonica type. During this 24 hrs experimental time, phenol gets oxidized into dark melanin pigment. Colour conversion occurs due to the presence of polyphenol oxidase (PPO) activity (a positive response) in the seed coat.34,35 In this present investigation, five rice genotypes showed phenol positive reaction and categorized as indica type but remaining one genotype (Sadanunia) showing phenol negative test indicating japonica type rice group. Signifying that this variety Sadanunia has a mutation in the locus Phr1 locus, and consequently not able to synthesize functional PPO enzyme. The PPO enzyme activity was not detected in variety Sadanunia. Thus phenol test protocol is an important attribute to identify the varieties for further protection and conservation.
Table 5: Summary of the caryopses ultrastructural characteristics studied under scanning electron microscope (SEM) of the six genotypes (CWR and farmer’s varieties) was depicted.
|
Rice varieties |
Thickness pericarp-testa (μm) |
Aleurone layer thickness |
Size of the Aleurone |
Bran Thickness |
Size AG(μm) |
Size CSG |
Size of PB(μm) |
Abundance in PB |
Barb size of Awn (μm) |
|
Weedy rice |
5.91 |
24.17 |
8.80 x |
29.55 |
1.08 – 3.04 |
5.88 -13.33 |
1.088 |
++ |
358.489 |
|
Wild rice |
6.96 |
23.89 |
10.95 x |
30.85 |
1.30 -3.91 |
5.42 – 16.26 |
1.53 |
++ |
329.169 |
|
Banni |
5.36 |
31.66 |
14.54 x |
37.029 |
0.86 – 2.60 |
3.53 – 13.748 |
0.692 |
+++ |
_ |
|
Sadaswarna |
6.73 |
20.95 |
7.04 x |
27.682 |
1.15- 3.26 |
6.708 – 15.12 |
1.48 |
+++ |
_ |
|
Yamuna |
8.17 |
28.15 |
8.98 x 11.66= |
36.327 |
1.38 – 3.05 |
7.69 – 23.07 |
0.846 |
+++ |
_ |
|
Sadanunia |
5.66 |
19.96 |
19.60 x 11.20= |
25.03 |
0.5 – 3.50 |
4.00 – 12.00 |
1.50 |
++ |
– |
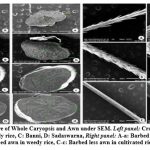 |
Figure 6: Ultrastructure of Whole Caryopsis and Awn under SEM. Left panel: Cross section of caryopsis, |
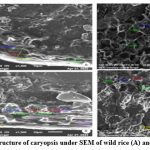 |
Figure 7: Ultrastructure of caryopsis under SEM of wild rice (A) and weedy rice (B). |
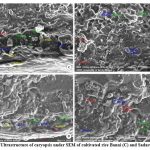 |
Figure 8: Ultrastructure of caryopsis under SEM of cultivated rice Banni (C) and Sadaswarna (D). |
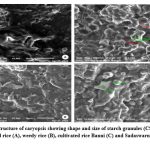 |
Figure 9: Ultrastructure of caryopsis showing shape and size of starch granules (CSG) under SEM of wild rice (A), weedy rice (B), cultivated rice Banni (C) and Sadaswarna (D). |
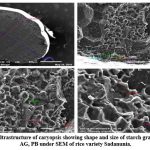 |
Figure 10: Ultrastructure of caryopsis showing shape and size of starch granules (CSG), AG, PB under SEM of rice variety Sadanunia. |
Fruit of the rice is caryopsis type where single seed is attached with the wall of the mature ripened ovary (named as pericarp) establishing a seed-like grain. Rice grain (paddy seed) comprising of two parts, outer protective covering husk (termed as hull) and inner edible part known as caryopsis.
Husk is surrounding by two slices, larger slice lemma and smaller slice palea, which protects the inner edible part caryopsis.30,31 Generally, hull part weight varies from 18-28% of the total weight of rice grain (rough). Based on histological observation, edible caryopsis part contains outer cuticular layer (CL), followed by aleurone layer (AL), and inner most endosperm layer (EL). Collectively these two layer (CL and AL) of the caryopsis is known as bran layer. It is usually removed while rough rice is processed through milling and polishing. Polished rice is designated as white rice (WR). Recent investigation is indicating that less polished (including aleurone, testa and pericarp) can lead to higher grain yield and health benefits keeping nutrients in these layers. Rice bran contains some distinctive phytochemicals with nutritional and medicinal values which are advantageous to human health. These phytochemicals may be used for nutraceutical development for cancer improvement, type 2 diabetes, immune regulatory processes and obesity. Rice bran also contains most valuable antioxidant gamma oryzanol, and vitamin-E (tocopherol and tocotriols). Several types of polyphenolics compounds are detected in rice bran such as salicylic acid, ferulic acid, and caffeic acid. Among the phytosterols, beta-sitosterol is the most common. All these components have health promoting properties. Different amino acids, many cofactors and various type of secondary metabolites available in the rice bran with medicinal values and considered as a whole food.17
Endosperm layer mainly composed of starch, starch granules may be polygonal or spherical in shape and size. Polygonal starch granules are with edges that ranged from smooth to highly angular and arranged tightly to form compact compound starch granule (CSG). Starch granules in weedy rice mostly spherical and with little angularity. Protein body (PB) may be present within the CSG region of the endosperm layer. In this study every variety has pin hole, wild rice have 0.897µm size pinhole, weedy rice has 0.846µm pinhole, Banni, Sadaswarna have 0.709µm pinhole,Yamuna has 0.92µm pinhole. Protein bodies are present while surrounding the compound starch granule. Wild rice and weedy rice show moderate distribution of protein body and their size are ranged from 1.538 µm and 1.088 µm respectively, cultivated varieties (Banni, Sadaswarna,Yamuna) have more protein bodies compare to that of wild and weedy rice but they are smaller in size 0.344µm,0.920µm, and 0.846µm. Awns are present in both wild rice and weedy rice, whereas among the cultivated varieties awn only observed in Banni. In this study it was observed that weedy rice has longest awn followed by wild rice and Banni. The major differences were between the awns of wild rice, weedy rice from that of cultivated rice that they have barbed awn whereas the cultivated rice is barbed less. Weedy rice has longest barbed (358.489 µm) compare to wild rice (329.169 µm). Histo-anatomical structure of caryopsis cross section was investigated using scanning electron microscopy to view details architecture of the grain at in situ position (Figure 6-10). Single seed weight ranges from 10 mg to 30 mg subject to the varietal variation in characteristics. Pericarp contribute 1-2 % weight of the caryopsis followed by seed coat and aleurone 5-6%, embryo provides 2-3 % weight of the caryopsis and starchy endosperm shares maximum part of the caryopsis weight ranges from 89 to 94%. Whole caryopsis contains following layers from outside to inside pericarp, testa, aleurone, embryo and starchy endosperm respectively. Endosperm part of the caryopsis mainly composed of starchy parenchyma cells and share almost 90% of the caryopsis weight which is the major source of food in this world.29 In our present investigation, mostly similar type of caryopsis ultrastructure has been observed in wild rice, weedy rice and in cultivars. Pericarp and testa may be fused and cannot be observed as separate layer under SEM study. Aleurone layer may be single cell layered with cuboidal structure or may be multi layers containing of thick cell wall. Structurally aleurone cells consisting of loosely packed and scattered aleurone grains (AG), including lipid bodies and remains intracellular voids (Fig. 7-10). Compound starch granules (CSG) size varies from smallest (3.53 μm in Banni) to largest in Yamuna (23.07 μm). Shape and arrangement of the CSG in the endosperm amylopast plays important role in grain quality. Generally, starch granules are consisting of amylose (0-30%) and amylopectin (70% or more than 70%). Thin cell wall contains very little amount of cell wall materials of the endosperm layer. Shape of the CSG was polyhedral to spherical with different size (3.53 μm to 23.07 μm) with minimum angularity and surrounded by many numbers of small size protein bodies (PB) (0.692 to 1.53μm). Compound starch granule (CSG) ranges from 5.88 to 13.33 μm with irregular spherical structure (Table 5; Figs. 7-10) in weedy rice. In wild rice, CSG is polyhedral structure without any angularity (5.45 μm to 16.26 μm in size). Microspore-pinhole was also present in all six rice genotypes (Figs. 7-10). CSG are various shape and size, spherical to polyhedral with moderate angularity (3.53 to 13.748 μm in size) in Banni. PB is moderately present with less impression of PB (0.692 to 1.53 μm in diameter) in all the rice genotypes (Table 5). It was detected that CSG ranges from polyhedral to spherical in shape and size from 3.53 to 23.07 μm (Table 5; Figs.7-10). Both wild rice (O. rufipogon) and weedy rice (O. sativa f. spontanea) have long awn with barbed features (329.169 to 358.489 μm) (Fig. 6). Bran thickness was highest in Banni (37.029 μm), followed by Yamuna (36.327 μm), wild rice (30.85 μm), weedy rice (29.55 μm), Sadaswarna (27.682), and Sadanunia (25.03 μm) (Table 5; Figs. 7-10). Specifically, the shattering seed habit and long dormancy period acquired by the feral weedy rice is a unique observation in this present investigation which is consistent with the earlier report.37,38 Pericarp colour is ranging from red, greenish, brown, that report also consistent with previous study.39
Conclusion
There was no report about the agro-morphological features of weedy rice (O. sativa f. spontanea) based on caryopsis ultrastructure using scanning electron microscopy (SEM). In the present study, comparison was made with respect to wild rice (O. rufipogon) and cultivated rice varieties (Yamuna, Banni, Sadaswarna, Sadanunia) for characterization of the rice germplasm. Germplasm characterization is one of the most important parameters for any breeding program to utilize as parental material. Agromorphological traits and physicochemical descriptions were considered to characterize the six rice genotypes including this weedy rice. Weedy rice (Oryza sativa f. spontanea), is an annual, self-pollinating plant and considered as feral conspecific to cultivated rice (O. sativa). Weedy rice appears to possess a wide range of variation in the phenotypic characteristics for adaptation in natural harsh climatic conditions. Many biotic and abiotic stresses tolerance traits have been accumulated slowly through natural evolution to withstand in the climatic fluctuation. Therefore, it needs conservation through on farm in situ process and utilization in the breeding program to develop climate resilient high yielding improved rice varieties with quality grain for sustainable food security.
Future perspective
Weedy rice may be used as good donor parental line to be used in breeding program to introgress genes/QTLs into the progeny lines which are associated with biotic or abiotic stress tolerance traits. Whole genome sequencing (WGS) can be performed to identify the SNPs variation related to agronomically important traits. The QTL mapping population can be developed to discover the trait specific QTLs of high yield potentiality to meet up the future demand of rice by 2050.
Acknowledgement
This study was supported by the University of North Bengal. SCR is thankful to the university authority for giving financial support to collect and conserve the rice species and varieties for utilization in breeding program to develop climate resilient improved varieties.
Conflict of interest
The authors declare that there is no conflict of interest in publishing research work.
References
- Wang, W. J., Zhao, M. H., Zhang, G. C., Liu, Z. M., Hua, Y. C., Jia, X. T., et al. (2020). Weedy rice as a novel gene resource: a genome-wide association study of anthocyanin biosynthesis and an evaluation of nutritional quality. Front. plant Sci. 11:878. doi: 10.3389/fpls.2020.00878.
CrossRef - Sun, J., Ma, D. R., Tang, L., Zhao, M. H., Zhang, G. C., Wang, W. J., et al. (2019). Population genomic analysis and de novo assembly reveal the origin of weedy rice as an evolutionary game. Mol. Plant 12, 632–647. doi: 10.1016/j.molp.2019.01.019.
CrossRef - Wing, R. A., Purugganan, M. D., and Zhang, Q. F. (2018). The rice genome revolution: from an ancient grain to green super rice. Nat. Rev. Genet. 19, 505–517. doi: 10.1038/s41576-018-0024-z.
CrossRef - Ellstrand, C. N., Heredia, M. S., Leak-Garcia, A. J., Heraty, M. J., Burger, C. J., Yao, L., et al. (2010). Crops gone wild: evolution of weeds and invasives from domesticated ancestors. Evol. Appl. 3, 494–504. doi: 10.1111/j.1752-4571.2010.00140.x.
CrossRef - Qiu, J., Jia, L., Wu, D. Y., Weng, X. F., Chen, L. J., Sun, J., et al. (2020). Diverse genetic mechanisms underlie worldwide convergent rice feralization. Genome Biol. 21, 1–11. doi: 10.1186/s13059-020-01980-x.
CrossRef - Meyer RS, Purugganan MD. (2013). Evolution of crop species: genetics of domestication and diversification. Nat Rev Genet. 14(12):840-52. https://doi.org/10.1038/nrg3605
CrossRef - Eizenga GC, Kim HJ, Jung JKH, Greenberg AJ, Edwards JD, Naredo MEB, Banaticla-Hilario MCN, Harrington SE, Shi Y, Kimball JA, Harper LA, McNally KL and McCouch SR. (2022). Phenotypic Variation and the Impact of Admixture in the Oryza rufipogon Species Complex (ORSC). Front. Plant Sci. 13:787703. doi: 10.3389/fpls.2022.787703.
CrossRef - Qiu J, Zhou YJ, Mao LF, Ye CY, Wang WD, Zhang JP, Yu YY, Fu F, Wang YF, Qian FJ, Qi T, Wu SL, Sultana MH, Cao Y-N, Wang Y, Timko MP, Ge S, Fan LJ, Lu YL (2017) Genomic variation associated with local adaptation of weedy rice during de-domestication. Nature Comm 8:1–12. https://doi.org/10.1038//ncomms15323
CrossRef - Li LF, Li YL, Jia Y, Caicedo AL, Olsen KM (2017) Signatures of adaptation in the weedy rice genome. Nature Genet 49:811–814. https://doi.org/10.1038/ng.3825.
CrossRef - Londo JP and Schaal BA (2007). Origins and population genetics of weedy red rice in the USA. Mol Ecol 16: 4523–4535.
CrossRef - Xia HB, Wang W., Xia H, Zhao W and Lu BR. (2011). Conspecific crop-weed introgression influences evolution of weedy rice (Oryza sativa f. spontanea) across a geographical range. PLOS One 6(1): e16189.
CrossRef - Ma, D., Li, M., and Sun, J. (2008). Studies on biological diversity and genetic differentiation of liaoning weedy rice. J. Shenyang Agric. Univ. 39, 265–269. doi: 10.1007/s10499-007-9164-4.
CrossRef - Pipatpongpinyo, W., Korkmaz, U., Wu, H., Kena, A., Ye, H., Feng, J., et al. (2019). Assembling seed dormancy genes into a system identified their effects on seedbank longevity in weedy rice. Heredity 124, 135–145. doi: 10.1038/s41437-019-0253-8
CrossRef - Nadir, S., Xiong, HB., Zhu, Q. et al. Weedy rice in sustainable rice production. A review. Agron. Sustain. Dev. 37, 46 (2017). https://doi.org/10.1007/s13593-017-0456-4
CrossRef - Juliano, B. O., and Tuaño, A. P. P. (2019). “2 – Gross structure and composition of the rice grain,” in Rice, 4th Edn, ed. J. Bao (Washington, D.C: AACC International Press), 31–53. doi: 10.1016/b978-0-12-811508-4.00002-2.
CrossRef - Yue-Ting, H. U., Wang, J. G., Liu, H. L., Sun, J., Sun, X. X., Zhao, H. W., et al. (2016). Functional nutrition quality analysis of japonica rice(Oryza sativa L.) varieties in the cold region. J. Plant Genet. Resour. 17, 840–845. doi: 10.13430/j.cnki.jpgr.2016.05.007
- Zarei, I., Brown, D. G., Nealon, N. J., and Ryan, E. P. (2017). Rice bran metabolome contains amino acids, vitamins and cofactors, and phytochemicals with medicinal and nutritional properties. Rice 10:24. doi: 10.1186/s12284-017-0157-2
CrossRef - Zhang Qifa (2021). Purple Tomatoes, Black Rice and Food Security. Nature Reviews Genetics. https://doi.org/10.1038/s41576-021-00359-3.
CrossRef - Prathepha P (2009) Seed morphological traits and genotypic diversity of weedy rice (Oryza sativa f. spontanea) populations found in the Thai Hom Mali rice fields of north-eastern Thailand. Weed Biol Manag 9:1–9. https://doi.org/10.1111/j.1445-6664.2008.00312.x
CrossRef - Han ZY, Li F, Qiao WH, Nong BX, Cheng YL, Zhang LF, Huang JF, Wang YY, Lou DJ, Ge JY , Xing M, Fan WY, Nie YM, Guo WL, Wang SZ, Liu ZR, Li DT, Zheng XM and Yang QW (2022). Identification of candidate genes and clarification of the maintenance of the green pericarp of weedy rice grains. Front. Plant Sci. 13:930062. https://doi.org/10.3389/fpls.2022.930062
CrossRef - Ziska LH, Gealy DR, Tomecek MB, Jackson AK, Black HL (2012) Recent and projected increases in atmospheric CO2 concentration can enhance gene flow between wild and genetically altered rice (Oryza sativa). PLoS One 7(5):e37522. https://doi.org/10.1371/journal.pone.0037522.
CrossRef - Suh HS (2003) Characterization of weedy rice germplasm. Wild Crop Germplasm Bank, Yeungnam University, pp 1007
- Chen LJ, Suh HS (2015). Weedy rice—origin and dissemination. Yunnan Publishing Group Corporation, Yunnan Science and Technology Press, China. pp 234.
- Fogliatto S, Ferrero A, Vidotto F. (2020). How Can Weedy Rice Stand against Abiotic Stresses? A Review. Agronomy. 10(9):1284. https://doi.org/10.3390/agronomy10091284
CrossRef - He, Q.; Kim, K.; Park, Y. (2017) Population genomics identifies the origin and signatures of selection of Korean weedy rice. Plant Biotechnol. J. 15, 357–366.
CrossRef - Little, Ruby R., Hilder, Grace B., Dawson, Elsie H. Differential effect of dilute alkali on 25 varieties of milled white rice. Cereal Chem. 35, 111 (1958).
- Sood, B.C. and Siddiq, E.A. (1978). A rapid technique for scent determination in rice. Indian J Genet Plant Breed. 38, 268–271.
- Juliano, B, and D. Bechtel. (1985). The Rice Grain and Its Gross Composition, In: B. Juliano, Ed., Rice Chemistry and Technology, 2nd Edition, American Association of Cereal Chemists, St. Paul, 1985, pp. 17-58.
- Yu, Xu-run., Zhou, Liang., Xiong, Fei., Wang, Zhong. (2014). Structural and histochemical characterization of developing rice caryopsis. Rice Science. 21(3), 142-149.
CrossRef - Wu, X., Liu, J. Li, D., Liu, C.M. Rice caryopsis development I: Dynamic changes in different cell layers. J Integr Plant Biol, 58(9): 772-785 (2016).
CrossRef - Usma A, Ahmad M, Zafar M, Sultana S, Ullah F, Saqib S, Ayaz A, Zaman W. (2022). Palynological Study of Weed Flora from Potohar Plateau. Agronomy. 12(10):2500. https://doi.org/10.3390/ agronomy 12102500.
CrossRef - Gross BL, Skare KJ, Olsen KM. Novel Phr1 mutations and the evolution of phenol reaction variation in US weedy rice (Oryza sativa). New Phytol. 2009 Dec;184(4):842-50. doi: 10.1111/j.1469-8137.2009.02957.x.
CrossRef - Shahil Kumar, S. K. Chakrabarty and Yogendra Singh. (2021). Variation in phenol colour reaction in grains of rice (Oryza sativa L.) varieties. Indian J. Genet., 81(3): 367-375 (2021) DOI: 10.31742/IJGPB.81.3.3.
- Chang TT and Bardenas EA. 1965. The morphology and varietal characters of the rice plant. Technical Bulletin, IRRI, Manila, Philippines, pp 40.
- Yu Y, Tang T, Qian Q, Wang Y, Yan M, Zeng D, Han B, Wu CI, Shi S, Li J. (2008). Independent losses of function in a polyphenol oxidase in rice: differentiation in grain discoloration between subspecies and the role of positive selection under domestication. Plant Cell. 20:2946–2959.
CrossRef - Cagampang G., Perez C., Juliano B. 1973. A gel consistency test for the eating quality of rice. Sci. Food Agri. 24: 1589-1594.
CrossRef - Wu, D. Y., Lao, S. T., and Fan, L. J. (2021). De-Domestication: an extension of crop evolution. Trends Plant Sci. 26, 560–574. doi: 10.1016/j.tplants.2021.02.003.
CrossRef - Sun, J., Qian, Q.,Ma, D.-R., Xu, Z.-J., Liu, D., Du, H.-B., et al. (2013). Introgression and selection shaping the genome and adaptive loci of weedy rice in northern China. New Phytol. 197, 290–299. doi: 10.1111/nph.12012
CrossRef - Yang, Q. W., Cheng, Y. L., Zhang, L. F., Han, Z. Y., Li, F., Zhang, W. X., et al. (2021). Discovery and study of a green pericarp germplasm in rice. J. Plant Genet. Resour. 23, 146–151.

