Introduction
In India, 70–75% of the population is directly or indirectly dependent on agriculture, which forms the backbone of the nation1. The Indian economy depends heavily on the oilseed industry because it is the world’s largest producer of all major oilseeds, such as groundnut, rapeseed, mustard, sunflower, safflower, sesame, soybean, castor, and linseed2. Groundnut is the most important food and cash crop in India3. The state of Gujarat supplies approximately 40% of India’s production4. The Solvent Extractors’ Association of India reported that the groundnut oil availabilityof the country for 2014-15 was 2,40,000 tonnes, which was reduced by 170,000 tonnes or 41.50% from 2013 (410,000 tonnes). According to estimates, groundnut crop production has decreased globally over the past ten years5.The enhancement in the crop yield is usually achieved by excessive use of chemical fertilizers in the present agricultural system6. However, overemployment of chemical fertilizers results in severe issues, such as soil degradation, nitrogen leaching, soil compaction, and reduction in organic matter in soil.
Sustainable biological approaches for the enhancement of crop production employing rhizospheric microorganisms, especially PGPR, are gaining immense popularity worldwide. These bacteria promote plant growth and development via plant root-microbial interactions, improving nutrient availability (nitrogen, phosphorous, and potassium), controlling the levels of phytohormones (gibberellins, cytokinin), and controlling phytopathogens through the production of secondary metabolites (HCN and chitinase production). PGPR have been broadly classified into two categories-intracellular or endophytic, iPGPR and extracellular or rhizopheric, ePGPR based on their association with plant roots7. The ePGPR displays enhanced interaction with several plants due to their free-living ability in comparison to the iPGPR. Extracellular PGPR includes Acetobacter, Azotobacter, Bacillus, Clostridium, Derxia, Enterobacter, Pseudomonas, Rhodopseudominas8, which have been isolated and extensively studied on several crops including groundnut, wheat, rice, maize and soybean9. In the present study, we have screened for potential PGPR in the groundnut rhizosphere, which can be employed as novel bio-inoculants for enhanced production of the crop.
Materials and Method
Collection of Sample and Isolation of Rhizobacteria
Rhizospheric soil samples were collected from four different agriculture fields of Kotdapitha 21.966728, 71.204532, Virnagar 22.043104, 71.113215, District Rajkot, Kalawad 22.206375, 70.377288, District Jamnagar, and Garani 21.924582, 71.136719, District Amreli, from the Saurashtra, Gujarat. The groundnut plants were uprooted, and shoots were cut off, and roots along with the rhizosphere soil were stored aseptically in sample bags. The soil samples were stored at 4°C until further use. The samples were serially diluted in the range 10-3 to 10-8 and colonies with morphological variations were isolated.
Characterization of the Isolates for PGP traits
All the isolates were tested for plant growth promoting traits: indole acetic acid (IAA), hydrogen cyanide (HCN), ammonia production, phosphate solubilisation, and gibberellin production. All the tests were performed in triplicate.
Indole Acetic Acid
Indole acetic acid production was performed according to the colorimetric method10. Briefly, isolates were transferred into 5 mL of nutrient broth (NB) containing 100 mg/mL of L-tryptophan. The tubes were incubated at 37°C for 48 h. After incubation, the broth was centrifuged at 10,000 rpm for 5 minutes. The supernatant (1 mL) was transferred into a fresh, sterile micro centrifuge tube and 2 mL of Salkowski’s reagent (0.5M ferric chloride+ 35% perchloric acid) was added. The tubes were gentlymixed and incubated for 30 minutes at room temperature, and a pink coloration of the solution was observed. The color change was recorded spectrophotometrically at 530 nm. The standard curve was plotted in the range of 20–200 µg/mL.
Ammonia production
All the isolates were analyzed for the production of ammonia11. The 24 h old bacterial cultures were inoculated in 10 mL peptone broth and incubated at 37°C for 48h. After incubation, 0.2 mL of freshly prepared Nessler’s reagent was added to test tubes. Ammonia production was observed by change in color from yellow to brown. Furthermore, the quantitative estimation of ammonia was spectrophotometrically measured at 600 nm12. The standard curve was plotted in the range of 10-100 µg/mL.
Hydrogen Cyanide Production
All the isolates were screened for the production of HCN by adapting the method as described by Alstrom in 198913. Briefly, 100 µL bacterial culture were streaked on nutrient agar medium containing 4.4 g/L glycine plates. Whatman filter paper no.1 was soaked in alkaline picrate solution (2% sodium carbonate in 0.5% picric acid) and placed at the top of the plates. The plates were sealed with parafilm to prevent volatilizationand incubated at 28°C for 4 days. Color changes of filter paper from yellow to light brown to reddish-brown indicated HCN production.
The HCN production by the rhizobacterial strain is determined using the picric acid method14. Briefly, media (NB) was supplemented with 4.4 g/Lglycine. The 3 mm strips of Whatman No.42 filter paper were sterilized and then soaked in a picrate alkaline solution. Later,filter paper strips were dried and placed in a test tube with 5 mL of inoculated bacterial culture, and the tubes were plugged with cotton to prevent volatilization. The tubes were incubated at 28 ± 2оC for 3–5 days. After the incubation period, a color change was observed, and strips were placed in fresh tubes with 10 mL of distilled water and mixed properly with a vortex. The optical density of the samples was measured at 515 nm. The standard curve plotted with potassium cyanide in range of 10-100 µg/mL15.
Phosphate Solubilization Test
All isolates were screened for their qualitative ability to solubilize calcium phosphate using Pikovskaya agar16. Briefly, isolates were spotted on Pikovskaya agar plates and incubated at 28±2°C for 7 days. The halo zone indicated phosphate solubilization.
Quantitative Analysis of Phosphate Solubilization
The amount of phosphate released was measured by the chlorostannous reduced molybdophosphoric acid blue method. Briefly, 1mL of bacterial culture was inoculated into 100mL sterile Pikovskaya broth in Erlenmeyer flask and incubated at 28±2°C for 11 days with shaking at 120 rpm. The uninoculated broth was used as a control. The whole experiment was performed in triplicates. Broth (10 mL) from each sample was withdrawn on the 3rd, 5th, 7th, and 10th day for measurement of soluble phosphorous and variation in pH. The cultures were centrifuged at 10,000 rpm for 15 minutes. The supernatant (100 µL) was added in the flask containing 10 mL of chloromolybdic reagent in a shaking condition and diluted with 40 mLof distilled water. Later, 5 drops of chlorostannous acid reagent were added along the sides of the flask and mixed properly. The final volume was made up to 50 mL with distilled water17. The resultant blue color was measured by spectrophotometrically at 660 nm against blank. The standard curve was plotted in the range of 10–50 µg/mL.
Gibberellin (GA) production
All isolates were screened for their quantitative ability to produce phytohormone-gibberellin. Briefly, the bacterial culture was inoculated in NB media containing 1mM of L-tryptophan and incubated at 37оC for 24 h at 150 rpm condition. The culture after incubation was centrifuged at 10,000rpm for 5 min and a cell free supernatant was collected and used for estimation of gibberellic acid18.
Gibberellin production was estimated with the Folin-Ciocalteu reagent19. Bacterial cell extract (1 mL) was added to the test tube, followed by the addition of 1mL Folin-Ciocalteu reagent and 1mLof concentrated hydrochloric acid into the test tubes. The mixture was boiled in a water bath for 5 min and then allowed to cool at room temperature. The greenish blue color produced was recorded using a spectrophotometer at 760nm. The standard was performed with gibberellic acid (GA3) in the range of 10–100 mg/mL.
Identification of Potent PGPR: The isolates RG12 and RGKP3 displayed all positivePGP traits. The isolate RGKP3was selected for further molecular identification.
Molecular identification of PGPR isolate by 16s rRNA sequencing
DNA was isolated from the overnight culture of RGKP3. Quantification of DNA was done by evaluating on 1.0% Agarose Gel to obtain a single band of high-molecular weight DNA was observed. The fragment of gene was amplified by PCR. A single discrete PCR amplicon band was observed on a resolving agarose gel. The PCR amplicon was purified by column purification to remove contaminants. The DNA sequencing reaction of the PCR amplicon was carried out with primer27F using the BDT v3.1 Cycle Sequencing Kit on an ABI 3730xl Genetic Analyzer. The gene sequence was used to carry out BLAST with the database of NCBI GenBank database. Based on the maximum identity score, the first ten sequences were selected and aligned using multiple alignment software programs. The gene sequences obtained were compared with sequences available in the GenBank databases using the NCBI and BLAST at https://blast.ncbi.nlm.nih.gov.Sequencing was done by SLS Research Private Limited, Surat, Gujarat. Sequences were submitted to the NCBI GenBank database, and accession number was obtained.
Results
The rhizospheric soil samples of groundnut were collected from Rajkot (RG1-21), Jamnagar (RGK1-RGK9), Virnagar (RGV1-RGV5), and Amreli (RGKP1-RGKP9) districts of Saurashtra, Gujarat. A total forty-two rhizobacteria were isolated from rhizospheric soil (Table 1) and were analysed for their PGP traits. The PGP traits, such as IAA, ammonia, HCN, gibberellin, and phosphate solubilization augments the plant growth and development.
 |
Figure 1: (a) Qualitative analysis of IAA production of thirty-three positive isolates; (b)Qualitative analysis of ammonia production of fifteen positive isolates. |
IAA is a pivotal phytohormone for the division and differentiation of plant cells and tissues. Furthermore, it supports plant root elongation. Figure1(a) shows the results for IAA production with respect to control. The quantification of IAA was done using spectrophotometric analysis (Fig.2). The results indicated that thirty-three isolates produced IAA in the range of 20.7–133 µg/mL. The isolate RG11, RG21,and RGV2 produced maximum concentration of IAA, which was 87% higher than the least IAA production by RG9.The potent PGPR KP3 produced IAA at a significantly higher levels, compared to IAA production reported in the literature20.
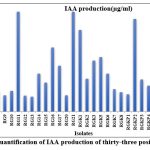 |
Figure 2: Quantification of IAA production of thirty-three positive isolates |
The ammonia production by the PGPR indirectly affects plant growth and development. The PGPR nitrogenous materials of peptones break down into ammonia, which is released into the soil and used by plants as their nutrient source21. Figure 1(b) shows the brown color formation, which depicts the production of ammonia in test tubes on addition of Nessler’s reagent. The spectrophotometric analysis of the brown color produced was observed in only seventeen isolates. Figure 3shows the maximum amount (55.5 µg/mL) of ammonia was produced by isolate RG5, while RG19 produced minimum amount of ammonia. The other isolates produced ammonia in the range of 21.4–55.5 µg/mL. Goswami et al. (2013) reported maximum ammonia production was 36 µg/mL which is 36% less than our findings22.
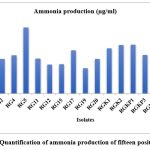 |
Figure 3: Quantification of ammonia production of fifteen positive isolate |
The HCN production is associated with bioremediation and as a bio control for growth enhancement and antagonistic activities. The qualitative estimation of HCN was confirmed by the change in coloration of filter paper soaked in sodium picrate solution from yellow to orange-brown. Twenty-six isolates produced HCN and showed orange to reddish brown coloration of solution (Fig. 5a) and total sixteen isolates were not able to produce HCN. Jadav et al. (2020) isolated only four HCN producing bacteria from the Limonium stocksii rhizosphere that supported our HCN trait finding23.
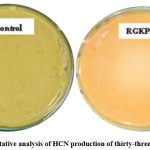 |
Figure 4: Qualitative analysis of HCN production of thirty-three positive isolates |
Phosphorous is the second-key nutrient after nitrogen for plant growth24. The results indicated that out of forty-two isolates, only 4 isolates demonstrated the capacity to solubilize phosphorous from an insoluble phosphate source present in the media.
Table 1: Plant growth promotional properties of PGPR isolates
| S. No. | Name of the Isolate | Indole acetic acid Production | Ammonia Production | Hydrogen cyanide Production | Phosphate solubilization | Gibberellins |
| 1. | RG1 | + | + | – | – | – |
| 2. | RG2 | + | + | + | – | – |
| 3. | RG3 | + | – | – | – | – |
| 4. | RG4 | – | + | – | – | – |
| 5. | RG5 | – | + | + | – | – |
| 6. | RG6 | + | – | + | – | + |
| 7. | RG7 | + | – | – | – | – |
| 8. | RG8 | – | – | + | – | – |
| 9. | RG9 | + | – | + | – | – |
| 10. | RG10 | + | – | + | – | – |
| 11. | RG11 | + | + | + | – | – |
| 12. | RG12 | + | + | + | + | + |
| 13. | RG13 | + | – | – | – | – |
| 14. | RG14 | + | – | – | – | – |
| 15. | RG15 | + | + | + | – | – |
| 16. | RG16 | + | – | – | – | – |
| 17. | RG17 | + | + | + | – | – |
| 18. | RG18 | – | – | – | – | – |
| 19. | RG19 | – | + | + | – | – |
| 20. | RG20 | + | + | + | – | – |
| 21. | RG21 | + | – | + | – | – |
| 22. | RGK1 | + | + | + | – | – |
| 23. | RGK2 | + | + | – | – | – |
| 24. | RGK3 | + | – | – | – | – |
| 25. | RGK4 | – | – | – | – | – |
| 26. | RGK5 | + | – | + | – | – |
| 27. | RGK6 | + | – | – | – | – |
| 28. | RGK7 | + | – | – | + | + |
| 29. | RGK8 | + | – | + | – | – |
| 30. | RGKP1 | + | + | – | – | – |
| 31. | RGKP2 | + | – | + | – | – |
| 32. | RGKP3 | + | + | + | + | + |
| 33. | RGKP4 | + | + | + | – | – |
| 34. | RGKP5 | + | – | + | – | – |
| 35. | RGKP6 | + | – | + | – | – |
| 36. | RGKP7 | + | – | + | – | – |
| 37. | RGKP8 | + | + | + | – | – |
| 38. | RGV1 | – | – | + | – | – |
| 39. | RGV2 | + | – | – | – | – |
| 40. | RGV3 | + | – | + | – | + |
| 41. | RGV4 | + | – | – | – | – |
| 42. | RGV5 | – | + | + | + | – |
 |
Figure 5: (a) Quantitative analysis of HCN production of isolates with compared to control (b) Quantitative analysis of Gibberellins (GA) production with compared to control. |
Furthermore, 4 positive isolates were studied for quantitative estimation of phosphorous using the colorimetric method. Phosphate solubilizing isolates shows blue color compared to yellow colored control on addition of chlorostannous reagent on the 5thday of the assay, the solubilization concentration of RGK7 was recorded to be maximum. The 4 isolates had potential to solubilize phosphate from Pikovskaya’s media in range of 65.6–259.5 µg/mL. On the 3rd and 5th days, phosphate is solubilized in a range of 65.6–108.5 µg/mL and on the 7thday, the isolate K7 had 259.5 µg/mL phosphate solubilization. After the 7thdays, the amount of free phosphate gradually decreases during phosphate solubilization by isolates25. The standard curve of TCP (tri-calcium phosphate) was plotted in the range of 50–500 µg/mL. Figure 7b shows that the RGKP3 had maximum solubilization after 10 days. Tahir et al.(2013) supported our findings reporting that Azospirillumstrain WS-1 solubilized 218.1 µg/mL phosphate, which is 16% less than our findings26.
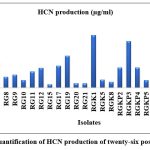 |
Figure 6: Quantification of HCN production of twenty-six positive isolates |
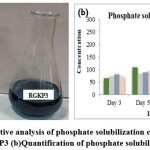 |
Figure 7: (a)Quantitative analysis of phosphate solubilization control with positive result of isolate RGKP3 (b)Quantification of phosphate solubilization of 4 isolates. |
Gibberellins are plant regulators and play a major role in germination and elongation of the stem27. Recent studies hypothesise that bacteria have developed an independent biosynthetic pathway for the production of gibberellins28. Only five isolates produced gibberellin in the range of 10.2–112.4 µg/mL. The isolate K7 produced the highest amount of gibberellin in the range of 112.4 µg/mL, while isolate V3 was found to produce the lowest amount of GA (10.2 µg/mL). Youssef et al. (2010) also found the gibberellin production in the range of 18.75–49.95 µg/mL, which is 43.35% less than our findings29.
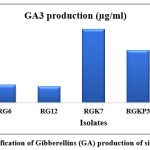 |
Figure 8: Quantification of Gibberellins (GA) production of six positive isolates |
Molecular identification of Potent PGPR
The most promising rhizobacteria isolate has multiple PGP traits that are positive. Gram staining revealed that potent PGPR is a gram-positive bacterium. The isolates were identified by 16S rRNA partial sequencing. The 16S rRNA sequence of RGKP3 and PGPR has been placed in GenBank with the accession number OP528743. Figure 9 displays the phylogenetic analysis of the identified PGPR RGKP3 isolate.
Moreover, using Genbank data, the KP3 PGPR isolate presented close homology with Priestia megaterium.
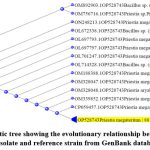 |
Figure 9: Phylogenetic tree showing the evolutionary relationship between RGKP3, a PGPR isolate and reference strain from GenBank database. |
Conclusion
In the present study, a total of forty-two isolates were obtained from the rhizospheric region of the groundnut crop. Qualitative and quantitative analysis for PGP traits found only two isolates with positive results for all multiple PGP traits. A potent RGKP3 strain was identified by 16S rRNA sequencing. The investigation suggests the potent PGPR must be studied further for its plant growth-promoting ability.
Acknowledgement
The authors would like to acknowledge the department of biotechnology and microbiology at Atmiya University in Rajkot, Gujarat, for providing research facilities and technical assistance.
Funding Sources
We are thankful to Government of Gujarat for providing financial assistance under its ‘SHODH SCHEME’
Conflict of Interest
There are NO conflict of interest.
References
- Searchinger T, Hanson C, Ranganathan J, et al. Creating a sustainable food future. A menu of solutions to sustainably feed more than 9 billion people by 2050. World resources report 2013-14: interim findings. World Resources Institute (WRI); World Bank Groupe-Banque Mondiale; United …; 2014.
- Variath MT, Janila P. Economic and academic importance of peanut. The peanut genome. Springer; 2017:7-26.
CrossRef - Behera BR, Paikaray RK, Jena S, Rath B, Mishra KN, Prasad B. Residual effect of integrated nutrient management in rice on growth and yield of groundnut (Arachis hypogaea L.). 2022;
- Varasani J, Shiyani R, Ardeshna N, Swaminathan B. Technical efficiency analysis of groundnut production in Saurashtra region of Gujarat. Int J Agric Sci. 2016;8(54):852-858.
- Bansal RK, Gondaliya V, Shaikh A. A review of the status of the groundnut production and export of India. Indian Journal of Economics and Development. 2017;13(2):369-374.
CrossRef - Akter R, Rahman MH, Chowdhury MAR, Manirujjaman M, Elshenawy SE. Advances of nanotechnology in plant development and crop protection. Applications of Computational Intelligence in Multi-Disciplinary Research. Elsevier; 2022:143-157.
CrossRef - Gray E, Smith D. Intracellular and extracellular PGPR: commonalities and distinctions in the plant–bacterium signaling processes. Soil biology and biochemistry. 2005;37(3):395-412.
CrossRef - Verma M, Mishra J, Arora NK. Plant growth-promoting rhizobacteria: diversity and applications. Environmental biotechnology: for sustainable future. Springer; 2019:129-173.
CrossRef - Pérez-Montaño F, Alías-Villegas C, Bellogín R, et al. Plant growth promotion in cereal and leguminous agricultural important plants: from microorganism capacities to crop production. Microbiological research. 2014;169(5-6):325-336.
CrossRef - Gordon S, Paleg L. Quantitative measurement of indole acetic acid. Physiol Plant. 1957;10:37-48.
CrossRef - Bhatt S, Dholakia PR, Sorathia DK, Solanki KJ, Dabhi NK, Dedakiya SK. Isolation & Biochemical Characterization of Halotolerant Plant Growth Promoting Rhizobacteria. The International Journal of Science and Technoledge. 2015;3(6):166.
- Demutskaya L, Kalinichenko I. Photometric determination of ammonium nitrogen with the Nessler reagent in drinking water after its chlorination. Journal of Water Chemistry and Technology. 2010;32(2):90-94.
CrossRef - Alström S, Burns RG. Cyanide production by rhizobacteria as a possible mechanism of plant growth inhibition. Biology and Fertility of Soils. 1989;7(3):232-238.
CrossRef - Sehrawat A, Sindhu SS, Glick BR. Hydrogen cyanide production by soil bacteria: Biological control of pests and promotion of plant growth in sustainable agriculture. Pedosphere. 2022;32(1):15-38.
CrossRef - Patel TS, Desai PB. Isolation and screening of PGPR from rhizospheric and non rhizospheric soil of Bt-cotton. Indo-American Journal of Agricultural and Veterinary Sciences. 2015;3:B1110-B1120.
- Pikovskaya R. Mobilization of phosphorus in soil in connection with vital activity of some microbial species. Mikrobiologiya. 1948;17:362-370.
- Thakur A, Parikh S. Isolation and characterization of phosphate solubilizing bacteria associated with groundnut rhizosphere. International Journal of Agricultural Science and Research (IJASR) Vol. 2017;6
- Ashry NM, Alaidaroos BA, Mohamed SA, Badr OA, El-Saadony MT, Esmael A. Utilization of drought-tolerant bacterial strains isolated from harsh soils as a plant growth-promoting rhizobacteria (PGPR). Saudi Journal of Biological Sciences. 2022;29(3):1760-1769.
CrossRef - Abou-Aly HE, Youssef AM, El-Meihy RM, Tawfik TA, El-Akshar EA. Evaluation of heavy metals tolerant bacterial strains as antioxidant agents and plant growth promoters. Biocatalysis and Agricultural Biotechnology. 2019;19:101110.
CrossRef - Agustiyani D, Purwaningsih S, Dewi T, et al. Characterization of PGPR isolated from rhizospheric soils of various plant and its effect on growth of radish (Raphanus sativus L.). IOP Publishing; 2022:012037.
CrossRef - Backer R, Rokem JS, Ilangumaran G, et al. Plant growth-promoting rhizobacteria: context, mechanisms of action, and roadmap to commercialization of biostimulants for sustainable agriculture. Frontiers in plant science. 2018:1473.
CrossRef - Goswami D, Vaghela H, Parmar S, Dhandhukia P, Thakker JN. Plant growth promoting potentials of Pseudomonas spp. strain OG isolated from marine water. Journal of plant interactions. 2013;8(4):281-290.
CrossRef - Jadav P, Rakholiya K, Kaneria M, Singh SP. Isolation and Characterization of Plant Growth Promoting Rhizospheric Bacteria From Limonium stocksii. 2020:
CrossRef - Gyaneshwar P, Kumar GN, Parekh L. Effect of buffering on the phosphate-solubilizing ability of microorganisms. World Journal of Microbiology and Biotechnology. 1998;14:669-673.
CrossRef - Gupta R, Kumari A, Sharma S, Alzahrani OM, Noureldeen A, Darwish H. Identification, characterization and optimization of phosphate solubilizing rhizobacteria (PSRB) from rice rhizosphere. Saudi Journal of Biological Sciences. 2022;29(1):35-42.
CrossRef - Tahir M, Mirza MS, Zaheer A, Dimitrov MR, Smidt H, Hameed S. Isolation and identification of phosphate solubilizer’Azospirillum, Bacillus’ and’Enterobacter’strains by 16SrRNA sequence analysis and their effect on growth of wheat (‘Triticum aestivum’L.). Australian Journal of Crop Science. 2013;7(9):1284-1292.
- Xia J, Hao X, Wang T, et al. Seed priming with gibberellin regulates the germination of cotton seeds under low-temperature conditions. Journal of Plant Growth Regulation. 2023;42(1):319-334.
CrossRef - Kang S-M, Khan AL, Hamayun M, et al. Gibberellin-producing Promicromonospora sp. SE188 improves Solanum lycopersicum plant growth and influences endogenous plant hormones. Journal of microbiology. 2012;50:902-909.
CrossRef - Youssef GH, Seddik WM, Osman MA. Efficiency of natural minerals in presence of different nitrogen forms and potassium dissolving bacteria on peanut and sesame yields. J Am Sci. 2010;6(11):647-660.

