Introduction
Spinach (Spinacia oleracea L.): Miraculous herb
Spinacia oleracea L. belongs to Chenopodiaceae family and is an annual herb. It is native of SouthWest Asia and is cultivated globally. It is grown on large scale due to its high nutritional content (iron, copper, zinc, phosphorus, vitamin B complex, folic acid, flavonoids, apocyanin and Omega-3-fatty acid). Cultivation of spinach is hiking because it grows relatively quick and is easy to maintain. But large amount of chemical fertilizers are applied to it and therefore much work has to be done in order to lower our dependency on such fatal fertilizers and encourage PGPR based organic fertilizers [1].
Spinach: A green leafy healthy bowl
A bowl of spinach is loaded with such high value nutrients which has myriads of medicinal importance. Spinach is used as a traditional medicine, due to presence of tannins, phenolic active phytochemicals, viz, steroids, glycosides, terpenoids and alkaloids. This make spinach a healthy bowl as it deals with leprosy, diabetes, asthma, lung inflammation, scabies, ringworm and many such diseases of brain and heart [1].
Why PGPR?
Several species of PGPR are found around the world which has certain growth effects on various plants. They mostly have beneficial effects on plants and can be used as biofertilizer, biopesticide, in forestry, microbe rhizoremediation and as a probiotics [2-5]. Pseudomonas and Bacillus are most widely accessible PGPR among Azospirillum, Agrobacterium, Azotobacter, Arthrobacter, Alcaligenes, Serratia, Enterobacter, Klebsiella, Clostridium, Vario-vovax, Xanthomonas and Phyllobacterium [6-10].
Mechanisms of PGPR enhancing growth of plant
A number of direct and indirect mechanisms have been discovered which are quiet complex yet encourage yield of plant. These mechanisms involve synthesis of certain metabolites (auxin, cytokinin and gibberellins), production of siderophore, hydrogen cyanide (HCN), antibiotics and volatile compounds. Competition, induced systemic resistance and mineral solubilization (e.g. phosphorus) are other factors that are carried out by Pseudomonas to increase yield of plant [11-13].
This species also shows biocontrol activity on pathogens. As noted, Pseudomonas strain 7NSK2 had inhibitory effect on growth of several phytopathogenic fungi by pyoverdin production [14].
Growth promoting species of Pseudomonas
Little work has been reported on association of Pseudomonas with spinach in concern with its use as biofertilizer. Certain reports are mentioned here, but more work is needed to be done in this association to get fruitful results.
Pseudomonas strain S2 and S4
It was reported by Chiun and Micallef (2017) [15] that root- colonizing strains of Pseudomonas S2 and S4 showed plant growth promotion in spinach alongwith lettuce and tomato. Spinach roots were inoculated with Pseudomonas in seedling stage. At 6 weeks of post-germination of spinach, growth promotion was investigated by Shoot dry weight (SDW). Root inoculation of spinach cv. ‘Tyee’ with Pseudomonas strain S2 or S4 resulted in 69% and 63% increase in SDW compared to non-inoculated controls (p<0.005 and p<0.01 respectively).
Pseudomonas putida and Pseudomonas fluorescens
Urashima and Hori (2003) [16], showed that fluorescent strains of pseudomonads, included in PGPR group, viz, Pseudomonas putida and Pseudomonas fluorescens had growth effects in spinach. Several criteria were selected for this study, as mentioned-
Small scale sterilized hydroponic culture bioassay system was implemented for promoting elongation of spinach root.
These fluorescent strains encouraged around 50% increase in shoot and root growth in host plant.
Type B diluted liquid culture was used which removed growth suppression of spinach, occurring at initial stage. Hence, it is noteworthy that growth promotion of spinach in type B culture was due to bacterial metabolite or its secretion.
The growth promotion attribute of potent PGPR also depends upon its colonization in seed or root of host which depends upon several inoculation methods. In this concern, it was reported by Urashima and Hori (2003) in case of spinach that lowering of bacterial population on seeds was quiet less when bacterized seeds were preserved at 40C. Lowering of fluorescent pseudomonads population on seeds of spinach was maintained by soaking it in 10g L^ < -1 > methyl cellulose (1000 of polymerization). Also, such population was maintained at high concentration for a long time (6 month) when methyl cellulose was applied. Alongwith, this growth of spinach seeds was enhanced mainly in hydroponic culture. Such PGPR population was increased when carriers (method of application) viz, sawdust horse feces compost, rice straw cattle feces compost were applied. Hence, high population of such PGPR was noted in soil with application of such organic materials. The merits of application of carriers were they provide protection to the strain from environmental stress, hence increasing life span of PGPR under field conditions.
Among, several mechanisms of spinach growth enhancement by Pseudomonas sp. as reported above, release of siderophore which chelated iron and made it available to spinach was observed. Uptake of iron in spinach, causing biofortification naturally in host plant was reported by Khare et al. (2018) [17]. Pseudomonas fluorescens chelated iron from soil upon siderophore synthesis and caused increase in root length of spinach (1.2 to 2.4cm), seed germination rate increased by 30%, shoot length increment upto 3.4 cm and leaf number. Such positive response of selected PGPR was observed solely, in absence of any other chemical fertilizer. This report encouraged use of such PGPR on large scale as it does not make use of any other combination of chemical fertilizers (Fig 1).
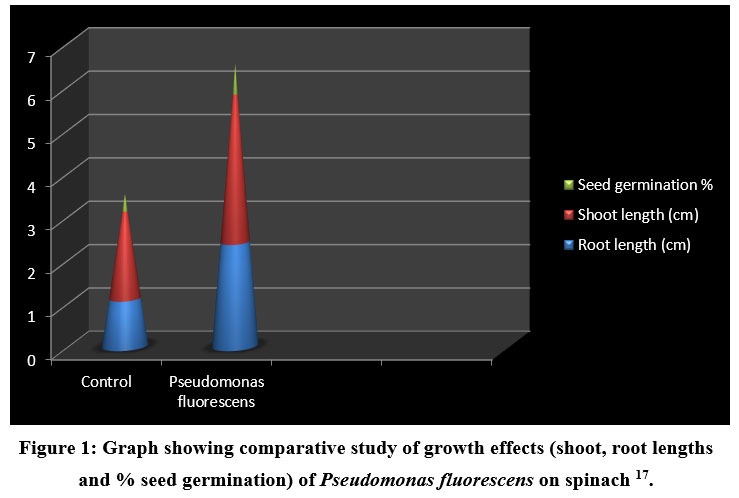 |
Figure 1: Graph showing comparative study of growth effects (shoot, root lengths and % seed germination) of Pseudomonas fluorescens on spinach 17. |
Pseudomonas putida strain (PTCC 1696)
Growth promotion activity of Pseudomonas sp. under stressed soil condition was reported by Bhlawan et al. (2020) [18] Pseudomonas putida (PTCC 1696) was inoculated in 3 kg saline soil in which spinach seeds were sown. After 90 days of planting, result showed higher level of chlorophyll a and Chlorophyll b alongwith root and shoot fresh and dry weights. This occurred due enhanced uptake of soil nutrients under influence of Pseudomonas putida (PTCC 1696) (Fig 2). Hence, it is understood that this strain increased growth of spinach by remediating soil present in saline condition alongwith cadmium pollution (Table 1).
Table 1: Representation of various beneficial effects of Pseudomonas species (PGPR) under different soil conditions in Spinach.
|
PGPR |
Abiotic stress/Plant growth |
Growth effects |
References |
|
Pseudomonas fluorescens |
Soil salinity stress |
Increased fresh and dry weight and Fe, Zn, Cu, Mn concentration in aerial parts of spinach |
[19] |
|
Pseudomonas aeruginosa MGPB31 |
Soil salinity stress |
Escalate root and shoot lengths |
[20] |
|
Pseudomonas putida RC06, Paenibacillus polymyxa RC35 |
Production of Indole-3-acetic acid (Normal soil condition) |
Increased root and shoot weights. Improved N and P nutrition. |
[21] |
|
Pseudomonas protogenes CHAO, Pseudomonas alloputida RUM 14 |
Soil contaminated with metribuzin herbicide ( 0, 50 and 100 grams/hectare) |
Increased tissue plant concentration of macronutrients (P-5583.30, K-83000.00, Ca-10886.70, Mg-10766.60 mg/kg), micronutrients (Cu-22.73, Zn-73.00, Fe-221.36 mg/kg dry matter) and dry weight of leaves (8.76 gm). Pseudomonas alloputida RUM 14 showed greatest alleviation of harmful effects of metribuzin. |
[22] |
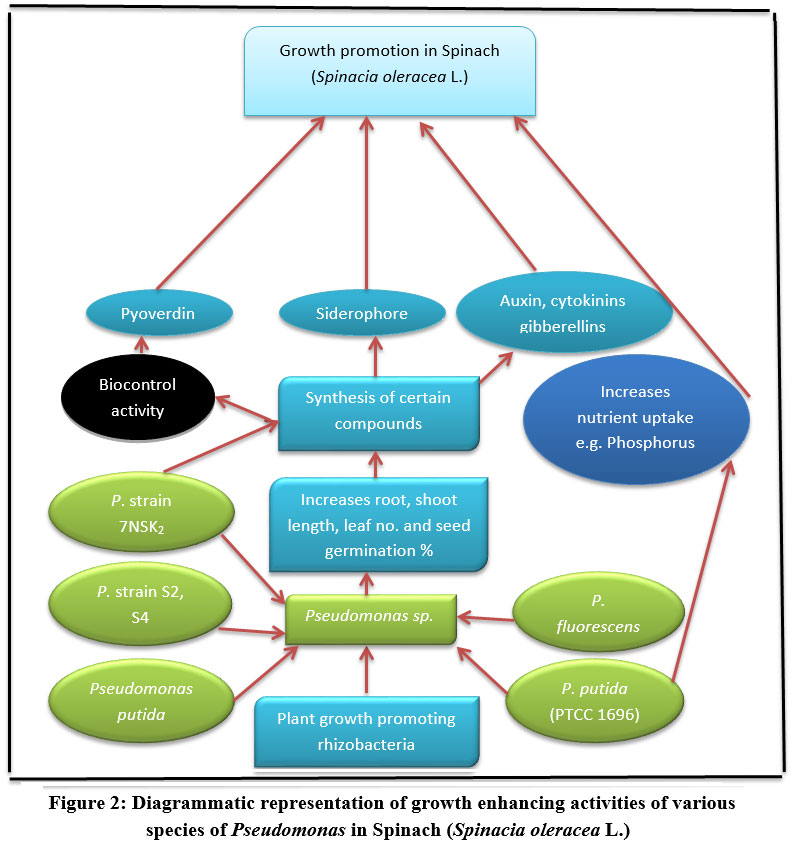 |
Figure 2: Diagrammatic representation of growth enhancing activities of various species of Pseudomonas in Spinach (Spinacia oleracea L.). |
Conclusion and Future Prospects
This review is just an attempt to acknowledge such PGPR (Pseudomonas sp.) which is needed to be studied more in concern with its application to increase productivity of spinach. In this work certain specific conditions (saline and cadmium polluted soil) and method of inoculation of Pseudomonas sp. has also been mentioned which gave some hopeful result and can be used for field application of spinach in replacement of chemical fertilizers when explored more. Thus, quiet good work is needed to be done to move productivity of spinach towards PGPR based organic and sustainable farming which makes Pseudomonas a future PGPR based fertilizer for spinach.
Globally present PGPR, Pseudomonas whose other aspects, viz, type of biofermentation, bioformulation and carrier molecule for its large scale field application is need to be studied. Such work should be accompanied as scarcely denoted here. But to increase the life span of such PGPR in field and their market demand, the underexplored attribute study is significant.
The future outlook of this work is one should focus on how Pseudomonas species deal with different abiotic stress (soil salinity) and contaminated (chemical herbicides and heavy metals) soil conditions. As reported here the strains in such conditions enhance spinach growth and alleviate detrimental effects of chemicals. The work on such aspects is quiet limited and need to be explored more. As this review is just an attempt to show positive relation between different species of Pseudomonas and spinach, under various soil and environmental situations.
Acknowledgement
The author would like to thanks Prof. Pooja Singh, H.O.D. Botany, Deen Dayal Upadhyaya Gorakhpur University, Gorakhpur, for helping in preparation of this manuscript.
Conflicts of Interest
The authors declare that there are no conflicts of interest regarding publication of this paper.
References
- Roughani A., Miri S.M. Spinach: An important green leafy vegetable and medicinal herb. Paper presented at: The 2nd International Conference on Medicinal plants, organic farming,Naturaland PharmaceuticalIngredients.2003,Iran.[https://www.researchgate.net/publication/ 329699312_ Spinach_An_Important_green_leafy_vegetable_and_medicinal_herb]. Accessed on 10 Feb 2023.
- Lucy M., Reed E., Glick B.R. Application of free living plant growth promoting rhizobacteria. Antonie van Leeuwenhoek; 2004; 86: 1-25.
CrossRef - Nicholson W.L. Roles of Bacillus endospores in the environment. Cell Mol. Life Sci;2002; 59: 410-416.
CrossRef - Quadt-Hallmann A. Hallmann J., Kloepper J.W. Bacterial endophytes in cotton: location and interaction with other plant-associated bacteria. Can. J. Microbiol;1997; 43: 254-259.
CrossRef - Ryu C., Farag M.A., Hu C.H., Reddy M.S., Wei H.X., Pare P.W., Kloepper J.W. Bacterial volatiles promote growth in Arabidopsis. Proc. Nat. Acad. Sci; 2003; 100: 4927- 4932.
CrossRef - Bullied W.J., Buss T.J., Vessey J.K. Bacillus cereus UW85 inoculation effects on growth, nodulation and N accumulation in grain legumes: Field studies. Can. J. Plant Sci; 2002; 82: 291-298.
CrossRef - De Freitas J.R., Germida J.J. Plant growth promoting rhizobacteria for winter wheat. Can. J. Microbiol; 1990; 36: 265- 272.
CrossRef - Kloepper J.W., Lifshitz R., Zablotowicz R.M. Free-living bacterial inocula for enhancing crop productivity. Trends Biotechnol;1989; 7: 39-44.
CrossRef - Saubidet M.I., Fatta N., Barneix A.J. The effect of inoculation with Azospirillum brasilense on growth and nitrogen utilization by wheat plants. Plant Soil; 2002; 245: 215-222.
CrossRef - Young C.S., Lethbridge G., Shaw L.J., Burns R.G. Survival of inoculated Bacillus cereus spores and vegetative cells in non-planted and rhizosphere soil. Soil Biol. Biochem; 1995; 27:1017-1026.
CrossRef - Glick B.R., Jacobson C.B., Schwarze M.M., Pasternak J.J. 1- Aminocyclopropane-1-carboxylic acid deaminase mutants of the plant growth promoting rhizobacterium Pseudomonas putida GR 12-2 do not stimulate canola root elongation. Can. J. Microbiol. ;1994; 40: 911-915.
CrossRef - Kloepper J.W. A review of mechanisms for plant growth promotion by PGPR. Paper presented at: 6th International PGPR Workshop. 5-10 Oct 2003; Calicut. India.
- Wu L., Estrada O., Zaborina O., Bains M., Shen L., Kohler JE., Patel N., Musch M.W., Chang E.B., Fu Y.X., Jacobs M.A., Nishimura M.I., Hancock., R.E.W., Turner J.R., Alverdy J.C. Recognition of Host Immune Activation by Pseudomonas aeruginosa. Science;2005;309 (5735):774-777.
CrossRef - Hofte M., Seong K.Y., Jurkevitch E., Verstraete W. Pyoverdin production by the plant growth beneficial Pseudomonas strain 7NSK2: Ecological significance in soil. Plant and Soil;1991; 130:249-57.
CrossRef - Chiun K, H., Micallef S.A. Plant-mediated restriction of Salmonella enterica on tomato and spinach leaves colonized with Pseudomonas plant growth-promoting rhizobacteria. Int. Journal of Food Microbiology; 2017; 259:1-6.
CrossRef - Urashima Y., Hori K. Selection of PGPR which promotes the growth of spinach. 2003.[https://www.researchgate.net/publication/340336389_selection_of_PGPR_which_promotes_the_growth_of_spinach]. Accessed on 12 Feb 2023.
- Khare A., More H., Madliya S., Patil V.D. Biofortification of Spinach (Spinacia oleracea) with plant growth promoting rhizobacteria- Pseudomonas fluorescens. JETIR; 2018; 5(8).
- Bhlawan H., Astaraei A., Lakzian A. Effect of the Pseudomonas putida inoculation in alleviation of saline soil cadmium stress on spinach plant. Int. J. of Advan. Sci. Technol; 2020; 29(06): 9450-9468.
- Bolhansani Z., Ronaghai A.M., Ghasemi R., Zarei M. Influence of rice-husk derived biochar and growth promoting rhizobacteria on yield and chemical composition of spinach in soil under salinity stress. Iranian J. of Soil Research; 2019; 33(3).
- Uchgaonkar P., Kudale S., Singh, Dasgupta D. Marine Pseudomonas aeruginosa MGPB31: A Potential Bioinoculum to alleviate salinity stress in Spinach (Spinacia oleracea ). Paper presented at: NCIFEH Conference Proceeding; Sep 2018; Navi Mumbai, Maharastra, India.
- Cakmakci R., Erat M., Erdogan U., Figen M. The influence of plant growth promoting rhizobacteria on growth and enzyme activities in wheat and spinach plants. J. of Plant Nut and Soil Sci; 2007; 170(2): 288-295.
CrossRef - Asadzadeh K. Effect of Plant Growth Promoting Rhizobacteria and Filter cake on Spinach. [https://jsw.um.ac.ir/article_40515_en.html]. Accessed on 12 March 2023.

