Introduction
Plant nutrition significantly impacts on cropping systems, environmental sustainability, and human and other animals’ health and well-being. Minerals are a vital requirement for a plant’s growth, metabolism, and completion of its life cycle. Mainly, plants require 14 elements for their development and they are categorized into macroelements and microelements. Six mineral elements, such as nitrogen (N), phosphorous (P), potassium (K), calcium (Ca), magnesium (Mg), and sulphur (S) are included in the macroelements category and are required in large quantities. Among macroelements, nitrogen (N), phosphorous (P), and potassium (K) are considered primary macronutrients and calcium (Ca), magnesium (Mg), and sulphur (S) are secondary macronutrients. At the same time, microelements are required in small quantities, which include chlorine (Cl), boron (B), iron (Fe), manganese (Mn), copper (Cu), zinc (Zn), nickel (Ni), and molybdenum (Mo)1. Plants require optimal mineral elements for good quantity and quality crop production. When the concentration of mineral elements increases exponentially harms plant growth and crop yield. Humans and other animals acquired essential minerals elements from plants through the food chain2.
The plants experience nutritional imbalance when they grow in areas with low phytoavailabilty. Such deficiencies are resolved by the use of fertilizers, through which they acquire essential elements. The invention of the energy-intensive Haber-Bosch process leads to synthetic fertilizers massive production. NPK is a widely used chemical fertilizer, and nitrogen is one of the essential elements for plant growth, whereas its excess accumulation causes detrimental environmental problems. N-fertilizer synthesis is one of the reasons for the production of greenhouse gaseous and N and P fertilizers contribute to the eutrophication process3,4. Similarly, excess rock phosphate and rock sulphate utilisation for fertilizer synthesis causes their extermination within the next 25-100 years. The chemical fertilizers’ efficiency was decreasing over time and excessive use causes serious soil degradation, nitrogen leaching, soil compaction, reduction in soil organic matter, and loss of soil carbon5. Hence in the future, sustainable fertilizer management is required in the field of crop production2,6.
Researches in the development of high-potential, environment-friendly fertilizers are very essential in the agriculture sector7. A group of fertilizers is called slow-release fertilizers (Eg: Urea formaldehyde (UF), Nitroform (UF derivative), methylene urea (MU)); they can hang on the soil after their application and is available for a significantly longer time. As a result, they didn’t quickly wash out from soil through rain and irrigation; hence it reduces eutrophication effects8. Slow-release fertilizers are safer, able to decrease soil toxicity, cause less damage to plant roots and also reduce the frequency of fertilization9. Thermoplastic resins such as polyolefin, polyvinylidene chloride, and co-polymers are used as a coating material for the synthesis of slow-release fertilizers because they cannot degrade easily10. Natural coating materials are more environmentally friendly and safer; hence researchers are now focused on this field. Mussel shell and cow urine are organic waste products, but they are rich in micro and macronutrients. Nowadays, mussels shell waste accumulation creates a high level of pollution. In this scenario, natural, slow-release fertiliser synthesis using mussel shells is considered an environment-friendly waste disposal method. The present study mainly focused on synthesising mineral-rich, natural, slow-release fertiliser using waste materials such as mussel shells and cow urine.
Materials and Methods
Sample collection
The raw Perna perna mussel shells wastes were collected from hotels and markets located 5 km around Kovalam, Thiruvananthapuram, Kerala, India (8.3988° N, 76.9820° E). Shells were thoroughly washed and removed debris attached to the shell surface. After proper drying, shells were crushed using an iron rod and powered in the mixer grinder. Then the powder was sieved in a brass frame sieve (Standard laboratory test sieves) with a 45-micron mesh size. Cow urine was collected from cattle farm without contamination and filtered urine was used for the study.
 |
Figure 1: Shell of Perna perna, A: Dorsal region, B: Ventral region |
Experimental procedure
Shell saturation experiment
In this experiment, shell powder remained constant and on every alternate day, urine changed. For this, 75 ml fresh cow urine was added to a 25 g mussel shell containing a beaker and given one hour of gentle stirring in a closed container. After stirring, the mixture retains without disturbance. On alternative days, urine in the beaker was removed and added fresh urine followed by one hour of gentle stirring. This procedure continues for 10 days. Every alternate day, 1 g of shell powder was washed with distilled water for removing excess urine content, dried thoroughly, and used for further analysis.
Urine filtration experiment
In this experiment, 25 g shell powder was added to 75 ml fresh cow urine and continuously stirred for one hour. In this setup, urine was constant, and shell powder was removed every alternate day. The experiment was conducted for 10 days, and every alternate day the removed shell powder was washed with distilled water to remove excess urine content and dried thoroughly and store for further analysis.
Shell leaching experiment
For this experiment, 25 g nutrient-impregnated shell powder was mixed with 75 ml distilled water, and the setup was kept for 30 days to measure the leaching of elements. Then, every 10 days 1 g shell powder was taken from the setup, dried and concentrations of elements were measured.
XRF analysis
Elemental concentration variation in the shell saturation experiment and urine leaching experiment was calculated by X-Ray fluorescence spectroscopy (Spectro-Ametek, SPECTRO XEPOS-XRF). We analysed the concentration variations of macro elements such as P, K, Ca, S, and Mg and microelements such as Fe, Zn, Cu, Mn, Mo, Cl, and Ni.
BET analysis
The specific surface area and pore size distribution of normal mussel shell, nutrient impregnated shell powder, and 30 days nutrient leached shell powder was measured by BET (Brunauer–Emmett–Teller) analyser (BELSORP-max) with N2 as the analysis gas.
Results and Discussion
Synthesis of natural slow-release fertilizers is a highly considerable aspect of research that diminishes the adverse effects of chemical fertilizers. Natural slow-release fertilizers provide a steady supply of essential elements to plants over a course of time. Runoff of elements from plants’ proximity is the major reason for nutrient scarcity in plants; hence the idea of slow release or controlled release fertilizer was developed. Cow urine and mussel shell powder are waste products but they are rich with essential elements necessary for plant growth. In the shell saturation experiment, except for calcium and magnesium, all other macronutrient (P, K, Ca, S) concentrations were significantly increased and the results presented as graph (Figure 2) which was plotted using log values of concentration (ppm). Calcium concentration was exponentially higher in the mussel shell; hence calcium was leached into the urine. Macronutrients are vital to plant nutrients found in every plant cell and are involved in cellular function and plant growth. They act as activators of many enzymes, are involved in metabolism, photosynthesis, and cellular multiplication, and play a critical role in the formation of the best quality and quantity yield from crops, etc.11. The interactions between nutrients are also needed for plant development. For example, sufficient potassium supply promotes N metabolism and facilitates the synthesis of amino acids and proteins12. Hence, an insufficient supply of a single nutrient adversely affects the activities of others.
Among macronutrients N, P, and K are the primary macronutrients. Nitrogen is an indispensable and unavoidable element that utilizes plants in the form of NO32- and NH4+. Nitrogen is an essential constituent of protein and chlorophyll synthesis; hence optimal nitrogen concentration increases photosynthesis, leaf area production, and leaf duration. These factors are directly linked with crop production. Phosphorous is mainly involved in plant growth, which is involved in proper plant maturation, helping to withstand harsh environmental conditions, cell division, seed development, etc.13. Similarly, potassium is involved mainly in the physiological processes of plants including nutrient transport, nitrogen metabolism, carbon assimilation, etc. which determine crop quality11. The secondary macronutrients are sulphur, magnesium, and calcium. Sulphur is mainly involved in synthesising sulfur-containing amino acids and is the critical component of many prosthetic groups. In plants, calcium acts as a structural component and is involved in cellular signalling and magnesium is the key component of chlorophyll. Hence the necessity of macronutrients in plants is vital and its deficiencies directly affect the quality and quantity of crop production14.
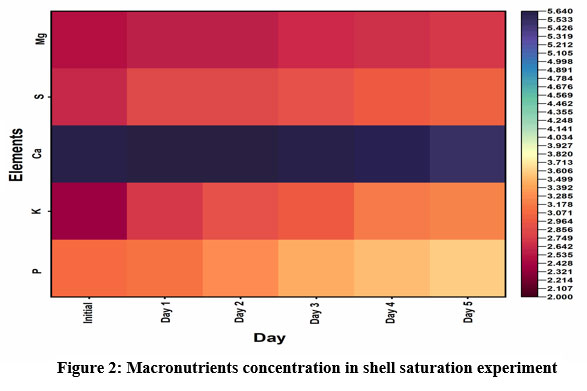 |
Figure 2: Macronutrients concentration in shell saturation experiment |
A significant concentration difference was not observed in the case of micronutrients (Table 1). Shell powder had more concentration of micronutrients than cow urine. The requirement and accumulation of micronutrients were traces, but they are essential for the better growth of plants. They play various physical and metabolic functions including pollen germination, photosynthesis, chlorophyll synthesis, and various hormonal and enzyme activities15. In plants, zinc is an important micronutrient that mainly regulates several enzyme activities including the activity of alcohol dehydrogenase, carbonic anhydrase, superoxide dismutase and also it is part of some enzyme structures such as Alkaline Phosphatase, phosphatides lipase, Carboxy peptidase, RNA polymerase, Dehydrogenase and Aldolase16,17,18. Similarly, Fe, Mn and Cl are involved in photosynthesis and carbohydrate synthesis and Cu is involved in protein and chlorophyll synthesis. The major function of Mo is nitrogen fixation and Ni is nitrogen metabolism; hence its deficiency causes stunted growth and chlorosis15. In the case of the urine filtration experiment significant nutrient scrubbing was not observed (Figure 3 and Table 2). Macronutrient concentration was significantly lower in the urine filtration experiment than shell saturation experiment. Similarly, in the case of micronutrients, nickel was absent in all samples from the urine filtration experiment. Hence the shell powder from the shell saturation experiment was richer with macro and microelements.
Table 1: micronutrients concentration in shell saturation experiment
| Sample | Fe (ppm) | Zn (ppm) | Cu (ppm) | Mn (ppm) | Mo (ppm) | Cl (ppm) | Ni (ppm) |
| Raw cow urine | 0.68±
0.02 |
0.52 ±
0.02 |
0.413 ±
0.15 |
3.64 ±
0.25 |
0.27 ±
0.029 |
1170.33 ±
250.52 |
0.62 ±
0.006 |
| Raw shell powder | 56.76 ±
9.37 |
1.58 ± 0.23 | 1.77 ±
0.09 |
0.085 ±
0 |
0.64±
0.09 |
287.42±
18.67 |
0.085 ±
0 |
| S1 | 56.59±
2.10 |
1.33±
0.35 |
1.50±
0.15 |
0.085 ±
0 |
0.58±
0.14 |
227.79±
4.42 |
0.085
0 |
| S2 | 58.43 ±
9.9 |
1.44±
0.30 |
1.66±
0.26 |
0.085 ±
0 |
0.53±
0.16 |
239.96±
1.70 |
2.91±0.29 |
| S3 | 60.01 ± 3.45 | 1.47 ± 0.41 | 1.74 ±0.17 | 0.085 ±
0 |
0.71 ± 0.17 | 289 ± 5.89 | 2.86 ± 0.15 |
| S4 | 60.24±1.04 | 1.56±0.2 | 1.81±0.11 | 0.085 ±
0 |
0.74±0.04 | 284.32±1.04 | 2.90±0.14 |
| S5 | 60.45±3.17 | 1.62±0.71 | 1.92±0.3 | 0.085 ±
0 |
0.68±0.11 | 295.17±2.47 | 2.91±0.2 |
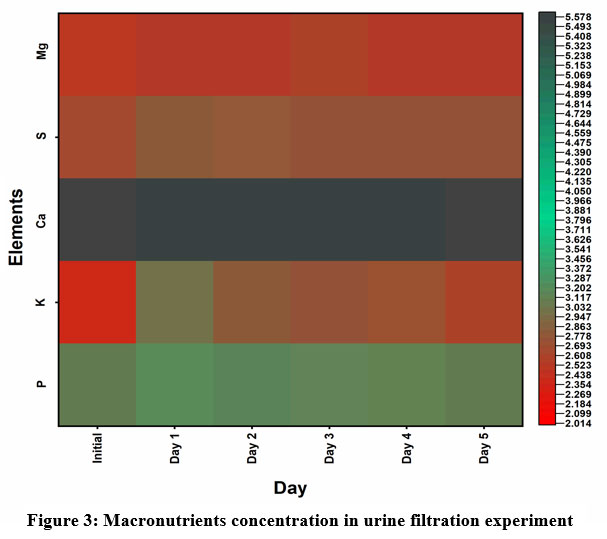 |
Figure 3: Macronutrients concentration in urine filtration experiment |
Table 2: Micronutrients concentration in urine filtration experiment
| Sample | Fe (ppm) | Zn (ppm) | Cu (ppm) | Mn (ppm) | Mo (ppm) | Cl (ppm) | Ni (ppm) |
| Raw cow urine | 0.68±
0.02 |
0.52 ±
0.02 |
0.413 ±
0.15 |
3.64 ±
0.25 |
0.27 ±
0.029 |
1170.33 ±
250.52 |
0.62 ±
0.006 |
| Raw shell powder | 56.76 ±
9.37 |
1.58 ± 0.23 | 1.77 ±
0.09 |
0.085 ±
0 |
0.64±
0.09 |
287.42±
18.67 |
0.085 ±
0 |
| US1 | 46.23±5.31 | 1.66±0.22 | 1.56±0.04 | 0.085 ±
0 |
0.51±0.01 | 314.21±10.41 | ND |
| US2 | 43.59±3.14 | 1.59±0.07 | 1.47±0.57 | 0.085 ±
0 |
0.6±0.12 | 297±9.34 | ND |
| US3 | 36.51±1.64 | 1.53±0.62 | 1.61±0.1 | 0.085 ±
0 |
0.59±0.04 | 305±4.12 | ND |
| US4 | 44.78±4.28 | 1.6±0.3 | 1.59±0.07 | 0.085 ±
0 |
0.58±0.11 | 284±8.54 | ND |
| US5 | 45.52±2.98 | 1.54±0.24 | 1.64±0.74 | 0.085 ±
0 |
0.67±0.74 | 297±5 | ND |
The formation of struvite crystals was an important highlight of these experiments (Figure 4). Struvite’s are transparent crystals formed of equimolar concentrations of magnesium, phosphorous, and ammonium with the formula NH4MgPO4·6H2O. Struvites are less soluble crystals; hence they can provide a continuous supply of magnesium, phosphorous, and nitrogen to the soil over a period of time. Cow urine is rich in potassium and ammonium, whereas magnesium concentration was limited. However, shell powder contains a sufficient quantity of magnesium; hence, when cow urine mix with shell powder, the magnesium from the shell act as an additional source for struvite formation. In the study of Liu et al. (2013)19 seawater and brine were used as additional magnesium sources for the struvite synthesis from urine. pHis another limiting factor for struvite formation. Struvites are formed only in alkaline conditions. Cow urine and shell powder have alkaline nature; hence they facilitate struvite synthesis20. In the past decade, many studies have been conducted to analyse the efficiency of struvite as fertilizers. Because of the slow-release nature of struvite could provide a long-term source of macroelements such as ammonium, phosphorous, and magnesium to plants26. Struvites are mainly used for phosphate recovery because phosphate rocks and non-renewable geological reserves are diminishing and hence they use as a major phosphate source in plants. The studies of Johnston and Richards (2003)21, Antonini et al. (2012)22, and Talboys et al. (2016)23 revealed that struvite could be used as a sustainable alternative to conventional P-based fertilizer.
 |
Figure 4: Struvite crystals |
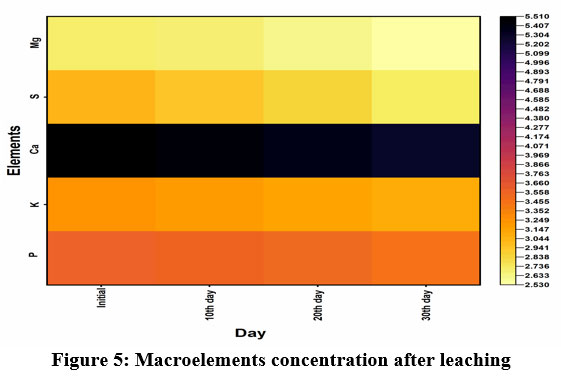 |
Figure 5: Macroelements concentration after leaching |
Table 3: Microelements concentration after leaching
| Sample | Fe (ppm) | Zn (ppm) | Cu (ppm) | Mn (ppm) | Mo (ppm) | Cl (ppm) | Ni (ppm) |
| Initial | 60.45±3.17 | 1.62±0.71 | 1.92±0.3 | 0.085 ±
0 |
0.68±0.11 | 295.17±2.47 | 2.91±0.2 |
| 10th day | 49.31±2.41 | 0.77±0.01 | 0.89±0.04 | 0.085 ±
0 |
0.14±0.005 | 164.34±2.11 | 1.03±0.07 |
| 20th day | 36.87±2.22 | 0.54±0.04 | 0.41±0.01 | 0.085 ±
0 |
0.085 ±
0 |
106.4±3.74 | 0.45±0.01 |
| 30th day | 29.45±4.14 | 0.37±0.02 | 0.085 ±
0 |
0.085 ±
0 |
0.085 ±
0 |
56.45±1.89 | 0.085 ±
0 |
Table 4: Bet analysis
| Measures | Normal shell | Nutrient immobilized shell | Nutrient leached shell |
| Surface area | 0.70858 m2/g | 0.37814 m2/g | 2.2811 m2/g |
| Pore size | 1.26 nm | 1.26 nm | 1.74 nm |
| Pore volume | 0.2246 m3/g | 0.17186 m3/g | 1.0682 m3/g |
Slow-release fertilizer development reduces drainage of fertilizer from soil and also environmental pollution. The leaching experiment results (Figure 5 and Table 3) revealed that more than half of the macroelements were retained in the shell after 30 days of continuous leaching. These results suggest that nutrient-impregnated shell powder can use as an excellent slow-release fertilizer. Nutrient impregnation and leaching were confirmed by BET analysis (Table 4), and the results highlighted that after nutrient impregnation, the surface area and pore volume of the shell decreased because nutrients used the space. However, surface area, pore size, and pore volume increase after leaching, because the nutrient-withdrawn area expands the surface. Many plants are intolerant to soil acidity and which causes reduced crop yield. Liming materials helps to increase the soil’s pH, facilitating excellent activity and composition of microbial organisms. Microbes play a critical role in the transformation and cycling of organic matter20. Traditionally, calcium hydroxide is used as a liming agent, but its production is more cost effective and power-consuming. Similarly, slow-release fertilizer production is relatively expensive24. Carbon base, slow-release fertilizers are very efficient for nutrient management because they have high porosity, surface area, and abundance of the functional group which helps to absorb nutrients and they are cost-effective25. The present work concept was the production of slow-release fertilizer from waste materials; hence it is a highly efficient, cost-effective, eco-friendly fertilizer and acts as the best liming material.
Conclusion
Several environmental hazards and economic losses widely occur due to excessive use of chemical fertilizers, unbalanced fertilization, and improper fertilization methods. Conventional mineral fertilizers are readily soluble in soil and they easily run out after rain leading to an increased risk of eutrophication. Hence, slow-release fertilizers have been developed to meet the sustainable nutrient availability to plants, increase yield and quality, maintain economic balance, and create environment-friendly agriculture. Mussel shells and cow urine are rich in minerals, but their independent application could not critical impact on soil fertility. Besides, mussel shell accumulation makes severe environmental pollution. Recycling waste mussel shells in the form of slow-release fertilizer becomes a pollution control strategy. The formation of struvite crystals provides a more slow-release nature to fertilizer and gives the regular flow of macronutrients such as nitrogen, phosphorous, and magnesium. The present slow-release fertilizer reduces environmental issues created by conventional mineral fertilizers. This new mineral-rich natural fertiliser has four-way action, (1) it reduces soil acidity, (2) provides micro and macronutrients, (3) gives long-term nutrient supply because of slow-release action, (4) Eco-friendly waste disposal of a mussel shell.
Acknowledgement
The authors gratefully acknowledge UGC-MRP project for providing funding and also the Calicut university central sophisticated instrumentation facility (CSIF) for providing infrastructural facility.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Sources
This work was supported by UGC-MRP project (University grand commission- Major research project) grant number F. No. 43-602/2014 (SR) 17-11-2015.
Reference
- Kumar S., Kumar S., Mohapatra T. Interaction between macro‐and micro-nutrients in plants. Frontiers in Plant Science. 2021;12:665583.
CrossRef - White P. J., Brown P. Plant nutrition for sustainable development and global health. Annals of botany. 2010;105(7):1073-1080.
CrossRef - Smith P., Martino D., Cai Z., Gwary D., Janzen H., Kumar P., … & Smith J. Greenhouse gas mitigation in agriculture. Philosophical transactions of the royal Society B: Biological Sciences. 2008;363(1492):789-813.
CrossRef - Conley D. J., Paerl H. W., Howarth R. W., Boesch D. F., Seitzinger S. P., Havens K. E., … & Likens G. E. Controlling eutrophication: nitrogen and phosphorus. Science. 2009;323(5917):1014-1015.
CrossRef - Lin W., Lin M., Zhou H., Wu H., Li Z., Lin W. The effects of chemical and organic fertilizer usage on rhizosphere soil in tea orchards. PloS one. 2019;14(5):e0217018.
CrossRef - Kesler S. E. Mineral supply and demand into the 21st century. In proceedings for a workshop on deposit modeling, mineral resource assessment, and their role in sustainable development. US Geological Survey circular. 2007;1294:55-62.
- Kampeerapappun P., Phanomkate N. Slow release fertilizer from core-shell electrospun fibers. Chiang Mai J. Sci. 2013;40(4):775-782.
- Neamţu C., Popescu M., Oancea F., Dima Ş. O. Synthesis optimization and characterization of microencapsulated NPK Slow-release fertilizers. Open Chemistry. 2015;13(1).
CrossRef - Trenkel M. E. Controlled-release and stabilized fertilizers in agriculture. Paris: International fertilizer industry association. 1997;11.
- Lawrencia D., Wong S. K., Low D. Y. S., Goh B. H., Goh J. K., Ruktanonchai U. R., … & Tang S. Y. Controlled release fertilizers: A review on coating materials and mechanism of release. Plants. 2021;10(2):238.
CrossRef - Xu X., Du X., Wang F., Sha J., Chen Q., Tian G., … & Jiang Y. Effects of potassium levels on plant growth, accumulation and distribution of carbon, and nitrate metabolism in apple dwarf rootstock seedlings. Frontiers in Plant Science. 2020;11:904.
CrossRef - Ruiz J. M., Romero L. Relationship between potassium fertilisation and nitrate assimilation in leaves and fruits of cucumber (Cucumis sativus) plants. Annals of applied Biology. 2002;140(3):241-245.
CrossRef - Zewide I., Reta Y. Review on the role of soil macronutrient (NPK) on the improvement and yield and quality of agronomic crops. Direct Res J Agric food Sci. 2021;9:7-11.
- de Bang T. C., Husted S., Laursen K. H., Persson D. P., Schjoerring J. K. The molecular–physiological functions of mineral macronutrients and their consequences for deficiency symptoms in plants. New Phytologist. 2021;229(5):2446-2469.
CrossRef - Zewide I., Sherefu A. Review Paper on Effect of Micronutrients for Crop Production. J. Nutrition and Food Processing. 2021;4(7).
CrossRef - Pandey N., Pathak G. C., Singh A. K., Sharma C. P. Enzymic changes in response to zinc nutrition. Journal of Plant Physiology. 2002;159(10):151-1153.
CrossRef - Hacisalihoglu G., Ozturk L., Cakmak I., Welch R. M., Kochian L. Genotypic variation in common bean in response to zinc deficiency in calcareous soil. Plant and soil. 2004;259(1):71-83.
CrossRef - Mousavi S. R., Galavi M., Rezaei M. Zinc (Zn) importance for crop production—a review. International Journal of Agronomy and Plant Production. 2013;4(1):64-68.
- Liu B., Giannis A., Zhang J., Chang V. W. C., Wang J. Wang. Characterization of induced struvite formation from source-separated urine using seawater and brine as magnesium sources. Chemosphere. 2013;93(11):2738-2747.
CrossRef - Tao W., Fattah K. P., Huchzermeier M. P. Struvite recovery from anaerobically digested dairy manure: A review of application potential and hindrances. Journal of environmental management. 2016;169:46-57.
CrossRef - Johnston A. E., Richards I. R. Effectiveness of different precipitated phosphates as phosphorus sources for plants. Soil use and management. 2003;19(1):45-49.
CrossRef - Antonini S., Arias M. A., Eichert T., Clemens, J. Clemens. Greenhouse evaluation and environmental impact assessment of different urine-derived struvite fertilizers as phosphorus sources for plants. Chemosphere. 2012;89(10):1202-1210.
CrossRef - Talboys P. J., Heppell J., Roose T., Healey J. R., Jones D. L., & Withers P. J. Withers. Struvite: a slow-release fertiliser for sustainable phosphorus management?. Plant and soil. 2016;401(1):109-123.
CrossRef - Wang C., Lv J., Xie J., Yu J., Li J., Zhang J., … & Patience B. E. Effect of slow-release fertilizer on soil fertility and growth and quality of wintering Chinese chives (Allium tuberm Rottler ex Spreng.) in greenhouses. Scientific Reports. 2021;11(1):1-14.
CrossRef - Rashid M., Hussain Q., Khan K. S., Alwabel M. I., Hayat R., Akmal M., … & Alvi S. Carbon-based slow-release fertilizers for efficient nutrient management: synthesis, applications, and future research needs. Journal of Soil Science and Plant Nutrition. 2021;21(2):1144-1169.
CrossRef - Valle S. F., Giroto A. S., Dombinov V., Robles-Aguilar A. A., Jablonowski N. D., & Ribeiro C. Struvite-based composites for slow-release fertilization: a case study in sand. Scientific reports. 2022;12(1):1-14.
CrossRef

