Introduction
According to Vedas and Indian Philosophy, the universe in which we live is composed of 5 basic elements or Panchbhutas which are Earth (Prithvi), Water (Jal), Fire (Agni), Air (Vayu), Sky (Akash) (Verma and George 2002). One of the major components of above is Earth or Prithvi which is mainly the hard surface of the planet mainly composed of different elements and this hard surface is basically habitat of different terrestrial plants and animals.
Soil is the fundamental and most basic substance by which this earth is made. This soil contain different inorganic and organic elements and compounds and different species ranging from bacteria to plant to animal. All the species of plant and animal are mutually interconnected, for example plants release oxygen (O2) as their byproduct and animals respire this oxygen as a living gas which they use in their metabolism. Similarly, animals release carbon dioxide (CO2) as byproduct that plants use to run their metabolism. So, this shows everything is somehow connected.
Plants are autotropic species which are a hub of different anabolic and catabolic reactions. In plants different activities are performed to sustain in the environment. Plants need different inorganic and organic elements to form macromolecules and micro molecules in the cells. But all the necessary elements are not present in the soil of a particular area, so we have to provide these essential elements like Nitrogen (N), Potassium (K), Phosphorus (P) in the form of chemicals to make the soil nutritious for plants and to grow the plants for the desired result. This chemical form of nutrition increases the yield of plants but they cause severe health hazards to plants1.
Because of different health hazard in plants the concept of Biofertilizer originated. Biofertilizers are the sum of everything like plant extracts, manures and dead remains of plant etc. Biofertilizer is a substance that basically contains different microorganisms which fixes nitrogen or mainly solubilizes phosphorus and potassium present in the upper layer of soil which is also called as rhizosphere, these microorganisms increase the basic nutrients in soil and stimulate the growth of plants. They increase the productivity of crop without harming the environment1, for example a biofertilizer with phosphate solubilizing and nitrogen fixing capacity can fix upto 40 kilograms of nitrogen per acre2. Biofertilizers can also be defined as microbial inoculants, which are made artificially to multiply the natural microorganism present in soil to improve the fertility and productivity of soil3. Different microorganisms like bacteria, algae and fungus help as biofertilizer and fix or solubilize different components present in soil and atmosphere.
Some of the bacteria which are used as biofertilizers are Azotobacter, Azospirillum, Rhizobium, etc. Some of the algae which are used as biofertilizers are species of Cyanobacteria or Blue- green algae (BGA)3. Some of the fungi which are used as biofertilizers are Ectomycorrhizae and Endomycorrhizae in symbiotic relationship with higher plants for example Basidiomycota, Ascomycota and Glomeromycota and Arbuscular mycorrhizal (AM) fungi.
Different types of biofertilizers are: 1. symbiotic nitrogen fixing spp. (Rhizobim spp.); 2. asymbiotic free nitrogen fixing spp. (Azotobacter); 3. Azospirillum, 4. Algal biofertilizers (BGA or BGA with a symbiotic relationship with Azolla), 5. phosphate solubilising bacteria, 6. Mycorrhizae4.
Nitrogen Fixing Bio-fertilizers
Free living – Azotobacter, Beijerinickia, Klebsiella, Anabaena, Nostac, Clostridium.
Symbiotic – Rhizobium, Anabaena azollae, Frankia.
Associative – Azospirillum.
P Solubilizing Bio-fertilizers
Bacteria – Bacillus circulans, B. megathecium var. phosphaticum, B. subtilis. Pseudomonas striata.
Fungi – Penicillium sp., Aspergillus awamori.
P Mobilizing Bio-fertilizers
Arbuscular mycorrhiza – Glomus sp., Gigaspora sp., Sclerocysis sp. Ecto mycorrhiza – Loccaria sp., Pisolithus sp., Boletus sp., Amaniata sp. Ericoid mycorrhiza – Pezizella ericae
Orchid mycorrhiza – Rhizoctonia solani.
Micronutrient Solubilizers
Silicate and Zinc Solubilizers – Bacillus sp.
(Source: Entrepreurial Training Manual, The Professor and Head of Department of Microbiology, Tamil Nadu Agriculture University, Coimbatore – 3)
In the era of food shortage during 1950’s in the developing countries the concept of green revolution originated. In green revolution, huge amount of chemical fertilizers was used like Urea which increased productivity for a short period of time but deteriorated the original level of productivity of soil. Biofertilizers are economically affordable and ecologically sound way of providing nutrition to plants. It is a renewable resource that can be used again and again. Because it is economically cheaper and affordable it is used by most of the marginalized and small peasants. It provides sustainable growth and development which is very essential in the era of global warming. In this age biofertilizers are not just an option but a necessity that should be used5.
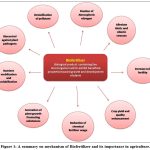 |
Figure 1: A summary on mechanism of Biofertilizer and its importance in agriculture. |
Review of Literature
The history on the study of Biofertilizers started in 1895, when Nobbe and Hiltner in laboratory made a culture of Rhizobia and launched it with the name of “Nitragin”. Following to Rhizobia, Azotobacter, Blue-green Algae and Azospirillum were discovered. In India N.V. Joshi started to study about Rhizobium symbiotic relationship with legume plants as biofertilizer. In India the commercial production of biofertilizers started in 1956. But in India during the ninth five-year plan, Ministry of agriculture set up National Project on Development and Use of Biofertilizers (NPDB) and this popularize the idea of biofertilizers in India. In India most commonly used biofertilizers are Rhizobium (RHZ), Azotobacter (AZS), Blue-green algae (BGA) and Azolla, Phosphate solubilizing or mobilizing biofertilizers (PSB)3. In the current scenario the development in the biofertilizers are as follows -:
Rhizobial Inoculants
The research on Rhizobial inoculants started at Taiwan in 1958. Firstly, the effective rhizobial strain is selected, isolated and then it is used to yield proper Pure rhizobial strains from different plants like peanut and soybean were isolated and then experiments were done to find out the most effective strain. Different isolates were stored in Culture Collection and Research Center (CCRC) of the Food Industry Research and Development Institute in Taiwan. When the isolated strain was used in field the soybean production increased from 5% to 134% 2.
Phosphate Solubilizing Inoculants
The research in the field of P Solubilizing Inoculants started in 1990’s at P solubilizing microbes are extracted from various plants like peanut and horticulture plant. These inoculants not only increased the productivity of vegetable plants but also reduced the amount of chemical fertilizers required in the field. In Taiwan PSB inoculants were used in muskmelons and tomato seeds.
The use of chemical fertilizers led to the increase deposition of excess minerals like Phosphorus etc. Hence more and more Biofertilizers should be used to protect the environment. The future research in this field is going on as -:
How the best variety of inoculants can be isolated which can make up a multifunctional and multidimensional
Control the quality of inoculants and large scale production of biofertilizers,
A specific law can be made to regulate biofertilizers and made them compulsory to use and provide it at a cheaper
A good quality of biofertilizer should be made which can be able to biodegrade and bioremediate several chemical wastes like DDT and make the environment
Bacteria as Biofertilizers
Azotobacter as Biofertilizer
Azotobacter as a biofertilizer is used since more than 100 years and in 1901 Martinus Beijerinck described Azotobacter 6. Azotobacter belongs to Pseudomonadaceae or Azotobacteraceae family and its class is Gammaproteobacteria which generally can be found in normal soil across the globe7. The most researched species of Azotobacter is A.vinelandii8. When compared with other bacteria they are generally big in size, and has an oval shape (about 3µm wide and 10µm long)9. This bacterium is gram negative in nature and produce pigments of different colour like reddish-violet, yellowish-green and brown-black. The Azotobacter which occurs naturally secrete large amount of sequester water and slime and during unfavorable environment they create cyst around them to protect themselves, but this cyst is unable to fix the nitrogen in environment8. Azotobacter is found in the rhizosphere of the soil and it is present in neutral to alkaline pH soil10. Azotobacter can fix atmospheric nitrogen in the free-living state without symbiotic association with any other plant discovered by Beijerinck in 1901.
Regarding Azotobacter as biofertilizer or plant growth promoter several research papers is available but the exact mechanism is not yet known so it is suggested that several mechanism like nitrogen fixation, plant growth hormone production and siderophore production plays a key role in growth of plant11. Microorganisms and plants both release some substance for growth known as plant hormones which either foist inhibitory or stimulatory impacts on various biological and physiological processes in plants and microorganisms both12,13. In the studies done by14, they stated that Azotobacter releases auxin or indol-3-acetic acid (IAA) on the addition of tryptophan into the medium and in the studies done by15, they found that indole-3-acetic acid is present in old culture of Azotobacter in small amount without the addition of tryptophan in the medium. Some of the other hormones like Gibberellins and Cytokinins in small amount are also released by these bacteria. These hormones help the plant root to expand in width which ultimately helps in plant growth. Usage of Azotobacter as biofertilizer showed that the dry weight of some plant like tomato, chickpea increased upto great extent16.
Nitrogen fixation is a very important biological activity and it maintains the nitrogen balance in the atmosphere. Ultimately it recycles the nitrogen on earth surface17. Nitrogen fixation helps in the improvement of soil fertility and crop production18. Azotobacter can convert the nitrogen present in atmosphere into ammonia which can be taken up by plants and gets utilized in plant body19. At the time when they are fixing the nitrogen the plant become resistant to oxygen because of production of nitrogenase20. Azotobacter can fix upto 20 kgN/ha/year21,22 .
Siderophores are a group of iron (Fe) chelating molecules that helps to suppress the plant pathogen attack23,24. Azotobacter produces this siderophore which help the plant from pathogen attack and help the plant to grow rapidly.
Azospirillum as Biofertilizer
Azospirillum as a biofertilizer is used since more than 43 years and in 1978 Johanna Dobereiner discovered it with his group. Azospirillum belongs to Azospirillaceae family and it belongs to the class Alphaproteobacteria. It is a gram-negative bacteria which is curve or rod in shape, it is non- fermentative in nature and it is a chemoorganotroph25. It is generally found in all the part of earth in the rhizosphere of the soil. It is mostly found in the roots of corn, sugarcane, rice, wheat, etc. For a very long time only 5 genera of this species were known but now 10-12 new species were discovered. Latest two species which were discovered are A.thiophilum and A.picis26,27. Azospirillum when discovered was known for the ability for fixing the atmospheric nitrogen to ammonia but later it was discovered that it also releases some phytochemicals or phytohormones like indole-3-acetic acid or auxin which helps in the growth of the plant. There is only one species of Azotobacter which is not capable of fixing the nitrogen which is A.palatum28. Azospirillum is a very good plant growth promoting rhizobacteria because it has the ability to fix the nitrogen and also it releases phytochemicals which help in plant growth also and it has the ability to solubilize the phosphate and it produces siderophore29.
Azospirillum is an endophytic bacteria and a ‘free- living nitrogen fixer’ which fixes the biological nitrogen. It lives in association with plants and they are unable to fix the nitrogen in ‘in-vitro’ condition. They do not form nodules. The most common test which can we do to test the nitrogen fixing ability is acetylene reduction test. The enzyme nitrogenase which helps in nitrogen fixation also helps to convert ethylene to acetylene. It is very sure that Azospirillum fixes the nitrogen but how much of this nitrogen is used by plant is not known till date and is a big question also30.
Azospirillum like other bacteria also releases phytochemicals which are important for plant growth. It releases some phytohormones like auxins, gibberellic acid, cytokinins, abscisic acid, polyamines, ethylene and nitrogen dioxide. Auxin has a major role in increasing cell division in the xylem cells and root cells. In Azospirillum the production of auxin is very high. The indole-3-acetic acid (IAA) produced by Azospirillum is the major reason responsible for the increase in root system29. The next hormone is gibberellic acid (GA) which is mainly responsible for the division of cell and elongation of cell, it also breaks the dormant stage during the seed germination31,32. Azospirillum also release cytokinin (CK) plant hormone that helps in cell division in roots and shoot of plant. Their main work is to grow the cell and also help in differentiation of the cell33. These cytokinin is present in root of the plant and they get transport to the shoot and it helps in the the shoot and root growth and cell division, increase the size of the leaf and mature the cell and chloroplast34. The next phytohormone which helps in growth of plant is Abscisic acid (ABA), which helps the plant in stress conditions when there is a very high heat, less water in soil, etc. This ABA is then transported to the leaves and it changes the osmotic potential of the plant so it prevents the loss of water for the plant35. One of the important phytothormone is Polyamine which ultimately helps in plant growth but the exact mechanism of polyamine is not known as how they help in growth of the plant but if polyamine is not produced it restricts the growth of the plant36. Azospirillum also produces ethylene which helps to break the dormancy of the seed and helps to elongate the root of the plant37. Next is Nitrogen Oxide (NO), which impose signal cascade effect in the plant leading to the development of adventitious roots in the plant38,39.
Just like bacteria fixes nitrogen it also solubilizes the insoluble phosphate present in the soil with the help of enzymes like phosphatases, phytases and some the organic acids40. But Azospirillum produces some different type of organic acids which helps to solubilize more phosphate in the soil depending on the sugar present in the soil29. It also produces siderophore which is an iron chelating compound that helps some bacteria to take the iron (Fe) present in the soil and restricts the pathogenic bacteria to grow around the soil which safeguards the plant from disease attack. Some species of bacteria which produces catechol type of siderophore are A.lipoferum and A.brasilense41.
 |
Figure 2: Azospirillum zaea N742 |
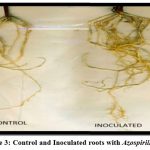 |
Figure 3: Control and Inoculated roots with Azospirillum29 |
Fungi as Biofertilizers
Mycorrhiza as biofertilizer
In nature, plant species are surrounded by both external and internal microorganisms. Some bacteria and mycorrhizal fungi play a key role in the growth and development of plants and can improve the quality of plant to a greater extent in stress condition and in favourable condition43,44,45. Arbuscular Mycorrhizal Fungi (AMF) make mutual symbiotic relation with 80% of plant46. What AMF do so it increase the area of roots, leading to increase in water and nutrient uptake47. AMF inoculation has shown increase in plant production and growth and also it protect the plant from the environmental stress48. AMF belongs to the phylum Glomeromycota and they are obligate symbiotic organism49. The mycelium present in AMF which originated from roots of the plant helps to get more nutrition by increasing the surface area where plant roots cannot access46. And, the hyphae of fungus are thinner in comparison to roots which can access the smaller area or pores47. Macromolecules like carbohydrate and some of the minerals get inside the roots of plant where AMF are present, this AM fungus hypha create a branched structure and make a dense colony near the root cortex, that is called as “arbuscules” which is the main site of nutritional exchange48. AMF also provides protection against flood, drought, high salinity, etc.49. Some metals are very important like iron (Fe), zinc (Zn), and copper (Cu) but if their concentration is high it becomes toxic, but this AMF reduces the metal toxicity and help the plants to take the metal in sufficient amount for growth50. A Zinc transporter is known in one species of AMF i.e, Glomus intraradices (GintZnT1)51. AMF has a high affinity phosphate transporter which helps to transport the phosphate from the soil to the plant root and it do this phosphate (pi) transport in symbiotic association but some studies found that AMF do this phosphate transport without growth effect on itself52,53,54. AMF directly helps to improve the structure of the soil and help to increase the productivity of the soil55. Arbuscular Mycorrhizal Fungi also help to reduce the emission of green house gas (GHG). GHG emission is a serious concern for the environment and it should be reduced and AMF reduce the emission of Nitrous oxide (N2O) which is one of the GHG leading to increase in temperature56. Thus it can be concluded that AMF is a great fungal biofertilizer which can be used for several benefits which were given above but because of ecosystem malfunctioning it is not present everywhere. So it should be naturally restored to compete with conventional chemical fertilizers57. There are several challenges when AMF is considered to be used as a biofertilizer in large scale but the need is very urgent. The production in large scale is difficult because AMF is an Obligate Symbiotic Organism and it cannot be produced in pure cultures so one of the common ways to extract and multiply the organism is that it should be extracted from the plant which possess it and it should be transferred to a new plant to multiply. But several attempts are being made so that it can be produced on a large scale and be used as a natural fertilizers57.
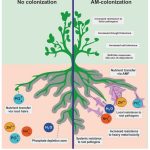 |
Figure 4: Effect of AM Colonies on Plant58. |
Trichoderma as Biofertilizer
Trichoderma is a genus of fungus which is used in agriculture which has many functions like it promote the growth of the plant also it provides protection against pathogens and it provide resistance against abiotic and biotic stress59,60,61. It is a free living fungus which interacts with soil, root and plant surface62. It is a symbiotic organism that is an endophyte and it establishes a direct relationship with plant by making a colony in the root system63. Many species of Trichoderma are being used in many agricultural field and are of great economic importance and it is one of the most used microbial inoculants to increase the plant growth and to defend the plant from pathogen64. The mass production of Trichoderma is possible through two processes which are solid fermentation and liquid fermentation65. But producing Trichoderma commercially in an agar medium which is solid in nature is not economically possible and feasible. To produce Trichoderma natural environment like roots of the crop, industrial wastes and stock waste can be a good environment where it can grow naturally66. Trichoderma based fertilizer (TBF) reduces the use of normal nitrogen, potassium and phosphorous based chemical fertilizers. Trichoderma increases the nutrition uptake of some essential micronutrients like Zinc (Zn), Copper (Cu), Iron (Fe), and Sodium (Na) and create a positive environment around the host plant. Trichoderma increase the uptake of the nutrients by increasing the surface area of root tissues. Trichoderma can be used with other biofertilizers like manure, phosphobacteria, azospirillum, B.subtilis e.t.c. Trichoderma should not be used when soil is dry, moisture is very important in the proper growth of Trichoderma67,68.
Algae as Biofertilizer
Blue-Green Algae or Cyanobacteria as Biofertilizer
Blue-green algae also known as cyanobacteria are most copious photosynthetic prokaryotes. They are autotropic organism69. It was the most primitive organism which evolved on the planet earth about 3.5 billion years ago. Some of the microfossils are found which are of precambrian era and was bigger from bacteria. It was the ancestor of cell organelle plastid and it was the only source of biogenic oxygen at the time when aerobic organisms started to originate70. It can do two major things in plant that is Photosynthesis and Nitrogen fixation. Cyanobacteria occurs in wide range of soil and present in both below and on the soil. It presents in sub-aerial environment and also present sporadically in wet surface such as paddy field. In majority of the paddy or rice field cyanobacteria occurs naturally which fixes nitrogen without any cost. Cyanobacteria are capable of converting atmospheric Dinitrogen (N2), Ammonia (NH3), Nitrites (NO2–), Nitrates (NO3–) into absorbable nitrogen form. Amino acids like asparagine, arginine and glutamine are the important source of nitrogen in plants71.
These are small and tiny oxygen releasing microorganism which are gram negative in strain and found in various ecological niches due to their structural conservation, plasticity and metabolism. Cyanobacteria are microorganism which can easily degrade several pollutants present in soil and increase the productivity of soil. World Health Organization (WHO) estimated the population to increase to 9.6 billion in the next 30 years and it has estimated that food production globally should be increase by 50% by the year 2029. Food production is increasing at a good pace but the use of chemical fertilizers is making the land “barren”. Thus the concept of green technology came to picture which focuses on how cyanobacteria can be used to increase the productivity of soil72. Diazotrophs are bacteria and archaea. They are a type cyanobacteria which are useful in making biofertilizers and are economically accessible which can fix the nitrogen for plant to provide protein to plant , provide vitamin B12, improve water holding capacity of the plant and improve soil aeration73.
Some of the important nitrogen fixing cyanobacteria are Scytonema Calothrix sp., Nostoc linkia, Anabaena variabilis, Aulosira fertilisima74.
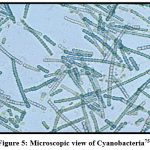 |
Figure 5: Microscopic view of use of Cyanobacteria75. |
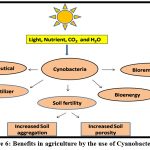 |
Figure 6: Benefits in agriculture by the Cyanobacteria76. |
Azolla and Anabaena as Biofertilizer
Anabaena azollae is a endophytic blue-green algae which lives in a symbiotic association with small water fern Azolla lam. to fix the nitrogen present in soil for itself and the host plant77. Anabaena azollae fixes the nitrogen for Azolla lam. and Azolla lam. provide protection from the outer environment and fixes the carbon for the alga77. Nitrogen is the most important element which is used in food production. The production of agriculture is dependent on the amount of nitrogen being given to the crops, in rice plants near about 21 kg N t-1 is absorbed to produce the grain and it is constant78. When chemical fertilizers (NPK) is used in the rice field the agro-ecosystem is disturbed and pollution is caused in the environment. This disturbed ecosystem and pollution can be treated through the use of biofertilizers79. But when we talk about the plant growth promoting hormones like auxin, gibberline, cytokinin, etc. it is not produced by Azolla- Anabaena and it should be studied further. There are 3 ways to inculcate the growth of Azolla- Anabaena, first as a monocrop, second as intercrop and third as deliberate or natural culture growth. The most compatible crop which can be grown in Azolla-anabaena biofertilizer is rice and it can apply on rice crop and mono or intercrop both80.
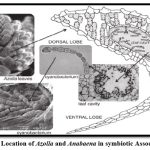 |
Figure 7: Location of Azolla and Anabaena in symbiotic Association81. |
Commercial Production of Biofertilizers in India
In 1920, N.V. Joshi started the use of Biofertilizers in India. Some of the marketed biofertilizers in India are-: 1). In nitrogen fixers, Azospirillum, Rhizobium, BGA, Azolla, Azotobacter, e.t.c. are being used. 2). In phosphate solubilizer, Pseudomonas, Aspergillus and Bacillus are being used. 3). In phosphate mobilizer VAM is used (VA-Mycorrhiza). 4). In potassium solubilizer, F.aurantia is used. 5). In plant growth promoting hormone biofertilizers Pseudomonas sp. is used. In India the requirement of biofertilizers is near about 5.5 lakhs metric tons and it will increase by 50-60k in near future82.
Table 1: Production of Biofertilizers (in Million Ton (MT)) in different states of India83.
| Indian States | Production (MT) | Azospirillum | Azotobacter | Rhizobium | P-solubalizing | Total
|
| Punjab | 2 | 0 | 0 | 1.47 | 0 | 1.47 |
| HP | 75 | 0 | 2.48 | 2.39 | 0 | 4.87 |
| Delhi | 1 | 0.037 | 0.39 | 0.17 | 0.284 | 0.881 |
| Haryana | 75 | 0 | 5.82 | 8.33 | 4.58 | 18.73 |
| Rajasthan | 75 | 0 | 17.11 | 13.9 | 13.87 | 44.88 |
| AP | 265 | 14.12 | 1.92 | 43.11 | 38.3 | 97.45 |
| UK&UP | 225 | 8.34 | 76.22 | 45.99 | 101.92 | 232.47 |
| Karnataka | 1,835 | 88.454 | 76.392 | 68.28 | 385 | 618.126 |
| Pondicherry | 75 | 7.79 | 0 | 0.725 | 7.3 | 15.815 |
| TN | 1,870.4 | 794.714 | 16.428 | 180.90 | 664 | 1656.042 |
| Kerala | 225 | 43.2 | 3.72 | 0.05 | 4.23 | 51.2 |
| Total | 4,723 | 956.65 | 200.48 | 365.32 | 1,219 | 2741.45 |
Conclusion
Benefits of using bio-fertilizers are described in this review and the side effects of using chemical fertilizers like NPK fertilizers in the agriculture sector which have caused several side effects on the environment such as reduced fertility of soil, accumulation of chemicals in the soil which is causing several harmful diseases in humans by contaminating the food and water. So, in this regard the use of biofertilizers comes into picture. Bio-fertilizers are the naturally occurring microorganism like Bacteria, Algae, Fungi, Pteridophytes or ferns which provide the essential minerals naturally from the environment by fixing or solubilizing some essential elements. In this review article some microorganisms of different domains are being studied like bacteria, algae and fungi. Bacteria acts as a very good natural bio-fertilizers because it is mainly concerned with fixing the nitrogen from atmosphere into ammonia which plant can take and use. It also solubilizes the insoluble phosphate present in soil to soluble form. Fungi also act as a good source of bio-fertilizers they also fixes nitrogen and solubilizes the phosphorous but their main role is that they accumulate in the roots of plant and help to uptake the nutrient present in the soil which plant cannot take by itself because plant roots are not thin enough that it can go into small pores and take the nutrients. These nutrients are easily accessible by hyphae of the fungus. Fungi such as Mycorrhiza and Trichoderma are also used to resist the abiotic and biotic stress like droughts and flooding, etc. Algae such as cyanobacteria or BGA and Anabaena azollae, also acts as a natural bio-fertilizer by fixing the nitrogen present in air. The positive effect of using bio-fertilizers in agriculture is that, they are economically feasible and provide immense support to the natural environment because they just not only increase the agricultural production but also bio-remediate and bio-degrade the harmful wastes which are present in soil. The commercial production of some bio-fertilizers is possible and are going on in whole world. But some of the bio-fertilizers like Mycorrhiza can mainly grow in natural environment where roots of plant are available. There commercial production in an agar medium or liquid medium is difficult as compared to bacterial bio-fertilizers. That is why the development in the field of bio-fertilizers is going on steadily and development is going on the commercial production and to find out which is the microorganism that can be produced commercially to increase crop production and to make the environment clean by remediation.
Acknowledgement
The authors are thankful to Head, Department of Botany, Guru Ghasidas Vishwavidyalaya, providing infrastructural facilities.
Conflict of Interest
There is not any conflict of interest including any financial, personal or other relationships with other people and organization.
Declaration of Funding Sources
The author declare that no fund, grant, or other support were received during the preparation of this manuscript.
References
- Bhattarcharjee , Dey U. Biofertilizer, a way towards organic agriculture: A review. African Journal of Microbiology Research; 2014; 8(24):1.
CrossRef - Aggani L. Development of Bio-Fertilizers and its Future Perspective. Scholars Academic Journal of Pharmacy (SAJP); 2013; 2(4):327-332.
- Ghosh N. Promoting Biofertilizers in Indian Agriculture. Economic and Political Weekly; 2003; 39(52): 5617-25.
- Patra , Singh J. A Review: Usage of Biofertilizer in Cereal Crops. Current Journal of Applied Science and Technology; 2019; 36(3): 1-8.
CrossRef - Pimental D., Houser D., Preiss E., White O., Fang H., Mesnick L., Barsky T., Tariche S., Schreck A. Water Resources: Agriculture, the Environment, and Society. Oxford University Press; 1996; 47( 2): 97-106.
CrossRef - Gerlach M., Vogel J. Nitrogen fixing bacteria. Zentralblatt fur Bakteriologie, Abt; 1902; 2:
CrossRef - Kennedy C., Dean D. The nifU, nifS, nifV gene products are required for activity of all three nitrogenise of Azotobacter vinelandii. Molecular and General Genetics; 1992; 231: 494-
CrossRef - Das K. Azotobacter as biofertilizer. Advances in Applied Microbiology; 2019; 108: 1-43.
CrossRef - Wilson W., Knight S.C. Experiments in bacterial physiology. Burguess: Minneapolis, USA; 1952: 49.
- Ramirez FLE., Mellado C.J. Bacterial biofertilizers. In: Siddiqui ZA, editor. PGPR: biocontrol and biofertilization.The Netherland: Springer; 2005; 143–172.
- Ansari R.A., Rizvi R., Sumbul A., Mahmood I. PGPR: Current Vogue in Sustainable Crop Probiotics and Plant Heath. Springer; 2017: 455-472.
CrossRef - Ansari A., Mahmood I. Plant Health Under Biotic Stress: Volume 1: Organic Strategies. Springer Singapore; 2019a; 1.
CrossRef - Ansari A., Mahmood, I. Plant Health Under Biotic Stress: Volume 2: Microbial Interactions. Springer Singapore; 2019b; 2
CrossRef - Brakel , Hilger F. Etude qualitative et quantitative de la synthese de substances de nature auxinique par Azotobacter chroococcum in vitro. Bull. Inst. Agron. Stns. Rech. Gembloux; 1965; 33:469–487.
- Blachere H., Hennequin J.R. Research on the synthesis of phytohormones and phenolic compounds by Azotobacter and bacteria of the In: Annales de I’Institut Pasteur; 1966; 111(3): 89.
- Sumbul A., Ansari R.A., Rizvi R., Mahmood I. Azotobacter: A potential bio-fertilizer for soil and plant Saudi Journal of Biological Sciences; 2020; 27: 3634-3640.
CrossRef - Wani A., Chand S., Wani M.A., Ramzan M., Hakeem K.R. Azotobacter chroococcum–a potential biofertilizer in agriculture: an overview. In: Soil Science: Agricultural and Environmental Prospectives. Springer, Cham; 2016: 333–348.
CrossRef - Vance P., Graham P.H. Nitrogen fixation in agriculture: application and perspectives. In: Nitrogen Fixation: Fundamentals and Applications. Springer, Dordrecht; 1995:77–86.
CrossRef - Prajapati , Yami K.D., Singh A. Plant growth promotional effect of Azotobacter chroococcum, Piriformospora indica and vermicompost on rice plant. NAST; 2008; 9: 85–90.
CrossRef - Hakeem K.R., Sabir M., Ozturk M., Akhtar M.S., Ibrahim F.H., Ashraf M., Ahmad M.S.A. Nitrate and nitrogen oxides: sources, health effects and their In: Reviews of environmental contamination and toxicology. Springer, Cham; 2016:183–217.
CrossRef - Kizilkaya R. Nitrogen fixation capacity of Azotobacter spp. strains isolated from soils in different ecosystems and relationship between them and the microbiological properties of J. Environ. Biol; 2009; 30 (1): 73–82.
- Esmailpour , Hassanzadehdelouei M., Madani A. Impact of livestock manure, nitrogen and biofertilizer (Azotobacter) on yield and yield components wheat (Triticum Aestivum L.). Cercetari Agronomice in Moldova; 2013; 46 (2): 5–15.
CrossRef - Wichard T., Bellenger P., Morel F.M., Kraepiel A.M. Role of the siderophore azotobactin in the bacterial acquisition of nitrogenase metal cofactors. Environ.Sci. Tech; 2009; 43 (19): 7218–7224.
CrossRef - Hayat , Ali S., Amara U., Khalid R., Ahmed I. Soil beneficial bacteria and their role in plant growth promotion: a review. Ann. Microbiol; 2010; 60 (4):579–598.
CrossRef - Tarrand J.J., Kreig N.R., Dobereiner J.A. Taxonomic study of the Spirillum lipoferum group with description of a new genus Azospirillum nov., and two species, Azospirillum lipoferum (Beijerinck) com nov. and Azospirillum brasilense sp. nov. Can J Microbiol; 1978; 24:967–980.
CrossRef - Lin S.Y., Young C.C., Hupfer H., Siering C., Arun A.B., Chen W.M., Lai W.A., Shen F.T., Rekha D., Yasin A.F. Azospirillum picis sp. nov., isolated from discarded tar. Int J Syst Evol Microbiol; 2009; 59:761–765.
CrossRef - Lavrinenko K., Chernousova E., Gridneva E., Dubinina G., Akimov V., Kuever J., Lysenko , Grabovich M. Azospirillum thiophilum sp. nov., a novel diazotrophic bacterium isolated from a sulfi de spring. Int J Syst Evol Microbiol; 2010; 60:2832–2837.
CrossRef - Zhou Y., Wei W., Wang X., Xu L., Lai R. Azospirillum palatum nov. isolated from forest soil in Zhejiang province, China. J Gen Appl Microbiol; 2009; 55:1–7.
CrossRef - Mehnaz S. Azospirillum: A Biofertilizer for Every Crop. Plants Microbes Symbiosis: Applied Facets; 2015; Doi: 1007/978-81-322-2068-8_15. Springer India.
CrossRef - Okumura R.S., Mariano D.C., Dallacort, R., Nogueira de Albuquerque A., Lobato A.K.S., Guedes M.S., Neto C.F.O., Oliveira da Conceicao H.E., Alves G.A.R. Azospirillum: a new and efficient alternative to biological nitrogen fixation in grasses. J Food Agric Environ; 2013; 2(1):1142–1146.
- Bacilio , Vazquez P., Bashan Y. Alleviation of noxious effects of cattle ranch composts on wheat seed germination by inoculation with Azospirillum spp. Biol Fertil Soils; 2003; 38: 261–266
CrossRef - Cassan , Maiale S., Masciarelli O., Vidal A., Luna V., Ruiz O. Cadaverine production by Azospirillum brasilense and its possible role in plant growth promotion and osmotic stress mitigation. Eur J Soil Biol; 2009a; 45:12–19.
CrossRef - Kieber J.J. Tribute to Folke Skoog: Recent Advances in our Understanding of Cytokinin Plant Growth Regul; 2002; 21(1):1-2.
CrossRef - Spaepen , Vanderleyden J., Okon Y.Plant growth promoting actions of rhizobacteria. Adv Bot Res; 2009; 51:283–320.
CrossRef - Bartels, , Sunkar, R. Drought and salt tolerance in plants. Crit Rev Plant Sci; 2005; 24: 23–58.
CrossRef - Bashan , de-Bashan L.E. How the plant growth promoting bacterium Azospirillum promotes plant growth – a critical assessment. Adv Agron; 2010; 108:77–136.
CrossRef - Glick R., Patten C.L., Holguin G., Penrose D.M. Biochemical and genetic mechanisms used by plant growth promoting bacteria. Imperial College Press, London; 1999: 125–140.
CrossRef - Pagnussat G.C., Simontacchi M., Puntarulo S., Lamattina L. Nitric oxide is required for root Plant Physiol; 2002;129: 954–956.
CrossRef - Pagnussat G.C., Lanteri M.L., Lamattina L. Nitric oxide and cyclic GMP are messengers in the indole acetic acid-induced adventitious rooting process. Plant Physiol; 2003; 132: 1241–
CrossRef - Reis V.M., Teixeira K.R.S., Pedraza R.O. What is expected from the genus Azospirillum as a plant growth promoting bacteria? In: Maheshwari DK (ed) Bacteria in agrobiology. Plant growth responses. Springer, Berlin/Heidelberg; 2011:123–138.
CrossRef - Shah S., Karkhanis V., Desai A. Isolation and characterization of siderophore, with antimicrobial activity, from Azospirillum lipoferum. Curr Microbiol; 1992; 25:347–351.
CrossRef - Mehnaz S., Weselowski B., Lazarovits G. Azospirillum zeae nov., diazotrophic bacteria isolated from rhizosphere soil of Zea mays . Int J Syst Evol Microbiol; 2007; 57(12): 2805–2809.
CrossRef - Brown M.E. Seed and root bacterization. Annu Rev Phytopathol; 1974; 12(1):181–197.
CrossRef - Levy Y., Dodd J., Krikun J. Effect of irrigation, water salinity and rootstock on the vertical mycorrhiza distribution of vesicular-arbuscular mycorrhiza in citrus roots. New Phytol; 1983; 95(3):397–403.
CrossRef - Creus C.M,. Sueldo R.J., Barassi C.A. Water relations in Azospirillum-inoculated wheat seedlings under osmotic Can J Bot; 1998; 76(2): 238–244.
CrossRef - Smith E., Read D.J. Mycorrhizal symbiosis. Academic, London ;1996.
- Allen F. Linking water and nutrients through the vadose zone: a fungal interface between the soil and plant systems. J.Arid Land; 2011; 3:155-163.
CrossRef - Balestrini R., Lumini , Borriello R., Bianciotto, V. “Plant-soil biotainteractions,” in Soil Microbiology, Ecology and Biochemistry, ed E.A. Paul (London: Academic Press; Elsevier); 2015: 311-338.
CrossRef - Auge M. Water relations, drought and vesicular-arbuscular mycorrhizal symbiosis. Mycorrhiza; 2001;11: 3-42.
CrossRef - Gohre V., and Paszkowski U. Contribution of the arbuscular mycorrhizal symbiosis to heavy metal Planta; 2006; 223: 1115-1122.
CrossRef - Gonzalez-Guerrero , Azcon-Aguilar C., Mooney M., Valderas A., MacDiarmid C.W., Eide D.J. Characterization of a Glomus intraradices gene encoding a putative Zn transporter of the cation diffusion facilitator family. Fungal Genet. Biol; 2005; 42: 130-140.
CrossRef - Pearson J.N., Jakobsen The relative contribution of hyphae and roots to phosphorous uptake by arbuscular mycorrhizal plants, measured by dual labelling with 32P and 33P. New Phytol; 1993;124: 489-494.
CrossRef - Smith S.E., Smith F.A., Jakobsen I. Mycorrhizal fungi can dominate phosphate supply to plants irrespective of growth Plant Physiol; 2003; 133:16-20.
CrossRef - Smith S.E., Smith F.A., Jakobsen I. Functional diversity in arbuscular mycorrhizal (AM) symbioses: the contribution of the mycorrhizal P uptake pathway is not correlated with mycorrhizal responses in growth or total P New Phytol; 2004; 162: 511-524.
CrossRef - Rillig M.C., Mummey D.L. Mycorrhizas and soil structure. New Phytol; 2006; 171: 41-53.
CrossRef - Bender S.F., Plantenga F., Neftel A., Jocher M., Oberholzer H.R., Kolh L. Symbiotic relationships between soil fungi and plants reduce N2O emissions from soil. ISME J; 2014; 8:1336-1345.
CrossRef - Berruti A., Lumini E., Balestrini R., Bianciotto V. Arbuscular Mycorrhizal Fungi as Natural Biofertilizers: Let’s Benefit from Past Front. Microbiol; 2016; 6:1559.
CrossRef - Catherine N., Jacott J.D., Christopher J.R. Positive effects of arbuscular mycorrhizal (AM) colonization ; 2017.
- Hermosa , Viterbo A., Chet I., Monte E. Plant-beneficial effects of Trichoderma and of its genes. Microbiology; 2012; 158:17–25.
- Brotman Y., Landau , Cuadros-Inostroza A., Takayuki T., Fernie A.R., Chet I., Viterbo A., Willmitzer L. Trichoderma plant root colonization: escaping early plant defense responses and activation of the antioxidant machinery for saline stress tolerance. PLoS Pathog; 2013; 9(3): e1003221.
CrossRef - Atanasova , Le Crom S., Gruber S., Coulpier F., Seidl-Seiboth V., Kubicek C.P., Druzhinina I.S. Comparative transcriptomics reveals different strategies of Trichoderma mycoparasitism. BMC Genomics; 2013; 14:121
CrossRef - Harman E., Herrera-Estrella A.H., Horwitz B.A., Lorito M. Special issue: Trichoderma–from basic biology to biotechnology. Microbiology; 2012; 158:1–2.
CrossRef - Martinez-Medina , Pozo M.J., Cammue B.P., Vos C.M. Below ground defence strategies in plants: the plant– Trichoderma dialogue. In: Vos C, Kazan K (eds) Belowground defense strategies in plants. Springer International Publishing, Cham; 2016a: 301–327.
CrossRef - Contreras-Cornejo H.A., Ortiz-Castro R., Lopez-Bucio J. Promotion of plant growth and the induction of systemic defence by Trichoderma: physiology, genetics and gene In: Mukherjee P, Horwitz B.A, Singh U.S, Mukherjee M, Schmoll M (eds) Trichoderma: biology and applications. CAB International, Wallingford, 2013: 175–196.
CrossRef - Oancea , Raut I., Şesan T.E., Cornea P.C. Dry flowable formulation of biostimulants Trichoderma strains. Agric Agric Sci Procedia; 2016;10: 494–502.
CrossRef - Isahak A., Doni F., Che Radziah C.M.Z., Wan Shiqin W.M., Wan Mohtar W.Y., Asmat A. In: Plant biodiversity-based research innovation and business opportunities (II). BioBiz Innovation Research Group, University Kebangsaan Malaysia, Bangi; 2014: 90–104.
- Benitez , Rincon A., Carmen L.M., Codon A.C. Biocontrol mechanisms of Trichoderma strains. Intl Microbiol; 2004; 7: 249-260.
- Ranveer K.K., Victor , Yogendra S.G., Vivek K. Trichoderma: a Most Common biofertilizer with Multiple Roles in Agriculture. Biomed J Sci and Tech Res; 2018; 4(5).
CrossRef - Pisciotta M., Zou Y., Baskakov I.V. Light-dependent electrogenic activity of cyanobacteria, PloS One; 2010; 5(5): e10821.
CrossRef - Pang K.E., Tang Q., Chen L., Wan B., Changtai N., Xunlai Y., Shuhai X. Nitrogen fixing heterocystous Cyanobacteria in the Tonian Current Biology; 2018.
CrossRef - Mishra , Pabbi S. Cyanobacteria: A Potential biofertilizer for rice. Resonance; 2004;9: 6-10.
CrossRef - Subramanian , Uma L. Cyanobacteria in pollution control. J.Sci.Ind.Res; 1996; 55:685-692.
- Paumann M., Regelsberger , Obinger C., Peschek G. The bioenergetic role of dioxygen and the terminal oxidase in cyanobacteria. Biochim. Biophys Acta Bioenerg; 2005.
CrossRef - Prasad C., Prasad B.N. Cyanbacteria as a source biofertilizer for sustainable agriculture in Nepal. J.Plant Sci. Bot. Orient. 2001;1:127-133.
- Aken V. A blue-green algae species – Cylindrospermum sp. Science Image; 1993.
- Chittora D, Meena , Barupal T., Swapnil P., Sharma K. Cyanobacteria as a source of biofertilizers for sustainable agriculture. Biochemistry and Biophysics Reports; 2020; 22; 100737.
CrossRef - Peters G.A. Studies on Azolla-Anabaena azollae Pages 592-610 in W.E . Newton and C.J. Nyman(ed.), Proceedings of the First International Symposium on Nitrogen Fixation. Vol. 2. Washington State University Press, Pullman; 1976.
- Hove V.C. Azolla and its multiple uses with emphasis on Africa. Food and Agriculture Organization, Rome; 1989.
- Madhusoodanan V., Sevichan P.J. Azolla microphylla Kaulfuss: An economically important biofertilizer for paddy fields of Kerala. Journal of Economic and Taxonomic Botany; 1992;16: 73-76.
- Wagner M. Azolla – A review of its biology and utilization. Bot. Rev; 1997; 63:1-26.
CrossRef - Carrapico Azolla as a Superorganism. Its implication in Symbiotic Studies; 2010.
CrossRef - Barman M., Paul S., Choudhary A.G., Roy P., Sen J. Biofertilizer as Prospective Input for Sustainable Agriculture in Int.J.Curr.Microbiol.App.Sci; 2017; 6(11): 1177-1186.
CrossRef - Sayyed Z., Reddy M.S., Deshmukh A.M., Gangurde N., Patel P.R., Yellareddygari S., Kumar K.V. Potential of Plant Growth Promoting Rhizobacteria for Sustainable Agriculture. Bacteria in Agrobiology: Plant Probiotics; 2012:287-313.
CrossRef

