Introduction
Citrus economic value has risen dramatically in recent years. Its prominence stems from its demand in the processed juice market, as well as its application in medicine and the pharmaceutical industry. The presence of a diverse variety of secondary metabolites and essential bioactive compounds has eventually widened the scope of its production. According to the Food and Agricultural Organisation of the United Nations (FAO), 143755.6 thousand tonnes of citrus were produced worldwide in 2019. Asia is leading the market with production of over 28920.0 thousand tonnes of citrus fruits, followed by South American countries with an expected output of 20189.8 thousand tonnes. 1.
Citrus production still needs to be improved to meet the present and future demands around the world. Unfortunately, certain environmental and biological constraints are posing a threat to citrus cultivation. Citrus crops are plagued with viral, bacterial, and fungal diseases. These natural agents have become a severe headache for farmers as their management is strenuous and can be expensive in the worst-case scenario. Bacterial infections such as Citrus bacterial spot (Xanthomonas euvesicatoria), Citrus greening (Candidatus liberibacter), Citrus Black pit (Pseudomonas syringae), Citrus canker (Xanthomonas spp.) are some of the economically significant diseases. Among all of them, citrus canker caused by Xanthomonas axonopodis is known to be the most feared one. It is endemic to Asian and South-East Asian countries and infects various citrus species 2. Citrus paradisi (Grapefruit), Citrus limon (Lemons), Citrus aurantifolia (Mexican lime) are all particularly susceptible to Citrus canker disease. When conditions are ideal for bacterial growth and development, infection causes lesions on the leaves, fruits and stems, as well as defoliation and fruit drop 3.
Although Citrus canker is associated mostly with the genus citrus, it has managed to infect many plants belonging to the family Rutaceae. Eradication and management of the disease rely on quarantining the healthy plants from the infected ones and planting disease-resistant varieties of crops. These techniques are helpful during initial exposure of the bacteria but are ineffective in the long run. Copper based bactericides are extensively used throughout the world as an effective technique to minimise infection 4. Copper treatment reduces canker formation in leaves, fruits and increases yield by reducing premature loss of fruits. However, the excessive use of copper containing chemicals to treat diseases has created a new problem. At higher concentrations, copper is severely toxic to living organisms. Moreover, copper resistant microbes isolated from treated areas show higher resistance to multiple antibiotics 5.
With recent technological advancements, numerous alternative techniques for reducing the use of copper as an antibacterial agent have been developed. Pesticide containing zinc provide comparable protection against canker even at lower metal concentration 6. In field trials, nano-formulated zinc oxides have demonstrated superior performance to cuprous oxide 7. Additionally, it has been discovered that hexanoic acid has low phytotoxicity and can effectively suppress the growth of Xanthomonas 8. Besides using chemical toxins to regulate the growth and transmission of bacterial diseases in plants, many bio-formulations have been developed that can effectively deal with such problems. Bio control agents generally include microbial antagonists that has the ability to control plant pathogens either directly by producing metabolites or indirectly by eliciting plant defence response. Compared to the chemical bactericides used in the field, bio-control agents are more suitable because they are non-toxic by nature 9. The detection of the canker is the first step in the overall process, followed by the application of biocontrol agents to various plant part. The current review will discuss about the bio-control agents used in the treatment of canker in Citrus plants.
Symptoms and Mode of Transmission
Citrus canker is highly prevalent in areas with warm temperatures and heavy rainfall. Symptoms can be generally observed in leaves, fruits and stems. At first, tiny blister like lesions can be visible, but as the infection progresses, 2 to 10 mm in diameter lesions start appearing within 7 to 10 days of initial infection 3. Lesion in the leaf is visible from both sides. Young lesions appear yellow in colour, but as they progress, they become brown and corky. Yellow or brown blister-like lesions form on stems and twigs, becoming elevated and spongy over time. These spongy pustules grow into brown corky cankers and gradually darken and thicken. Under wet conditions, the lesion generally has a water-soaked appearance 2.
Transmission of citrus canker occurs mostly through wind and rain. Heavy wind or rain can transmit the disease to nearby plants. After exposure, the bacterium can easily penetrate the plant through stomatal openings and wounds created by unfavourable environmental circumstances. Spread of the disease over long distances is also observed during storms, hurricanes and tornadoes 4. Human activities such as transportation of infected fruit, leaves or twigs have resulted in the transmission of the disease to uninfected areas.
Effective Biocontrol Agents
Bacillus spp.
The genus Bacillus is included in the group of bacteria that encourage Plant Growth (PGPB). They are known for producing a wide range of secondary metabolites that prevent infections caused by numerous bacterial and fungal strains. Bacillus strains such as Bacillus subtilis, B. amyloliquifaciens, B. licheniformes and B. velezensis have been extensively used by researchers to treat variety of plant diseases. Against canker-causing Xanthomonas, Bacillus subtilis, Bacillus velezensis and Bacillus amyloliquifaciens are the strains that have been widely used as a biocontrol agent.
Bacillus amyloliquefaciens for example have shown efficient suppression of canker when tested in navel orange leaves. The frequency and size of canker lesions decreased by about 77% in B. amyloliquefaciens– treated leaves compared to the untreated one 10. Another study conducted by Daungfu 11 also reported the effectiveness of crude and cell suspension extract of B. amyloliquefaciens in controlling canker in Citrus aurantifolia leaves. The study suggested that treatment with B. amyloliquefaciens can completely control the disease incidence on lime plants in greenhouse condition. Additionally, because Bacillus can create endospores, it can endure extreme environments for an extended period of time.
Many locations where streptomycin is continuously applied to treat canker have produced isolated strains of Xcc that are resistant to the antibiotics. Streptomycin works by preventing bacterial ribosomal subunit from doing their job. However, it has been demonstrated that point mutations in the bacterial strB gene and rpsL gene change the ribosomal proteins normal structure, conferring resistance to antibiotic streptomycin 12. Nurul Islam13 studied the impact of endophytic bacteria against wild and streptomycin resistant Xanthomonas strains. Two isolated bacteria TbL-22 and TbL-26 later found to be Bacillus thuringiensis inhibited both wild and streptomycin resistant strains of Xanthomonas. Ethyl acetate extract of Tbl-22 inhibited XccW3 (wild) and XccM6 (streptomycin resistant) strains with the highest zone of inhibition of 20.64± 0.96 and 19.91± 0.87 mm, respectively.
Potential of Bacillus thuringiensis in controlling canker has not been utilised to its fullest extent. In one study a microbial bioformulation composed of Pseudomonas, Bacillus thuringiensis, Bauveria and Trichoderma were applied on canker incidence. The bio formulation was found to be effective in field trials, although such trials cannot determine the precise part played by B. thuringiensis in suppressing the canker 14.
Bacillus velezensis, another endophytic bacterium from the genus Bacillus, has demonstrated antimicrobial activity against both wild and streptomycin resistant Xcc strains. The MIC for streptomycin resistant (33.88 ±1.3mm) Xcc was found to be greater than that for wild strains (29.28±0.6 mm). The Xcc cell was distorted and lysed by the endophytic bacteria, thanks to the secretion of certain unknown antimicrobial chemicals 15.
Despite the fact that copper-based bactericides are no longer favoured due to the emergence of copper-resistant microbes 5. A number of recent studies have demonstrated the efficacy of using copper and microbes together to treat canker. Experiments have shown that combining B.velezensis cell free extract with 0.01% CuCl2 provide better results in controlling citrus canker 16.
Combination of Bacillus subtilis and copper to treat canker has also been reported. YE Ibrahim 17 reported decrease in Xanthomonas infection in citrus seedlings when treated with a formulation of Serenade® MAX containing 107 cells/ml-1 Bacillus subtilis QST 713. Combination of copper with Serenade® MAX showed 87.79% reduction in disease compared to 85.55% against Copper. Bioactive copper has the ability to induce SAR (Systemic acquired resistance) in plants by activating genes that encode the production of β-1,3 glucanase proteins, making it a preferred treatment option for Canker when combined with Bacillus subtilis 18.
Even in direct in-vivo field trials, administration of Bacillus subtilis aqueous suspension (2.7× 109 cells/ml) on Citrus aurantifolia trees demonstrated a significant reduction in the presence of canker in the leaves. The quick colonisation of the leaf area within 20 days of spraying leaves little chance for the Xcc to induce infection in the plant 19.
Pseudomonas spp
Pseudomonas species are gram negative rhizospheric bacteria that are well known for assisting plant growth and development. Pseudomonas is frequently used as a biocontrol agent throughout the world (Table 1). As an opponent to chemotherapy, different strains of Pseudomonas have already been used to kill plant pathogenic bacteria such as Phytopthora infestans, Rhizoctonia solani, Botyris cinerea and Ralstonia solanacearum 20, 21, 22, 23. Additionally, Pseudomonas increases plants ability to withstand biotic and abiotic stress and controls plants growth by producing Indole acetic acid, Hydrocyanic acid and siderophore 21. These attributes of Pseudomonas make it a perfect choice to be used as a biocontrol agent against various plant diseases. Numerous earlier investigations have supported Pseudomonas spp. effectiveness against Xanthomonas strains. Ota 24 observed the potential antagonistic activity of Pseudomonas against citrus canker bacterium Xanthomonas campestris pv. citri both in-vivo and in-vitro. Two inhibitory substance CLP-3 and CLP-5 later isolated by TLC from the same experiment were assumed to be Phytoalexins 25. When tested by the Agar plug method Pseudomonas fluoresecence successfully inhibited the growth of Xanthomonas in-vitro. Out of the four species of bacteria, the second largest zone of inhibition (14.77 mm) was observed for P. fluorescens 26.
Table 1: Microbes used as Bio-control agents against Citrus Canker.
| Sl. No | GENUS | SPECIES | CONDITION | PLANT | XANTHOMONAS
strain |
REFERANCE |
| 1. | Bacillus | Bacillus amyloliquefaciens QC-Y | In-vitro | Navel orange leaves | Xac | Qian et al. 2021 |
| Bacillus amyloliquefaciens LE109 | Greenhouse | Citrus aurantifolia leaves | Xcc | Daungfu et al. 2019 | ||
| Bacillus thuringiensis TbL-22,TbL-26 | In-vitro, MIC | Citrus spp. | Xccstr | Islam et al. 2019 | ||
| Bacillus velezensis | Greenhouse, MIC | Citrus spp. | Xccstr | Rabbee et al. 2019 | ||
| Bacillus velezensis | In-Vitro | Citrus aurantifolia seeding and tree | Xcc | Sudyong et al. 2019 | ||
| Bacillus subtilis | In-vivo | Citrus aurantifolia | Xcc | Das et al. 2014 | ||
| 2. | Pseudomonas | Pseudomonas spp | In-vitro/ in-vivo | Citrus spp. leaves | Xcc* | Ota, 1983a |
| Pseudomonas fluorescence | In-vitro/ in-vivo | Citrus limon
Citrus aurantifolia |
Xcc* | Kalita et al. 1996;
Patel et al. 2020 |
||
| Pseudomonas protegens CS1 | In-vitro/in vivo | Citrus limon leaves | Xcc | Michavila et al. 2017 | ||
| Pseudomonas aeuroginosa | Rajesh et al. 2015 | |||||
| Pseudomonas entomophila | In-vivo | Citrus limon | Xcc | Villamizar et al. 2020 | ||
| Pseudomonas putida, P.fluorescence | In-vitro | – | Xac | Badiger et al. 2016 | ||
| Pseudomonas geniculata | In-vivo | Duncan grapefruit | Xcc | Riera et al. 2018 | ||
| 3. | Kosakonia | Kosakonia cowanii GN223 | In-vivo | Naval orange | Xcc | Jiahao et al. 2021 |
| 4. | Staphylococcus | Staphylococcus pasteuri, S. warnei | In-vitro | Rangpur lime | Xcc | Nugroho et al. 2022 |
| 5. | Burkholderia | Burkholderia territorri A63, B. metallica A53 | In-vivo | Duncan grapefruit | Xcc | Riera et al. 2018 |
| 6. | Bacteriophage | Bacteriophage spp | In-vivo/ Field trials | Citrus aurantifolia | Xccco | Ibrahim et al. 2017 |
| Podophage, Siphophage, T4 phage | In-vitro | Hamlin sweet orange | Xac | Le (2019) | ||
| Filamentous phage XacF1 | In-vitro | – | Xac | Ahmad et al. 2014 |
Recent discoveries of numerous novel Pseudomonas strains may offer fresh information in this area. The use of Pseudomonas protegens as a biocontrol agent against Xcc is relatively very new. This species CS1 strain generates the pyochelin enantiomer and ROS necessary to block Xcc in both in-vitro and in-vivo conditions 27. Pseudomonas entomophila, a powerful pesticide, inhibits Xcc by creating certain secondary metabolites. The species was found to cure canker completely in in-vivo conditions within 21 days of inoculation 28.
Rajesh 29 evaluated the antagonistic activity of six Pseudomonas spp. against Xanthomonas axonopodis pv. citri. P. aeruginosa Rambhas-2 (PaRS) showed the maximum zone of inhibition (18.67mm) followed by P. fluorescens Navs`ari-2 (PfNC) and P. aeruginosa Navsari-1 (PaNS). Least inhibition (9mm) was observed in the isolate P. fluorescens Rambhas-1 (PfRB). The antagonistic activity was assumed to be due to the production of secondary metabolites or cell wall degrading enzymes.
Recent in-vitro experiments, however, revealed that Pseudomonas fluorescens efficacy was much inferior to that of Bacillus subtilis. Badiger 30 noted that Bacillus subtilis had a substantially higher MIC against Xac (16.16mm) then did Pseudomonas fluorescens (14.63mm) and Pseudomonas putida (7.42mm).
In a different, opposing report, Pseudomonas fluorescens administration in an in-vivo setting resulted in decrease in disease spots on Citrus aurantifolia leaf and fruit. The outcome was found to be comparable to chemical canker control (streptomycin sulphate + copper oxychloride) 31. Application of Pseudomonas geniculata strain 95 in Duncan grapefruit root reduces Xanthomonas infection by increasing the expression of salicyclic acid genes such as PR1, PR2, PR5 and SAM-SCAM and reactive oxygen species in aerial tissues 32.
Other Endophytic Bacteria
A gram-negative endophytic bacterium called Kosakonia cowanii has recently been identified as the disease-causing agent in Soybean plants (Glycine max). Although, the presence of these bacteria in environment could be dangerous, it is also known that non-pathogenic strains of these bacteria exist in nature 33. The effectiveness of this bacterial species as a biocontrol agent is less known to the scientific community. When tested against Citrus canker on adult trees, the strain Kosakonia cowanii GN223 inhibited Xcc growth on seedlings and Navel orange by 40.0% and 50.15 respectively. The effectiveness of GN223 in reducing disease severity was found to be equivalent to copper hydroxide treatment 34. The endophytic bacteria Kosakonia cowanii strain GN223 inhibits citrus canker formation by inducing a host defensive enzyme. GN223 has been shown to enhance the activity of catalase (CAT) and peroxidase (POD), effect of which can significantly decrease the prevalence of Canker in plants 35.
Staphylococcus species, Staphylococcus pasteuri and Staphylococcus warnei are effective in preventing the formation of Xcc as revealed by in-vitro studies and in-vivo studies as well. The bacteria produce certain unknown secondary metabolites that are toxic to Xcc. This can be confirmed by cell free supernatant (CFS) treatment, which showed clear MIC of 7.23mm and 6.22mm against Xcc. The CS and CFS extracts of the bacterial strains also significantly decreased 50% of the canker infection in leaves within 28 days of inoculation 36.
When sprayed to the roots, two novel rhizobacterial strain Burkholderia territorri and Burkholderia metallica enhances plant defence response against Citrus canker 32. Due to their innate tendency to induce infections like Cystic fibrosis and pneumonia in immuno-compromised humans, the usage of these bacterial strains are restricted 37.
Bacteriophage Virus
Bacteriophage viruses are natural bacteria killer. In a number of investigations, the bacteriophage has been shown to be effective in managing variety of plant disease in addition to citrus canker. However, the main problem in utilising bacteriophage is their inability to survive long enough in plant surface mostly because of their low tolerance level in UV light. However, it has been demonstrated that microencapsulated bacteriophages exhibit greater tolerance to changing pH, UV and temperature conditions 38. Formulating the bacteriophage with Riboflavin, ascorbic acid or skimmed milk drastically reduces the effect of UV light on the virus 39. Formulated bacteriophages have been used extensively to treat Citrus canker with great success. Administration of Bacteriophage in combination with acibenzolar-S-methyl (ASM) reduced the incidence of Asiatic Citrus canker (ACC) on leaves from 75.2% to 18.3% in greenhouse condition. Field trials also showed similar results. Application of formulated phage (to protect them from environmental damage) with ASM showed higher inhibition of Citrus canker by 82-86% 40. However, in a contrasting report published by Balogh 41 it was shown that formulation of phage (with skim milk) compromised the effectiveness in treating Citrus canker. When phages are administered without skim milk, they significantly reduce disease severity.
A growing trend is phage therapy, which involves using the bacteriophage virus to treat bacterial diseases. Bacteriophages are now a more effective treatment option for citrus canker than chemotheraputic methods thanks to successful in-vitro studies of phages against the condition 42. In his research Le 43, isolated three bacteriophage that were successful in preventing canker. KMV-Like podophages, siphophage and T4 like phage were identified as the isolated phages. Phage cocktail comprising Podophage and siphophage when applied on Hamlin sweet orange leaves showed reduction in canker lesion. According to the in-vitro research, pre-treating leaves with Phage cocktail yielded greater result than post infection therapy, with canker reduction rates of 52.7% and 47.4% respectively.
Filamentous phage XacF1 inhibits canker expansion by drastically reducing extracellular polysaccharide production in host cells. Reduction in motility of XacF1-infected host bacterial cells was also observed 44.
Plant based Extracts/oils
People utilise plant extracts and oils extensively because of their medicinal and flavouring benefits. The extracts/ oils of root, leaf, stem, flower and other parts of the plants contain a variety of bioactive compounds such as phenols, terpenes, alkaloids etc 45. The presence of these bioactive compounds has revealed the antibacterial, anti-pesticidal and anti-insecticidal potential of plant-based product 46 47 48. The use of these plant-based solution is more appropriate since, unlike bacterial or fungal bio-control agents, they have no known negative effects on people or the plant itself.
Both neem (Azardiricta indica) extract and neem oil have numerous uses. The existence of various secondary metabolites in neem extracts can be used to explain their appeal as an antibacterial agent 49. In-vitro tests have suggested that, both aqueous and alcoholic neem leaf extract have potential to be used against various strains of Xanthomonas that cause citrus canker 50 51. When applied externally to plant infected with Xanthomonas, the oil extracted from neem significantly reduced canker incidence, demonstrating its in-vivo anti canker potential 52. Streptomycin combined with A.indica also effectively combats citrus canker in green house conditions. Reduction of disease can be observed within 45 days of administration 53.
Both in-vitro and in-vivo studies on the treatment of canker with alcoholic and aqueous extract of Allium cepa showed a reduction in canker incidence. 50 54. In comparison to numerous chemical alternatives, like streptomycin, copper oxychloride and validamycin, onion extract is found to be superior. Interestingly, applying onion extracts to fruit and leaf surface produces superior outcomes for treating canker than do Bacillus species 52.
Except neem oil as discussed above, oil extracted from Ginger (Zingiber officinale), Common yarrow (Achillea millefolium), Common sage (Saliva officinales), Summer savory (Satureja hortensis) and True cardamom (Elettaria cardamomum) are equally effective against Xcc pathotype A* under greenhouse and laboratory conditions 55. Essential oil extracted from Citrus aurantium and C. aurantifolia also inhibit canker by damaging cell wall of Xcc. The presence of secondary metabolites such as α-terpineol, citronellal, geraniol and linalool may be the cause behind its antibacterial effect 56. Clove essential oil can be recommended as an alternative sanitisation product for decontamination of citrus fruits. The anti-bactericidal effect of clove oil against Xcc can be explained by the presence of high concentration of eugenol in it 57.
Mechanism of Action of Biocontrol Agents Against Xanthomonas
Bacillus and Pseudomonas are regarded as plant rhizospheric bacteria. They are known to secret a variety of metabolites and substances that either directly or indirectly aid in plant growth as shown in Fig.1. Both species of bacteria produces Siderophores, IAA, lytic enzymes, organic acid, oxalate oxidase and Hydrocyanic acid 21 58 16. Particularly crucial are the siderophores made by rhizospheric bacteria because they aid in chelating Fe3+ from environment and make it available to plant cells, rendering them inaccessible to pathogenic bacteria 44. Pseudomonas protegens produces a major siderophore called pyochelin enetiomer and also ROS in plants infected with Xcc 59. Bacillus velezensis produces high amount of Siderophore and IAA against Xcc in-vitro 16.
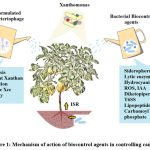 |
Figure 1: Mechanism of action of biocontrol agents in controlling canker. |
Note: Xac: Xanthomonas axonopodis subp. citri., Xcc: Xanthomonas citri subp. citri., Xcc*: Xanthomonas campestris subp. citri., Xccstr: Streptomycin resistant Xanthomonas citri subp. citri., XccCo: Copper resistant Xanthomonas citri subp. citri.
Bacillus and Pseudomonas also increases ISR (Induced systemic resistance) in plants infected with Xanthomonas (Fig.1). As discussed earlier, exposure of plant with Xcc induces P. geniculata strain to increases expression of genes such as PR1, PR2, PR5, SAM-SCAM and Phenylalanine ammonia lyase 1 which are related to Salicylic acid signalling pathway (Riera et al. 2018). Pseudomonas spp. also produces different antimicrobial compounds against Bacteria. One such example is the production of diketopiperezine and T6SS (Type 6 secretion system) by P. entomophila 28. The T6SS is an essential tool of gram-negative bacteria to deliver toxic compounds in host cell and subsequently plays important role in inter-bacterial competition in environment 60.
In addition to ISR, Bacillus also produces lipopetides such as surfactins, iturin and fengycin. Lipopeptides mode of action against bacteria includes cell wall disruption and pore formation. These lipopeptides can further be classified into a number of sub classes, and each has a unique mode of action against variety of microbes 61 11. Bacillus amyloliquefaciens for example produces Iturin A/ Mycosubtilin, Iturin B, Surfactin A/B and Fengycin A/B against Xanthomonas spp.62. Bacillus velezensis show antagonistic activity against Xanthomonas strains by releasing four antimicrobial compound bacillibactin, fengycin, surfactin and bacillomycin D 63.
Inhibition of Xanthomonas quorum sensing by bacillus and pseudomonas species have also been reported recently. Quorum sensing is mediated by DSF (diffusible signal factor), which is released by Xcc and encoded by the rpf gene cluster. The bacteria need this cell-cell signalling pathway in order to be virulent. Bacillus and Pseudomonas species contains genes homologous to carA and carB that encode DSF degrading enzyme carbamoylphosphate synthase, which allows them to successfully inhibit Xcc Quorum sensing 64.
The mechanism of action of Virus on the other hand is different from the techniques we have discussed earlier. After infecting bacteria, a virus passes through either a lytic or lysogenic cycle depending upon environmental condition. To infect virus uses receptors preset on Gram negative and gram-positive bacteria. Transmembrane protein OmpA, Porins such as OmpC /OmpF, pili, flagella and lypopolysaccharide serve as receptor for the attachment of bacteriophage virus in bacteria 65. Against Xanthomonas spp. Bacteriophage FoX2 and FoX6 recognise specific lipopolysaccharide present on the surface of the bacterial cells wall 66. In lytic cycle, the bacteriophage initially adheres to the surface of the host bacteria before injecting its genetic material into the cell. Virus after producing new virions by hijacking host cells replicative machinery, brusts out to release the new virions. In lysogenic cycle, the genetic material of bacteriophage after insertion into host cell gets integrated with the host cell chromosome. The bacterial cell reproduces normally along with phage genetic material.
The production of antimicrobial peptide is a quality of bacteria but not virus. Since virus contains no known mechanism to produce proteins by itself so such findings are very rare. However, in one recent study, a novel Xanthomonas citri infecting jumbo virus XacN1 has been isolated which encodes some special lytic enzymes such as lipases, chitinase, cell wall hydrolase and M23 family peptidase (ORF118, ORF322, ORF423 and ORF322) that break slimy polysaccharide matrix (xanthan gum) produced by the bacteria 67. The Filamentous phage XacF1 similarly aims to prevent Xanthan formation. The bacterium needs Xanthan to endure biotic and abiotic stress. Inability to produce xanthan by Xac leads to the cessation of Citrus canker 68.
Acknowledgement
Department of Applied Biology, School of Biological Sciences, University of Science and Technology, Meghalaya, Baridua-793101, India
Conflitct of Interest
Authors have no Conflict of Interest
References
- Citrus Fruit Fresh and Processed Statistical Bulletin 2020. https://www.fao.org/markets-and-trade/commodities/citrus/en/.
- Das, A.K. (2003). Citrus canker – A review. J. Appl. Hort. 5(1):52-60.
CrossRef - Thakre, B., Soni, U., Gour, C.L., Vishwakarma, R., & Jashwani, N. (2017). Field identification, Eradication and Current Management of Citrus canker caused by Xanthomonas campestris citri in Satpura Platun of Madhya Pradesh, India. Plant Archives. 17(1): 371-374.
- Gottwald, T.R. (2002): Citrus Canker: The Pathogen and Its Impact. Plant health progress. 3(1): 15. Doi:10.1094/PHP-2002-0812-01-RV
CrossRef - Behlau, F., Gochez, A.M., Jones, J.B. (2020). Diversity and copper resistance of Xanthomonas affecting citrus. Tropical Plant Pathology. 45(3): 200-212. https://doi.org/10.1007/s40858-020-00340-1
CrossRef - Maxwell, T.J., Rajasekaran, P., Das, S., Campos, M., Young, M.I., Mendis, H., Ozcan, A., Gerberich, K.M., Myers, M.E., Graham, J.H., Johnson, E.G., and Santra, S., (2019). Control of Citrus Canker in Greenhouse and Field with a Zinc, Urea and Peroxide Ternary Solution. J. Agric. Food Chem. 67(45): 12393-12401. DOI: 10.1021/acs.jafc.9b05108
CrossRef - Graham, J. H., Johnson, E. G., Myers. E., Young, M., Rajasekaran, P., Das. S., Santra, S., (2016). Potential of Nano-Formulated Zinc Oxide for Control of Citrus canker on Grapefruit trees. Plant Dis. 100: 2442-2447.
CrossRef - Caccalano, M.N., Dilarri, G., Zamuner, C.F.C., Domingues, D.S., Ferreira, H. (2021). Hexanoic acid: a new potential substitute for copper-based agrochemicals against citrus canker. J Appl Microbiol. 131 (5): 2488-2499. Doi: 10.1111/jam.15125.
CrossRef - Bora, P., Saikia, K., Hazarika, H. and Ragesh, G. (2019). Exploring potential of bacterial endophydes in disease management of horticultural crops. Current Horticulture 7(2): 32–37.
CrossRef - Qian, J., Zhang, T., Tang, S., Zhou, L., Li, K., Fu, X., Yu, S. (2021). Biocontrol of citrus canker with endophyte Bacillus amyloliquefaciens QC-Y. Plant Protection Science, 57 (1): 1–13
CrossRef - Daungfu, O., Youpensuk, S., Lumyong, S., (2019). Endophytic Bacteria Isolated from Citrus Plants for Biological Control of Citrus Canker in Lime Plants. Tropical Life Sciences Research, 30(1), 73–88.
CrossRef - Hyun, J., Kim, H., Yi, P., Hwang, R., Park, E. (2012). Mode of Action of Streptomycin Resistance in the Citrus Canker Pathogen (Xanthomonas smithii citri) in Jeju Island. Plant Pathol. J. 28(2): 207-211. http://dx.doi.org/10.5423/PPJ.OA.08.2011.0159.
CrossRef - Nurul Islam, M., Ali, M.S., Choi, S.J., Hyun, J.W., and Baek, K.H. (2019). Biocontrol of Citrus Canker Disease Caused by Xanthomonas citri citri using Endophytic Bacillus thuringiensis. Plant Pathol. J. 35(5): 486-497. https://doi.org/10.5423/PPJ.OA.03.2019.0060
CrossRef - Saikia, S., Bora, P., Bora, L.C., (2021). Bioagent mediated management of citrus canker. Indian Journal of Agricultural Sciences 91 (2): 198–201.
CrossRef - Rabbee, M.F., Ali, M.S., Baek, K. (2019). Endophyte Bacillus velezensis Isolated from Citrus spp. Controls Streptomycin- Resistant Xanthomonas citri citri That causes Citrus Bacterial Canker. Agronomy, 9: 470. Doi: 10.3390/agronomy 9080470.
CrossRef - Sudyoung, N., Tokuyama, S., Krajansang. S., Pringsulaka, O., Sarawaneeyaruk, S. (2019). Bacteria antagonists and their cell-free cultures efficiently supress canker disease in Citrus lime. Journal of Plant disease and Protection. 127:173-181.
CrossRef - Ibrahim, Y.E., Saleh, A.A., Komy, M.H., and Al-Saleh, M.A. (2016). Bacillus subtilis QST 713, copper hydroxide, and their tank mixes for control of bacterial citrus canker in Saudi Arabia. iocv_journalcitruspathology_30994. DOI 10.5070/C431030994
CrossRef - Ramos, Y.G., Duin, I.M., Lopes da silva, R.M., Leite Junior, R.P. (2022): Bioactive copper and Bacillus subtilis for the control and Resistance induction against Citrus canker in Sweet orange [Citrus sinenesis(L.) Osbeck] orchard establishment. Scientia Horticulturae. 303: 111238.
CrossRef - Das, R., Mondal, B., Mondal, P., Khatua, D.C., Mukherjee, N. (2014). Biological management of citrus canker on acid lime through Bacillus subtilis (S-12) in West Bengal, India. JBiopest 7:38-41.
- Vrieze, M., Germanier, F., Vuille, N., Weisskopf, L. (2018). Combining different Potato-Associated Pseudomonas Strains for Improved Biocontrol of Phytopthora infestans. Front.Microbiol, 9:2573. Doi: 10.3389/fmicb.2018.02573.
CrossRef - Pandey, S., & Gupta, S. (2020). Evaluation of Pseudomonas sp. for its multifarious plant growth promoting potential and its ability to alleviate biotic and abiotic stress in tomato (Solanum lycopersicum) Scientific report. 10:20951. https://doi.org/10.1038/s41598-020-77850-0
CrossRef - Wang, X., Zhou, X., Cai, Z., Guo, L., Chen, X., Chen, X., Liu, J., Feng, M., Qiu, Y., Zhang, Y., Wang, A. (2020). A Biocontrol Strain of Pseudomonas aeruginosa CQ-40 Promote Growth and Control Botrytis cinerea in Tomato. Pathogens, 10, 22. https://doi. org/10.3390/pathogens10010022
CrossRef - Mohammed, A.F., Oloyede, A.R., Odeseye, A.O. (2020). Biological control of bacterial wilt of tomato caused by Ralstonia solanacearum using Pseudomonas species isolated from the rhizosphere of tomato plants, Archives of Phytopathology and Plant Protection. 53(1-2): 1-16 DOI: 10.1080/03235408.2020.1715756.
CrossRef - T., (1983). Interactions in vitro and in vivo between Xanthomonas campestris pv. citri and Antagonistic Pseudomonas sp. Japanese journal of Phytopathology 49(3): 308-315.
- T., (1983). Production of Phytoalexin-like Substances in the Citrus Leaves Inoculated with a Bacterium. Ann. Phytopath. Soc. Japan 49: 676-682
- Kalita, P., Bora, L.C., Bhagabati, K.N. (1996). Phylloplane microflora of citrus and their role in management of Citrus canker. Indian phytopathology 49(3): 234-237.
- Michavila, G., Adler, C., De Gregorio, P.R., Lami, M.J., Caram Di Santo, M.C., Zenoff, A.M., Mirzaei-Najafgholi, H., Tarighi, S., Golmohammadi, M., Taheri, P., (2017). The Effect of Citrus Essential Oils and Their Constituents on Growth of Xanthomonas citri subsp. citri. Molecules. 22: 591. Doi:10.3390/molecules22040591
CrossRef - Villamizar, S., Ferro, J.A., Caicedo, J.C., and Alves, L.M.C. (2020). Bactericidal Effect of Entomopathogenic Bacterium Pseudomonas entomophila Against Xanthomonas citri Reduces Citrus Canker Disease Severity. Front. Microbiol. 11:1431. Doi: 10.3389/fmicb.2020.01431
CrossRef - Waghunde, R., Sabalapra, A. (2015). Morphological Characterisation and Antagonistic Effect of Native Pseudomonas Spp. Of south Gujrat. Journal of pure and Applied Microbiology. 9: 615-621.
- Badiger, N., Yenjerappa, S.T., Naik, M.N., Patil, M.B., Patil, M.G. (2016). Evaluation of antibiotics, antibacterial chemicals and bio-agents against citrus canker caused by Xanthomonas axonopodis citri (Hasse). International Journal of Plant Protection. DOI : 10.15740/HAS/IJPP/9.2/566-569.
CrossRef - Patel, R.A., Patel, B.K., Patel, M.V. (2020). Effectiveness of Pseudomonas fluorescens against bacterial canker Xanthomonas campestris citri in Acid lime cv. kagzi lime. International Journal of Recent Scientific Research, 11(5): 38453-38456. DOI: 10.24327/IJRSR.
CrossRef - Riera, N., Wang, H., Li, Y., Li, J., Stelinski, K.P., Wang, N. (2018). Induced systemic resistance against citrus canker disease by rhizobacteria. Phytopathology 108:1038-1045. http://dx.doi.org/10.1094/PHYTO-07-17-0244-R
CrossRef - Krawczyk, K., Borodynko-Filas, N. (2020). Kosakonia cowanii as the New Bacterial Pathogen Affecting Soybean (Glycine max ). Eur J Plant Pathol. 157:173–183.
CrossRef - Lai, J., Kuang, W., Liu, B., Song, S. (2021). Identification of endophytic bacterial strain GN223 and its effectiveness against citrus canker disease in navel orange under field conditions. Biocontrol Science and Technology. 32(1): 14-29. DOI: 10.1080/09583157.2021.1958302
CrossRef - Lai, J., Shuilin, S., Bing, L. (2020). Effects of three Endophytic bacteria with characteristics of Controlling citrus canker on the activity of several defensive enzymes in Navel Orange. Acta Agriculturae Zhejiangensis. 32 (11): 1994-2000
- Nugroho, Y.A., Suharjono, S., Widyaningsih, S. (2022). Biological control of citrus canker pathogen Xanthomonas citri citri using Rangpur lime endophytic bacteria. Egyptian Journal of Biological Pest Control 32:63 https://doi.org/10.1186/s41938-022-00561-3.
CrossRef - Lord, R., Jones, A.M., Horsley, A. (2020). Antibiotic treatment for Burkholderia cepacia complex in people with cystic fibrosis experiencing a pulmonary exacerbation. Cochrane Database of Systematic Reviews, Issue 4. Art. No.: CD009529. DOI: 10.1002/14651858.CD009529.pub4.
CrossRef - Ramirez, K., Cazarez-Montoya, C., Lopez-Moreno, H.S., Campo, N.C. (2018). Bacteriophage cocktail for biocontrol of Escherichia coli O157:H7: Stability and potential allergenicity study. PLoS ONE 13(5): e0195023.https://doi.org/ 10.1371/journal.pone.0195023.
CrossRef - Orynbayeva, A., Dzhalilova, F.S.U., Ignatov, A.N., (2020). Improved efficacy of formulated bacteriophage in control of black rot caused by Xanthomonas campestris campestris on cabbage seedlings. Archives of phytopathology and plant protection. 53(7-8): 379-393. DOI: 10.1080/03235408.2020.1745487
CrossRef - Ibrahim, Y.E., Saleh, A.A., Al-Saleh. M.A. (2017). Management of Asiatic citrus canker under field conditions in Saudi Arabia using bacteriophages and acibenzolar-S-methyl. Plant Disease. 101(5): 761-765. http://dx.doi.org/10.1094/PDIS-08-16-1213-RE.
CrossRef - Balogh, B., Canteros, B. I., Stall, R. E., and Jones, J. B. (2008). Control of citrus canker and citrus bacterial spot with bacteriophages. Plant Dis. 92:1048-1052.
CrossRef - Kajal, G., Sangita, A., Rajan, W. (2021). Isolation and study of bacteriophage of citrus canker causing bacterial pathogen. Int. J. of Life Sciences, 9 (2):175-180.
- Le, T.T.B. (2019). Bacteriophage: A potential treatment for Citrus canker. Thesis paper.
- Ahmed, E., Holmström, S.J.M. (2014). Siderophores in environmental research: roles and applications. Microbial Biotechnology7(3): 196–208. doi:10.1111/1751-7915.12117
CrossRef - Awuchi, C.G. (2019). The Biochemistry, Toxicology, and Uses of the Pharmacologically Active Phytochemicals: Alkaloids, Terpenes, Polyphenols, and Glycosides. J.Food Pharm.Sci. 7(3), 131-150
CrossRef - Srinivasana, P.V., Senthil-Nathana, S., Ponsankara, A., Thanigaivela, A., Edwina, E., Selin-Rania, S., Chellappandiana, M., Pradeepaa, V., Lija-Escalinea, J., Kalaivanib, K., Hunterc, W.B., Duraipandiyand, V., Al-Dhabid, N.A, (2017). Comparative analysis of mosquito (Diptera: Culicidae: Aedes aegypti Liston) responses to the insecticide Temephos and plant derived essential oil derived from Piper betle L. Ecotoxicology and Environmental Safety 139: 439–446
CrossRef - Abdelatti, Z.A.S., and Hartbauer, M., (2020). Plant oil mixtures as a novel botanical pesticide to control gregarious locusts. Journal of Pest Science. 93:341–353 https://doi.org/10.1007/s10340-019-01169-7
CrossRef - Ganie, S.A., Ghani, M.Y., Nissar, Q., Rehman, S. (2013). Bio efficacy of plant extracts and biocontrol agents against Alternaria solani. African Journal of Microbiology Research. 7(34): 4397-4402, 23. Doi: 10.5897/AJMR2013.5901.
- Loganathan, T., Barathinivas, A., Soorya, C., Balamurugan, S., Nagajothi, T.G., Ramya, S., Jayakumararaj, R., (2021). GCMS Profile of Bioactive Secondary Metabolites with Therapeutic Potential in the Ethanolic Leaf Extracts of Azadirachta indica: A Sacred Traditional Medicinal Plant of INDIA. Journal of Drug Delivery & Therapeutics; 11(4-S):119-126
CrossRef - Kharat, V.M., Bramhankar, S.B., Thakur, K.D., Isokar, S.S., Pillai, T., Dinkwar, G.T., (2020). Management of citrus canker bacterium by using botanicals and chemicals. International Journal of Chemical Studies. 8(2): 1878-1882
CrossRef - Tahir, H.A.S., Sahi, S.T., Habib, A., Haq, I.U., Ahmad, A., Ashraf, W., (2016). Evaluation OF Plant extracts as biocontrol agents against Xanthomonas axonopodis pv. citri the cause of Citrus canker. Pak. J. Phytopathol. 28 (01). 35-43.
- Rehman, M.A., Ali, S., Afzal, M.B.S., Khan, M.N., Ali, M., (2020a). Efficacy of Chemicals and Botanical Extracts to Control Citrus Canker on Kinnow in Sargodha Region. Journal of Innovative Sciences, 6(2): 101-107.
CrossRef - Atiq, M., Khan, M.A., Sahi, S.T., Ahmad, R., Younas, M., Shafiq, M., Ali, Y., (2018). Appraisal of plant extracts and streptomycin sulfate against citrus canker disease, Archives of Phytopathology and Plant Protection, 51:15-16, 824-833, DOI: 10.1080/03235408.2018.1500855
CrossRef - Rehman, M.A., Ali, S., Khan, M.N., Ahmad, S., Ali, M.A. (2020). Comparative Efficacy of different Antibiotics, Fungicides and Botanical extract for the control of Citrus canker of Kinnow mandarin. Pak. J. Phytopathol., Vol. 32 (02). 113-119
CrossRef - Rezaei, R. (2020). Effect of 8 essential oil on bacterial Citrus canker disease in Citrus. Plant Pathology Science. 9(1):30-39. DOI: 10.2982/PPS.9.1.30.
CrossRef - Mirzaei-Najafgholi, H., Tarighi, S., Golmohammadi, M., Taheri, P. (2017). The Effect of Citrus Essential Oils and Their Constituents on Growth of Xanthomonas citri subsp. citri. Molecules. 22: 591; doi:10.3390/molecules22040591
CrossRef - Battagin, T.S., Caccalano, M.N., Dilarri, G., Zamuner, C.F.G., Alleoni, N., Saldanha, L.L., Bacci Jr, M., Ferreira1, H. (2021). Syzygium aromaticum (Clove) essential oil: an alternative for the sanitization of citrus fruit in packinghouses. Journal of Food processing and Preservation.45(9): e15496.
CrossRef - Kumar, P., Dubey, R.C., Maheshwari, D.K., (2012). Bacillus strains isolated from rhizosphere showed plant growth promoting and antagonistic activity against phytopathogens. Microbiological Research 167 (2012) 493– 499.
CrossRef - Michavila, G., Adler, C., De Gregorio, P.R., Lami, M.J., Caram Di Santo, M.C., Zenoff, A.M.,
- Bernal, P., Allsopp, L.P., Filloux, A., Llamas, M.A. (2017). The Pseudomonas putida T6SS is a plant warden against phytopathogens. The ISME journal 11(4): 927-987.
CrossRef - Zhao, H., Shao, D., Jiang, C., Shi, J., Li, Q., Huang, Q., Rajoka, M.S.R., Yang, H., Jin, M. (2017). Biological activity of lipopeptides from Bacillus. Appl Microbiol Biotechnol. DOI 10.1007/s00253-017-8396-0.
CrossRef - Wang, X., Liang, L., Shao, H., Ye, X., Yang, X., Chen, X., Shi, Y., Zhang, L., Xu, L., Wang, J. (2022). Isolation of the Novel Strain Bacillus amyloliquefaciens F9 and Identification of Lipopeptide Extract Components Responsible for Activity against Xanthomonas citri subsp. citri. Plants. 11: 457. https://doi.org/ 10.3390/plants11030457.
CrossRef - Mácha, H., Marešová, H., Jurˇíková, T.; Švecová, M., Benada, O., Škríba, A., Baránek, M., Novotný, ˇC., Palyzová, A. (2021). Killing Effect of Bacillus velezensis FZB42 on a Xanthomonas campestris campestris (Xcc) Strain Newly Isolated from Cabbage Brassica oleracea Convar. capitata (L.): A Metabolomic Study. Microorganisms 9, 1410. https://doi.org/ 10.3390/microorganisms9071410.
CrossRef - Caicedoa, J.C., Villamizara, S., Ferroa, M.I.T., Kupperb, K.C., Ferroa, J.A. (2016). Bacteria from the citrus phylloplane can disrupt cell–cell signalling in Xanthomonas citri and reduce citrus canker disease severity. Plant Pathology 65, 782–791
CrossRef - Rakhuba, D.V., Kolomiets, E.I., Szwajcer dey, E., Novik, G.I. (2010). Bacteriophage Receptors, Mechanisms of Phage Adsorption and Penetration into Host Cell. Polish Journal of Microbiology 2010, Vol. 59, No3, 145–155
CrossRef - D., Fortuna, K.J., Moons, L., Broeckaert, N., Backer, L.E., Venneman, S., Rombouts, S., Lippens, L., Baeyen, S., Pollet, S., Noben, J., Oechslin, F., Vallino, M., Aertsen, A., Maes, M., Vaerenbergh, J.V., Lavigne1, R., Wagemans, J., (2022). The potential of bacteriophages to control Xanthomonas campestris pv. campestris at different stages of disease development. Microbial Biotechnology. 15(6). 1762–1782 doi:10.1111/1751-7915.14004
CrossRef - Yoshikawa, G., Askora, A., Blanc-Mathieu, R., Kawasaki, T., Li, Y., Nakano, M., Ogata, H., & Yamada, T. (2018). Xanthomonas citri jumbo phage XacN1 exhibits a wide host range and high complement of tRNA genes. Scientific Reports. 8: 4486 | DOI:10.1038/s41598-018-22239-3
CrossRef - Ahmad, A.A., Askora, A., Kawasaki, T., Fujie, M., and Yamada, T. (2014).
The filamentous phage XacF1 causes loss of virulence in Xanthomonas axonopodiscitri,the causative agent of citrus canker disease. Frontiers in Microbiology. Volume 5 | Article 321 | 2. doi: 10.3389/fmicb.2014.00321
CrossRef

