Introduction
The chickpea or chick pea (Cicer arietinum) is the most primitive crop that goes to the pea family. Chickpea has been cultivated in different parts of the world for so many years and is consumed as a dry pea or vegetable greens. Unlike fat and cholesterol chickpeas have a high amount of protein. Chickpeas are also containing different forms of carbohydrates, vitamins, minerals, and variant of fiber; hence it helps ease malnutrition and enhances human health.7 Chickpeas are one of the legumes that can grow on minimal moisture which offers farmers the chance to involve in multiple cropping, where it is cultivated around the termination of the showery season just next to the assembly main crop.
Chickpeas are one of the well-known crop types that are cultivated rotationally with cereals. This facilitates exhaustive and economic use of lands, predominantly in places where land insufficiency is observed. 4,7
Chickpeas have nodules in which atmospheric nitrogen is converted into ammonia; nitrogen which is essential for proper growth and productivity of the crop. The root nodules in legume plants are produced due to the symbiotic interaction of rhizobia with the host plant. Based on current literature, chickpea can produce as far as 140 kg of nitrogen per hectare from the atmosphere, this is a relatively huge amount of nitrogen when compared with other known legumes, consequently improving the fertility status of the soil for succeeding crops.4,7 In addition to nitrogen, the left-over chickpea also adds a considerable mass of organic matter to the soil that can sustain and upsurge soil health and fertility.4 This saves a huge amount of fertilizers cost and at the same time, it is environmentally friendly.
Chickpea has a very precise symbiotic association, with a distinctive set of rhizobia required for the establishment of nodules and nitrogen fixation. The absence of compatible strains and low population, symbiotically ineffective indigenous rhizobia bring difficulties in nodules formation.11 To shun insecurity about natural inoculation, legume seed ought to be inoculated every time. According to Romdhane et al. 2009.,15 chickpea yields can be enhanced by inoculation with competitive rhizobia.
Inoculation of chickpea seeds with appropriate and effective elite rhizobia inoculants in soils that lack symbiotically effective wild rhizobia is a very worthwhile practice for successful root nodulation and yield improvement of the crop.14 Inoculation raises soil nitrogen along with the upsurge in root and shoots nitrogen.1 Henceforth, this activity envisioned to assess in what way the inoculation of chickpea with selected rhizobial biofertilizers enhances its productivity in the study area.
Materials and Methods
Nodule sample collection, isolation, purification, and authentication test
Sample nodules were obtained from the main chickpea cultivating localities of Ethiopia in August 2016 and 2017. Isolation and purification of the isolates were also done at Holeta Agricultural Research Center Microbial Biotechnology Laboratory.
However, authentication of the isolates was completed at the National Agricultural Biotechnology Research Center greenhouse during the 2017 and 2018 growing seasons. Specific nodule collection points were geo-referenced using the UTM (Universal Transverse Mercator) coordinate system. Aseptic safety measures were worked out to circumvent the uncleanness of the samples pending they arrive at the microbiology laboratory.
Characteristics of the experimental site
The field trial was done on the Ginchi sub-station at the Dendi area of Ethiopia in the major cropping periods of 2019 and 2021, correspondingly. These trial spots were not inoculated with any form of inoculant for the past five years. The trial sites were located between 611213.30 Easting and 100200744.30 Nothing in the 6Z UTM Zone at an elevation of 2200 meters overhead of the water level. The experimental site is dominated by Vertisol having a characteristic of swelling and shrinking properties depending on moisture content. The usually cultivated crops in the trial site are teff, barley, and wheat. The typical minimal and maximal temperatures and precipitation of the trial sites are shown in Figure one below.
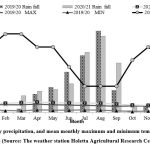 |
Figure 1: Monthly precipitation, and mean monthly maximum and minimum temperature patterns of the trial sites (Source: The weather station Holetta Agricultural Research Center, Ethiopia). |
Soil sample collection, examination, and experimental conditions
Merged soil representatives were gathered from random spots of the trial plots at a depth of 0-20 cm earlier in field preparation. Milled soil representatives were allowed to pass through a 2 mm mesh. Using standard analytical procedures, the soil samples’ chemical assets were done in the Holetta Agricultural Research Center soil chemistry laboratory (Table 1). Six highly effective indigenous chickpea rhizobial isolates, CP-16, CP-26, CP-28, CP-41, CP-17 (local standard check), and CP-100 were evaluated under field conditions at Dendi in contrast to the positive and negative controls. The trials were conducted using RCBD design per triple repetitions on a plot size of 12m2. The space between plots and blocks was enlarged to 0.5 and 1 m, respectively. The space between plants and rows was 10 and 30 cm, respectively. Each of the trial plots received a basal application of 20 kg P/ha from Triple Superphosphate during planting time. Arerti variety; planting material was used in the trial.
Inoculants preparation and use
Chickpea inoculant, which was prepared by mixing 20 ml broth culture with 100 g lignite carrier, was stirred well, transferred to the seed lot, and uniformly coated under the shade. Coated seeds were sown immediately after inoculation. All rhizobial isolates were evaluated for grain yield and shoot dry weight before and after harvesting.
Data assembly and examination
The uppermost chickpea yield-enhancing biofertilizers were determined based on analyzed agronomic, soil, and economic data that were collected from the Ginchi substation; Dendi districts of Ethiopia. The studied indicators of plant and soil were available phosphorus, organic carbon, total nitrogen, above-ground biomass yield (AGBY), Haulm yield (HY), grain yield (GY), and, soil pH. SAS statistical platform version 9.3 was used for analysis. The Least Significance Difference (LSD) at p= 5% was used to compare means. 16
Result and Discussion
Isolation, purification, and authentication of rhizobial isolates
In the isolation, purification, and authentication test overall 40 isolates were obtained from nodules that were collected from the central high lands of Ethiopia. Among these forty isolates, only 20 isolates (50%) of them passed the preliminary screening test. Among these 20 pure preliminarily screened rhizobial isolates only 10 (50%) of them successfully nodulate the host plant and passed the authentication test (Figure 2). However, among these 10 authenticated and symbiotically evaluated isolates only six of them; CP-16, CP-26, CP-28, CP-41, CP-17, and CP-100 were considered for the field trial at Dendi district based on their superiority in symbiotic effectiveness; isolates CP-16, CP-28 and CP-100 were effective(E), and isolates CP-26, CP-41 and CP-17 were highly effective (HE).
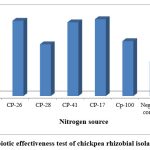 |
Figure 2: Symbiotic effectiveness test of chickpea rhizobial isolates on the sand. |
Soil analysis
As it is accessible in Table 1, the experimental sites have low total nitrogen in the soil. The mean soil pH of the test locations was 6.79, this means it is slightly acidic and idyllic for the cultivation of many field crops sown on fields. 17
The soil test result also displayed that the average available phosphorus (P) was above the critical levels (15.3 ppm). The phosphorus rating will be in the low ranges, which is sub-optimal for chickpea production which demands 14 kg ha-1 P2O5 in 1.5 tons of grain production. 3,10,17
Table 1: Major soil physicochemical properties of Vertisols of Ginchi.
| Parameter | Mean | Range | Test Methods |
| Total N (%) | 0.084 | 0.07-0.1 | Modified Kjeldhal11 |
| pH | 6.79 | 6.46-7.11 | 1:2.5 H2O |
| Available P (ppm) | 15.3 | 8.79-20.15 | Bray II |
| OC (%) | 1.13 | 0.78-1.91 | Walkley and Black17 |
The organic carbon content of the testing soil samples’ mean is 1.13%, this is rated as moderate and gives average structural condition and stability to the soil. 5
Response of chickpea to inoculants at Ginchi sub-station in 2019/20
Substantial statistical alterations (p ≤ 0.05) in AGBY and GY were observed among treatments at the Ginchi substation in 2019/20 (Table 2). Inoculant CP-41 showed significantly a higher AGBY than other treatments except for inoculant CP-16. Even though no substantial statistical alteration was detected amongst treatments except with the negative control, inoculant CP-100 showed a higher GY (2329.7 kg/ ha). Nevertheless, there were no substantial statistical alterations among treatments, inoculant CP-26 showed a higher HY. The higher GY score by inoculant CP-100 (2329.7 kg/ha) was 35% and 25% superior to the corresponding yields of the negative control (1630.2 kg/ha) and positive control (1806.6 kg/ha).
Table 2: Response of Chickpea to rhizobial inoculation in 2019/20 at Ginchi sub-station.
| Treatment | AGBY (kg/ha) | GY (kg/ha) | SDBM (kg/ha) |
| Negative control | 1078.1bc | 1630.2b | 4175.3 |
| positive control | 1006.6bc | 1806.6ab | 4106.3 |
| CP-16 | 1316.2ab | 1802.5ab | 4106.3 |
| CP-26 | 1090.3bc | 1995.8ab | 4589.4 |
| CP-28 | 890.6c | 1821.7ab | 3719.8 |
| CP-41 | 1460.9a | 2085.1ab | 4347.8 |
| CP-17 | 1046.3bc | 1768.4ab | 4106.3 |
| CP-100 | 1102.8bc | 2329.7a | 3961.4 |
| CV (%) | 16 | 20 | 24 |
| LSD (P<0.05) | 315.7 | 681.5 | ns |
| Mean | 1124 | 1905 | 4139.1 |
AGBY=Aboveground biomass yield, SDBY= Shoot dry biomass yield, GY=Grain Yield.
This comparative higher response of the inoculant in the Ginchi sub-stations soil condition could be credited to their competence of availing high N to the host through BNF.2,9 Similar to these results, Minalku and Mitiku13 testified that inoculation and application of starter nitrogen to chickpea amplified AGBY and GY meaningfully as equated to the negative control. Parallel outcomes were also attained from inoculation of chickpea with native rhizobial isolates in the central highlands of Ethiopia.13
Chickpea response to inoculation at Ginchi sub-station in 2020/21
As (Table 3) depicts treatments that showed substantial statistical alterations (p ≤ 0.05) in aboveground biomass and grain yield. However, a substantial statistical variance was not detected amongst CP-17, CP-26, CP-28, and the positive control on aboveground biomass yield, inoculant CP-17 showed a superior aboveground biomass yield (3671 kg/ ha) than them. Over the other treatments, inoculant CP-26, the positive control, CP-41, and CP-17 showed a higher significant statistical difference in GY (2576.9 kg/ ha), (2563.3 kg/ ha), (2482.5 kg/ ha) and (2147.6 kg/ ha), correspondingly. The higher GY scored by CP-26 was 22% better than the corresponding yield of the negative control.
Table 3: Response of chickpea to rhizobial inoculation at Ginchi substation in 2020/21
| Treatment | AGBY (kg/ha) | GY (kg/ha) | SDBM (kg/ha) |
| Negative control | 2345.6bcd | 2073.8bc | 3425.4 |
| Positive control | 3183.9ab | 2563.3a | 4177.8 |
| CP-16 | 1785.1d | 1943.5c | 4177.8 |
| CP-26 | 3258.9ab | 2576.9a | 4622.2 |
| CP-28 | 3044.4abc | 1963.1c | 3822.2 |
| CP-41 | 1913.4cd | 2482.5ab | 4400 |
| CP-17 | 3671a | 2147.6abc | 4177.8 |
| CP-100 | 2143.6bcd | 1974.3c | 4044.4 |
| CV (%) | 26 | 12 | 22 |
| LSD (P<0.05) | 1250 | 477.66 | ns |
| Mean | 2701.6 | 2215.6 | 4106 |
AGBY=Aboveground biomass yield, SDBY= Shoot dry biomass yield, GY=Grain Yield.
The rhizobial isolates CP-26 and CP-41depict comparative substantial dominance (p≤ 0.05) over the rest of the treatments, on GY. This relative superior performance of the inoculants in Ginchi soil condition is could be credited to their capability of availing high N to the host through BNF.2,9 Similar to the Ginchi case, Mnalku and Mitiku13 also reported that rhizobial strains’ inoculation and application of starter nitrogen to chickpea increased significantly as compared to the uninoculated and unfertilized control. Similar results were also obtained from inoculation of chickpea with indigenous rhizobial isolates in the central highlands of Ethiopia. 13
The average response of chickpea to inoculation at the Ginchi sub-station
In the two following years, statistical analysis results in Table 4 showed that there were significant statistical differences (p ≤ 0.05) among the treatments on AGBY and GY. Rhizobial inoculants CP-26 and CP-41 showed superior performance on GY (2286 kg/ ha) and (2283 kg/ ha). Although there were no statistical alterations among treatments on shoot dry biomass yield, the inoculants that showed a superior grain yield also scored relatively a higher shoot dry biomass yield (4606kg/ ha) and (4374 kg/ ha). The higher GY (2286 kg/ ha) and (2283 kg/ ha) scored by inoculants CP-26 and CP-41 were (27.4% and 27.3%) and (9.2% and 9.1%) higher than the grain yield of the negative control (1735 kg/ ha) and positive control (2085 kg/ ha), respectively.
In general, the combined analysis confirmed that CP-26 and CP-41 showed superior GY of chickpea at the Ginchi substation as equated to the controls. Accordingly, the aforementioned elite native chickpea rhizobial inoculants that showed a superior performance both in grain and shoot dry biomass yield are the best candidates for further verification to find elite chickpea inoculants that suit the central highlands of chickpea growing areas of Ethiopia.
Table 4: Response of chickpea to rhizobial inoculation at Ginchi sub-station in 2019/20-2020/21.
| Treatment | AGBY (kg/ ha) | GY (kg/ ha) | SDBY (kg/ ha) |
| Negative control | 1711.9bc | 1735.3b | 3800.3 |
| positive control | 2095.2abc | 2085ab | 4142 |
| CP-16 | 1550.6c | 1856.3ab | 4142 |
| CP-26 | 2174.6ab | 2286.4a | 4605.8 |
| CP-28 | 1967.5abc | 1892.4ab | 3771 |
| CP-41 | 1687.2bc | 2283.8a | 4373.9 |
| CP-17 | 2358.6a | 1958ab | 4142 |
| CP-100 | 1623.2bc | 2152ab | 4002.9 |
| LSD (P<0.05) | 594.96 | 492.8 | ns |
| Year | |||
| 2019/20 | 1124b | 1875.8b | 4139.1 |
| 2020/21 | 2668.2a | 2186.5a | 4106 |
| LSD (P<0.05) | 297 | 246 | ns |
| CV (%) | 27 | 21 | 22 |
| Mean | 1896 | 2031 |
4122.5 |
AGBY=Aboveground biomass yield, SDBY= Shoot dry biomass yield, GY=Grain Yield.
Benefit-cost analysis
The fractional financial examination outcome shows the uppermost net profit (ETB 44250 per hectare) was gotten from the use of 500 g of CP-26 per hectare (Table 5). The dominance analysis showed that except for the positive control all inoculants were not dominated. That means all inoculants are economically feasible one after the other in the following descending order; CP-26, CP-41, CP100, CP-17.CP-28 and CP-16. Since no beneficiary will prefer an alternative that gives lower net benefits than one with higher net benefits and lower total variable expenses, a treatment that showed lower net benefits (Birr per hectare) than other treatments; the positive control in this study, was eliminated out of the partial budget examination.6
Table 5: Partial budget analysis of rhizobial isolates experiment on chickpea, 2019-2021.
| Treatment | GY
(kg ha-1) |
Adj. yield -15% (kg ha-1) | Gross benefit (Birr ha-1) | TVC
(Birr ha -1) |
Net benefit
(Birr ha-1) |
Dominance
(Birr ha-1) |
MC
(Birr ha-1) |
MNB
(Birr ha -1) |
MRR (%) |
| No inoculation | 1735 | 1475 | 44250 | 0 | 44250 | ||||
| CP-16 | 1856 | 1578 | 47336 | 160 | 47176 | ND | 160 | 2925.5 | 1828 |
| CP-28 | 1892 | 1609 | 48256 | 160 | 48096 | ND | 160 | 3846.05 | 2404 |
| CP-17 | 1958 | 1664 | 49929 | 160 | 49769 | ND | 160 | 5518.85 | 3449 |
| CP-100 | 2152 | 1829 | 54876 | 160 | 54716 | ND | 160 | 10465.85 | 6541 |
| CP-41 | 2284 | 1941 | 58237 | 160 | 58077 | ND | 160 | 13826.75 | 8642 |
| CP-26 | 2286 | 1943 | 58303 | 160 | 58143 | ND | 160 | 13893.05 | 8683 |
| 18 kg N/ha | 2085 | 1772 | 53168 | 1400 | 51768 | D |
GY=grain yield, Adj= adjusted yield, TVC= total variable cost, MC=marginal cost, MNB=marginal net benefit, MRR= marginal rate of return, ND=none dominated D= dominated.
The outcome from the MRR specifies that for every ETB 1.00 investment in chickpea production using CP-26, CP-41, CP100, and CP-17.CP-28 and CP-16 inoculation on Vertisol, the producer can get an additional return of ETB 86.83,86.42,65.41,34.49,24.04 and 18.28 respectively. The lowest satisfactory rate of return supposed in this trial was 100% and hence all strains were profitable options. Though, in comparative terms inoculation of chickpea with CP-26 and CP-41gave the highest marginal rate of return (8683 %) and (8642%), respectively. Thus, these rhizobial inoculants are the best promising candidates for further confirmation on Vertisol in the farmers’ field at different agro-ecologies to consider them as candidates for the preparation of marketable chickpea rhizobial inoculants in Vertisol chickpea growing areas of Ethiopia.
Conclusion and Recommendations
Depending on the average field results on chickpea inoculation at the Ginchi substation; Dendi district due to their practical preeminence in grain and shoot dry biomass yield CP-26 and CP-41 became the best promising chickpea inoculants for further verification. The investigative results of the soil were found to be sub-optimal for the production of chickpea except for phosphorus. This confirms that producing chickpea using rhizobial isolate CP-26 and CP-41 along with 46 kg P2O5 on Ginchi soil condition is reasonably forfeiting in terms of grain and shoot dry biomass yield. Therefore, it is suggested that extra confirmation of the inoculants should be carried out in replicated conditions on Vertisol of different farmers’ fields at different agro-ecologies to identify candidates for the development of commercial chickpea rhizobial inoculants that outfit for chickpea cultivating parts of the central highlands of Ethiopia.
Acknowledgment
I am thankful to all of the colleagues who contributed their best during the preparation of this manuscript.
Conflict of Interest
The authors do not have any conflict of interest.
Funding Sources
The author(s) received no financial support for the publication of this manuscript.
References
- Ahmed, Z.I., Tariq, M, and Anjum, M.S (2008). Effect of different Rhizobium inoculation methods on the performance of lentils in pothowar region. Int. J.Agri. Bio. 10: 81-84.
- Andrade, D.S., Murphy P.J., and Giller, K.E (2002). The diversity of Phaseolus-nodulating rhizobium populations is altered by liming of acid soils planted with Phaseolus vulgaris in Brazil. Appl Environ Microbiol. 2002; 68:4025–34.
CrossRef - Aulakh, M.S(1985). Content and uptake of nutrients by pulses and oilseed crops. Ind. J. Ecol., 12(2): 238–242.
- Beyene, S., Tena, W., Hidoto, L., and Fikre, A (2015). Chickpea (Cicer arietinum L.) production in the Southern Nations, Nationalities, and Peoples’ Region of Ethiopia.
- Charman, P.E.V., and Roper M.M (2007). Soils-their properties and management. Oxford University Press, pp: 276-285.
- CIMMYT (2015). Financial products for farmers and servise providers report Ethiopia.
- Corp, M., Machado, S., Ball, D., Smiley, R., Petrie, S., Siemens, M., and Guy, S (2004). Chickpea Production Guide.
- Getachew, A., and Sinebo, W (2012). Drainage, sowing date, and variety effects on chickpea grown on a Vertisol in Ethiopia. Archives of Agronomy and Soil Science 56: 1-13.
- Getachew, A., Chilot, Y., and Teklu, E (2019). Soil acidity management. Ethiopian Institute of Agricultural Research (EIAR). Addis Ababa, Ethiopia. pp: 21.
- Jones, J.B. (2003). Agronomic Handbook: Management of crops, soils, and their fertility. CRC Press. Florida.
CrossRef - Kantar, F., Hafeez, F.Y., Shivakumar, B.G., Sundaram, S.P., Tejera, N.A., Aslam, A., Bano, A., and Raja, P (2007). Chickpea: Rhizobium management and nitrogen fixation. Chickpea Breed. Mgt, pp: 179-192.
CrossRef - Kjeldahl, J (1883). A New Method for the Determination of Nitrogen in Organic Matter. Zeitschrift für Analytische Chemie, 22, 366-382.
CrossRef - Mnalku, A., and Mitiku, G (2019). Response of Chickpea (Cicer arietinum) to Indigenous Rhizobial Isolates Inoculation on Vertisol of Central Ethiopian Highland. Ethiop. J. Agric. Sci. 29(2)109-117.
- Muhammad, A., Ahmed, H.K., Himayatullah, M., Ayaz, E., Ahmed, A., Sagoo, G., Inayatullah, A.,Hussain, A., Manzoor, M (2010). Nodulation, grain yield, and grain protein contents as affected by Rhizobium inoculation and fertilizer placement in chickpea cultivar b Bittle-98. Sarhad J. 26(4): 467-474.
- Romdhane, S.B., Mustapha, T., Mohamed, E.A., Ph de L, R.M (2009). The diversity of rhizobia nodulating chickpea (Cicer arietinum) under water deficiency as a source of more efficient inoculants. Soil Biology & Biochemistry. 41: 2568– 2572.
CrossRef - SAS Institute (2002). SAS/STAT User’s Guide. Version 8, 6th Edition, SAS Institute, Cary, 112.
- Tekalign, T., Haque, I., Aduayi, E.A (1991). Soil, plant, water, fertilizer, animal manure, and compost analysis manual. Working Document, No.13.
- Walkley, A., and Black, C.A (1934). An examination of different methods for determining soil organic matter demands the proposed modification by the chromic acid titration method. Soil 37: 29-38.
CrossRef

