Introduction
Colocasia esculenta (L.) Schott, commonly known as taro, belongs to the plant family Araceae. Taro is a nutritionally and pharmacologically important tuber crop, domesticated in ancient times.1 The two primary categories of taro cultivars are dasheen and eddoe varieties. The eddoe variety has several cormels and a small corm, while the dasheen type has a large corm and a few cormels.2,3 Intermediate varieties with the combinations of eddoe and dasheen characters also exist in natural populations.
Studies show that taro originated in tropical region that is spanning from India to Indonesia.4 Hence, high diversity of taro is expected in this area at the intraspecific level. African and Asian countries contribute more than 90% of the world’s taro production. During the period from 2018 to 2022, there was a 5% (843,940.98 ton) increase in global taro production.5 In India, major taro producing states include Kerala, Tamil Nadu, Andhra Pradesh, Telangana, Madhya Pradesh, Orissa, Uttar Pradesh, Bihar, Assam, Meghalaya and West Bengal.6,7
Taro flour is a gluten-free food with high carbohydrate content.8 Hence, it is a good alternative for people suffering from gluten-related diseases like non-Celiac gluten sensitivity (NCGS) and Celiac disease (CD), which require a gluten-free diet.9,10 Taro corms also exhibit anti- cancerous, antidiabetic and immunostimulatory properties. Consumption of taro tubers helps to fight against diseases and infections.11
Taro corm contains 20-35% starch on a fresh weight basis and 81% on a dry weight basis.12,13 Taro leaves are rich in protein and act as a source of essential amino acids. Taro leaves also contain minerals like potassium, iron, phosphorous, magnesium and zinc.14,15
Taro is important in hunger management because it alleviates hunger and addresses nutrient deficiency of many people in the world.16 Nowadays, the world population is growing rapidly, but at the same time, agricultural land area is decreasing. Climate change is also becoming a new threat. Alleviating world hunger has become a massive task.
New high-yielding, nutrient-rich, stress-tolerant, disease and pest-resistant crop plants can be developed through different methods like plant breeding or genetic engineering. Naturally available and potentially useful germplasm of the crop species is the basis of all these plant breeding programs. The availability of suitable germplasm has a major impact on the success rate of plant breeding efforts. Therefore, it is very important to explore and characterise naturally available germplasm accessions.
Taro is an underutilized and underexploited tuber crop even though it is a nutritionally rich and cost-effective food source. Only a little research has been done on taro at the intraspecific level. In addition, the characterisation of naturally available germplasm is a preliminary step to conserve the genetic diversity of crop species. The major aim and objectives of the present study include analysis of growth and yield characters of five taro cultivars of Kerala state of India and their performance analysis.
Materials and Methods
Five different cultivars of taro (CULT 1, CULT 2, CULT 3, CULT 4 and CULT 5), cultivating in the Kerala state of India, were selected for the current study. The experiment was carried out under uniform conditions in the experimental net house of the Department of Botany and Research Centre, Govt. Brennen College, Thalassery, Kannur District, Kerala, India.
The experiment was laid out in randomised block design (RBD) with 3 replications and 18 plants per plot. Taro seed tubers were collected directly from farmers and taken as the base material for the experiment. The experiment was carried out during the crop season 2023-2024. Taro seed tubers were planted during the third week of May. Planting, manuring, weeding, and earthing up were done in accordance with the package of cultivation practices recommended by Kerala Agricultural University. Twenty-two characters, including both plant growth and yield characters, were recorded and analysed. Mean values for each character for each cultivar were found out. The significance of the difference between cultivar means was tested statistically through analysis of variance (ANOVA). The F and P values of each character were determined and compared with the standard F table and alpha P value of 0.01.17 To determine the significance of the variation in cultivar means, the Critical Difference value was also computed.
Performance indices were computed for each agronomic character as per the following equation.18 Then cumulative performance index was calculated, and overall performance of all cultivars was analysed.

In the case of sinus width and sinus length, reciprocal of the above formula was used since short sinus width and sinus length are desirable characters.
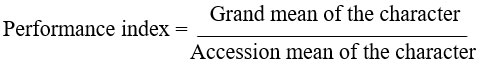
Results and Discussion
Twenty-two characters of five native taro cultivars were statistically analysed in this study (Table 2 and Table 3). There is highly significant variation among these five cultivars, according to the P-value for each character (P < 0.01). Based on the F-value, all characters exhibit considerable variation at the 1% level of significance, with the exception of secondary cormels length. The length of secondary cormel was significant at 5% significance level. Morphological characteristics of five taro cultivars are listed in Table 1.
The comparative performance of eight growth characters, including plant height, number of leaves, number of suckers, petiole length, leaf lamina length, lamina width, sinus depth and sinus width were analysed (Table 4). CULT 4 possess highest performance index for the character plant height. In CULT 4, average plant height was 107.28±4.13 cm. Plant height was minimum in CULT 2 (78.04±2.45 cm). Maximum number of leaves seen in CULT 3 followed by CULT 4. CULT 5 is characterised by the least number of leaves. At the same time, leaf lamina length and leaf lamina width were maximum in CULT 5.
Among five cultivars, the longest petiole was observed in CULT 4 (104.54±4.31 cm) followed by CULT 5 (103.14±3.33 cm) and the smallest petiole in CULT 2 (81.47±2.13 cm). Taro leaves and petioles are rich in fiber, minerals, protein and vitamins like vitamin C, vitamin B1, vitamin B2 and vitamin B3.19,20 Taro leaves and petioles also contain anti-nutrients like calcium oxalate, which can be eliminated through appropriate processing methods, thereby allowing the leaves and petioles to be used as vegetables.
The number of suckers was minimum in CULT 1 and maximum in CULT 3. Prior studies revealed a strong positive correlation between tuber yield and the number of suckers in taro.21,22 Photosynthetic area of taro leaves decreases with increase in sinus width and sinus depth. Hence, short sinus width and sinus length are desirable characters in the case of taro. Sinus width and sinus length were minimum in CULT 3 and maximum in CULT 4.
Fourteen yield characters, including total yield, corm fresh weight, cormels fresh weight, corm length, corm thickness, number of primary, secondary and tertiary cormels, length of primary, secondary and tertiary cormels and thickness of primary, secondary and tertiary cormels, were recorded and statistically analysed (Table 3). Based on yield characteristics, the comparative performance of each cultivar was analysed (Table 4).
CULT 5 produced the highest total yield, followed by CULT 2 and CULT 4. Corm fresh weight and cormel fresh weight were also maximum in CULT 5 (135.61±8.3 gm and 183.22±14.11 gm, respectively). Corm fresh weight was minimum in CULT 1, and cormel fresh weight was minimum in CULT 4. Length and width of leaf lamina and petiole length show a significant positive correlation with total yield.23 High petiole length, leaf width and leaf length of CULT 5 also may have contributed to its best overall yield.
Corm thickness and number of primary cormels were maximum in CULT 5, while corm length was maximum in CULT 4. Maximum number of secondary cormels observed in CULT 1. Meanwhile, CULT 2 possesses the highest number of tertiary cormels. Previous studies indicate a positive correlation between the number of cormels and the total yield of taro.24,25
CULT 5 shows the highest primary cormel thickness with a mean value of 2.87 ± 0.08 cm. The lengths of secondary and tertiary cormels were maximum in CULT 3, while their thicknesses were maximum in CULT 2.
The performance of five taro cultivars were analysed based on the performance index calculated for twenty-two characters of taro. Out of these five taro cultivars, CULT 5 ranked first based on the cumulative performance index. Maximum cormel fresh weight, corm fresh weight, total yield, corm thickness, number of primary cormels and thickness of primary cormels were reported in CULT 5. CULT 2 comes in the second position, followed by CULT 3. The number of tertiary cormels, thickness of secondary cormels and thickness of tertiary cormels were maximum in CULT 2. CULT 4 ranked fourth and CULT 1 ranked fifth. The elite cultivars mentioned in this study can be popularised among farmers for extensive cultivation. Future plant breeding projects can utilise these cultivars to produce promising taro varieties.
Conclusion
The development of high-yielding varieties and utilisation of naturally available germplasm of crop species are the key methods to overcome hunger and malnutrition. Growth and yield characteristics of five traditional taro cultivars were analysed in this experiment. Highly significant variation exists among these five cultivars. Taro cultivars were ranked based on the cumulative performance index. Among these five cultivars, CULT 5 ranked first, followed by CULT 2 and CULT 3. Maximum cormel fresh weight, corm fresh weight, corm thickness, number of primary cormels and primary cormel thickness were reported in CULT 5. CULT 5 also exhibited the highest leaf breadth and length. In the case of total yield, CULT 2 comes in the second position. Elite cultivars mentioned in this study can be utilised for large-scale cultivation and future plant breeding experiments.
Table 1: Morphological characteristics of taro cultivars.
| Cultivar Character | CULT 1 | CULT 2 | CULT 3 | CULT 4 | CULT 5 | |
| Leaf blade colour | Green | Green | Green | Purple green | Green | |
| Leaf blade margin | Green | Green | Purple | Purple | Green | |
| Petiole junction colour | Green | Green | Purple | Purple | Green | |
| Petiole colour | Top | Light green | Light green | Light purple | Purple green | Light green |
| Middle | Green | Green | Purple brown | Blackish purple | Light purple brown | |
| Basal | Green | Green | Purple brown | Blackish purple | Purple brown | |
| Arrangement of primary cormels | Sparsely budding | Densely budding | Densely budding | Sparsely budding | Sparsely budding | |
| Corm shape | Round | Round | Conical | Conical | Conical | |
| Corm cortex colour | Light purple | White | White | Purple | Light purple | |
| Corm central part colour | White | White | White | White | White | |
| Shape of cormels | Elongated and bulged at one end. | Obovate | Obovate | Round to obovate | Obovate | |
Table 2: Mean value of growth characters of five taro cultivars.
| Growth Characters | CULT 1 | CULT 2 | CULT 3 | CULT 4 | CULT 5 | Grand mean of the character | P value | CD @ 5% | CD @ 1% |
| Plant height (cm)* | 89.48 ±2.54 |
78.04 ±2.45 |
85.66 ±3.25 |
107.28 ±4.13 |
104.57 ±3.22 |
93.01 | 1.1 x 10-8 | 3.37 | 4.8 |
| Number of leaves * | 4.72 ± 0.16 |
5.22± 0.13 | 7.33 ± 0.2 |
5.39± 0.14 | 4±0 | 5.33 | 4.3 x 10-7 | 0.49 | 0.7 |
| Number of suckers * | 0.06 ± 0.06 |
0.56± 0.12 | 1± 0.23 |
0.89± 0.18 | 0.78 ± 0.15 |
0.66 | 0.00032 | 0.32 | 0.45 |
| Petiole length (cm)* | 89 ± 3.66 |
81.47 ±2.13 |
86.39 ±3.1 |
104.54 ±4.31 |
103.14 ±3.33 |
92.91 | 1.34 x 10-7 | 3.53 | 5.04 |
| Leaf lamina length (cm)* | 34.86 ±1.31 |
36.86 ±0.73 |
30.48 ±1.22 |
37.8 ±1.54 |
43.55 ±1.7 |
36.71 | 6.11 x 10-6 | 2.77 | 3.96 |
| Leaf lamina width (cm)* | 26.56 ±1.27 |
26.34 ±0.52 |
20.18 ±0.77 |
29.73 ±1.45 |
37.23 ±1.27 |
28.01 | 0.0002 | 3.37 | 4.82 |
| Sinus depth (cm)* | 5.26 ±0.13 |
5.9 ±0.11 |
4.42± 0.18 | 7.13 ±0.32 |
5.72 ± 0.19 |
5.69 | 4.12 x 10-8 | 0.29 | 0.42 |
| Sinus width (cm)* | 12.32 ±0.45 |
9.66 ± 0.29 |
7.52± 0.38 | 13.23 ±0.51 |
10.29 ±0.18 |
10.6 | 1.93 x 10-5 | 1.33 | 1.9 |
*F-value significant at 1% significance level.
Table 3: Mean value of yield characters of five taro cultivars.
| Yield Characters | CULT 1 | CULT 2 | CULT 3 | CULT 4 | CULT 5 | Grand mean | P value | CD @ 5% | CD @ 1% |
| Corm fresh weight (g)* | 45.89 ±5.63 |
62.17 ±3.59 |
50.78 ±2.95 |
126.78 ±12.03 |
135.61 ±8.3 |
84.25 | 2.05 x 10-6 | 14.33 | 20.45 |
| Cormel fresh weight (g)* | 91.44 ±14.51 |
162.28 ±15.15 |
114.61 ±10.27 |
77.33 ±5.89 |
183.22 ±14.11 |
125.78 | 0.0001 | 22.45 | 32.04 |
| Corm length (cm)* | 4.19± 0.14 | 4.24 ± 0.14 |
4.86 ± 0.16 |
9.89 ± 0.67 |
5.8 ± 0.17 |
5.8 | 5.4 x 10-10 | 0.47 | 0.67 |
| Corm thickness (cm)* | 3.79± 0.19 | 4.6 ± 0.14 |
3.87 ± 0.09 |
4± 0.19 |
5.68 ± 0.13 |
4.39 | 3.15 x 10-6 | 0.38 | 0.54 |
| No. of primary cormels* | 4.61± 0.28 | 4.89 ± 0.33 |
5.78 ± 0.34 |
2.72 ± 0.23 |
6.22± 0.32 | 4.84 | 0.0001 | 1 | 1.43 |
| No. of secondary cormels * | 5.56± 0.78 | 5.17 ± 0.53 |
4.89 ± 0.69 |
0.61 ± 0.24 |
3.67 ± 0.32 |
3.98 | 3.21 x 10-6 | 0.97 | 1.39 |
| No of tertiary cormels* | 0.17± 0.09 | 1.11 ± 0.31 |
0.94 ± 0.3 |
0±0 | 0.17 ± 0.09 |
0.48 | 0.0002 | 0.41 | 0.59 |
| Primary cormel length (cm)* | 8.36± 0.81 | 4.76 ± 0.24 |
3.62 ± 0.18 |
4.37 ±0.28 |
4.77 ± 0.28 |
5.18 | 6 x 10-5 | 1.23 | 1.76 |
| Primary cormel thickness (cm)* | 1.65± 0.08 | 2.6 ± 0.06 |
2± 0.06 | 2.53 ±0.16 |
2.87 ± 0.08 |
2.33 | 6.45 x 10-5 | 0.33 | 0.48 |
| Secondary Cormel length (cm) | 2.76± 0.28 | 2.76 ± 0.17 |
3.64 ± 0.29 |
3.12 ±0.29 |
2.25 ±0.21 |
2.91 | 0.01 | 0.50 | 0.71 |
| Secondary Cormel thickness (cm)* | 1.38± 0.07 | 2.34 ± 0.08 |
1.94 ± 0.05 |
1.72 ±0.11 |
2.04 ±0.12 |
1.88 | 5.99 x 10-5 | 0.24 | 0.34 |
| Tertiary cormel length (cm)* | 1.36± 0.02 | 2.01 ± 0.17 |
2.15 ± 0.08 |
0±0 | 1.5 ±0.02 |
1.4 | 0.0001 | 0.7 | 1 |
| Tertiary Cormel Thickness (cm)* | 1.42± 0.08 | 2.06 ± 0.09 |
1.79 ± 0.06 |
0±0 | 1.87 ± 0.01 |
1.43 | 9.07 x 10-7 | 0.36 | 0.51 |
| Total Yield (g)* | 85± 6.99 | 224.44 ±18.17 |
165.39 ±12.49 |
206.33 ±16.61 |
318.83 ±20.45 |
200 | 1.06 x 10-8 | 14.06 | 20.08 |
*F-value significant at 1% significance level.
Table 4: Performance index of five taro cultivars.
| Growth and Yield Character | CULT 1 | CULT 2 | CULT 3 | CULT 4 | CULT 5 |
| Plant height | 0.96 | 0.84 | 0.92 | 1.15 | 1.12 |
| Number of leaves | 0.89 | 0.98 | 1.37 | 1.01 | 0.75 |
| Number of suckers | 0.09 | 0.85 | 1.52 | 1.35 | 1.19 |
| Petiole length | 0.96 | 0.88 | 0.93 | 1.13 | 1.11 |
| Leaf lamina length | 0.95 | 1.00 | 0.83 | 1.03 | 1.19 |
| Lamina width | 0.95 | 0.94 | 0.72 | 1.06 | 1.33 |
| Sinus depth | 1.08 | 0.96 | 1.29 | 0.8 | 0.99 |
| Sinus width | 0.86 | 1.1 | 1.41 | 0.8 | 1.03 |
| Corm fresh weight | 0.54 | 0.74 | 0.6 | 1.5 | 1.61 |
| Cormel fresh weight | 0.73 | 1.29 | 0.91 | 0.61 | 1.46 |
| Corm length | 0.72 | 0.73 | 0.84 | 1.71 | 1 |
| Corm thickness | 0.86 | 1.05 | 0.88 | 0.91 | 1.29 |
| No. of primary cormels | 0.95 | 1.01 | 1.19 | 0.56 | 1.28 |
| No. of secondary cormels | 1.4 | 1.3 | 1.23 | 0.15 | 0.92 |
| No. of tertiary cormels | 0.36 | 2.32 | 1.97 | 0 | 0.36 |
| Primary cormel length | 1.62 | 0.92 | 0.7 | 0.84 | 0.92 |
| Primary cormel thickness | 0.71 | 1.12 | 0.86 | 1.09 | 1.23 |
| Secondary Cormel length | 0.95 | 0.95 | 1.25 | 1.07 | 0.77 |
| Secondary Cormel thickness | 0.73 | 1.24 | 1.03 | 0.91 | 1.08 |
| Tertiary cormel length | 0.97 | 1.43 | 1.53 | 0 | 1.07 |
| Tertiary Cormel Thickness | 0.99 | 1.44 | 1.25 | 0 | 1.31 |
| Total Yield | 0.43 | 1.12 | 0.83 | 1.03 | 1.59 |
| Cumulative PI | 18.7 | 24.21 | 24.06 | 18.71 | 24.6 |
| RANK | V | II | III | IV | I |
Acknowledgement
The authors would like to thank CSIR-Council of Scientific and Industrial Research, Govt. of India, New Delhi for Junior Research Fellowship and Senior Research Fellowship.
Funding Sources
The authors received no financial support for the research, authorship, and publication of this article.
Conflict of Interest
The authors do not have any conflict of interest.
Data Availability Statement
All data underlying this study are available as part of the article and no additional source data are required.
Ethics Statement
This research did not involve human participants, animal subjects, or any material that requires ethical approval.
Author Contributions
Aiswarya Thekkayil – Primary investigator. Participated in exploration of genetic resources, cultivation of taro plants, data collection and data analysis. Writing – Original Draft.
Sumisha Chennappoyil and Swedha Madhavan Mavally – Participated in exploration of genetic resources and data collection.
Chandramohanan Kotten Thazhath – Conceptualization and supervision of the study.
References
- Grimaldi I. M., Muthukumaran S., Tozzi G., Nastasi A., Boivin N., Matthews P. J., Van Andel T. Literary evidence for taro in the ancient Mediterranean: A chronology of names and uses in a multilingual world. PLoS One, 2018; 13(6). doi: 1371/journal.pone.0198333
CrossRef - Ferdaus M. J., Chukwu-Munsen E., Foguel A., da Silva R. C. Taro roots: An underexploited root crop. Nutrients, 2023; 15(15): 3337.
CrossRef - Okonkwo C. A. C. Polymorphism in taro (Colocasia esculenta (L.) Schott) and its implication in taro breeding and production in Nigeria. Journal of horticultural science, 1987; 62(3): 395-400.
CrossRef - Matthews P. J. Genetic diversity in taro, and the preservation of culinary knowledge. Ethnobotany Research & Applications, 2004; 2: 55–71.
CrossRef - Food and Agriculture Organization of the United Nations. FAOSTAT statistical database. https://www.fao.org/faostat/en/#data/QCL. 2022. Updated on October 7, 2024. Accessed on 17 October 2024.
CrossRef - Raju J., Shiny R., Byju G. Change in climate and climate suitability of major taro [Colocasia esculenta (L.) schott] growing regions of India. Journal of Root Crops, 2022; 48 (1 & 2): 47-56.
CrossRef - Srinivas T., Nedunchezhiyan M., Misra R. S. Marketing of taro in India. National Seminar on Climate change and Food Security: Challenges and Opportunities for Tuber Crops – NSCFT proceedings, 2011; 609-612.
CrossRef - Saxby S., Lee C., Li Y. Nutritional, physicochemical, and functional properties of five varieties of Taro (Colocasia esculenta). Current Developments in Nutrition, 2021; 5: 607.
CrossRef - Cenni S., Sesenna V., Boiardi G., Casertano M., Russo G., Reginelli A., Esposito L., Strisciuglio C. The role of gluten in gastrointestinal disorders: A review. Nutrients, 2023; 15(7): 1615.
CrossRef - Dilek N. M., Bilgiçli N. Effect of taro [Colocasia esculenta (L.) Schott] flour and different shortening ratio on physical and chemical properties of gluten‐free cookie. Journal of Food Processing and Preservation, 2021; 45(11), e15894.
CrossRef - Pereira P. R., Mattos, E. B. A., Correa A. C. N. T. F., Vericimo M. A., Paschoalin V. M. F. Anticancer and Immunomodulatory Benefits of Taro (Colocasia esculenta) Corms, an underexploited tuber crop. International Journal of Molecular Sciences, 2020; 22(1), 265.
CrossRef - Agama‐Acevedo E., Garcia‐Suarez F. J., Gutierrez‐Meraz F., Sanchez‐Rivera M. M., San Martin E., Bello‐Pérez L. A. Isolation and partial characterization of Mexican taro (Colocasia esculenta) starch. Starch‐Stärke, 2011; 63(3): 139-146.
CrossRef - Wills R. B., Lim J. S., Greenfield H., Bayliss‐Smith T. Nutrient composition of taro (Colocasia esculenta) cultivars from the Papua New Guinea highlands. Journal of the Science of Food and Agriculture, 1983; 34(10): 1137-1142.
CrossRef - Ejoh A. R., Mbiapo F. T., Fokou E. Nutrient composition of the leaves and flowers of Colocasia esculenta and the fruits of Solanum melongena, 1996; Plant Foods for Human Nutrition: 49: 107-112.
CrossRef - Mergedus A., Kristl J., Ivancic A., Sober A., Sustar V., Krizan T., Lebot V. Variation of mineral composition in different parts of taro (Colocasia esculenta) corms. Food chemistry, 2015; 170: 37-46.
CrossRef - Kapoor B., Singh S., Kumar P. Taro (Colocasia esculenta): Zero wastage orphan food crop for food and nutritional security. South African Journal of Botany, 2022; 145: 157-169.
CrossRef - Fisher R. A., Yates F. Statistical Tables for Biological, Agricultural and Medical Research, England: Longman, 1963: 356.
CrossRef - Amaravenmathy V. S., Srinivasan C. S. Phenotypic and genotypic variations for yield and plant architecture in some hybrid progenies of Arabica coffee. Journal of Coffee Research, 2003; 31(2): 99-105.
CrossRef - Mitharwal S., Kumar A., Chauhan K., Taneja N. K. Nutritional, phytochemical composition and potential health benefits of taro (Colocasia esculenta) leaves: A review. Food Chemistry, 2022; 383:132406.
CrossRef - Shekade D. P., Patil P. D., Sahoo G. V. Potential Use of Dragon Fruit and Taro leaves as functional food. European Journal of Engineering Science and Technology, 2018; 1(1):10-20.
CrossRef - Boampong R, Boateng S. K., Adu Amoah R., Adu Gyamfi B., Aboagye L. M., Ansah E. O. Growth and yield of taro (Colocasia esculenta (L) Schott) as affected by planting distance. International Journal of Agronomy, 2020; (1): 8863099. https://doi.org/10.1155/2020/8863099.
CrossRef - Devi H. S., Singh V. Correlates of genetic and phenotypic attributes of taro [Colocasia esculenta (L.) Schott]. Indian Journal of Hill Farming, 2019; https://epubs.icar.org.in/index.php/IJHF/ article/view/94736
CrossRef - Velayudhan K. C., Liji R. S., Rajlakshmy C. Correlation and path analysis in Taro (Colocasia esculenta (L.) Schott) morphotypes. Journal of Root Crops, 2000; 26(2): 36-39.
CrossRef - Paul K. K., Bari M. A., Islam S. M. S., Debnath S. C. Genotypic and phenotypic correlation coefficient studies for taro (Colocasia esculenta (L.) Schott). Bangladesh Journal of Botany, 2014; 43(1), 113-117.
CrossRef - Singh M., Yadav G. C. Correlation and path coefficient analysis for yield and horticulture traits in different genotypes of Colocasia (Colocasia esculenta antiquorum (L.) Schott). Journal of Pharmacognosy and Phytochemistry, 2018; 7(1S), 288-292.
CrossRef

