Introduction
Sphaeranthus indicus Linn., is a medicinal herb known for its round purple flowers and commonly known as “Gorakhmundi” and “East Indian globe thistle”, is a highly valuable medicinal plant that contain various medicinal properties including antiviral, antimicrobial, anti-inflammatory and anticancer activities. The plant is rich in secondary metabolites such as flavonoids, sesquiterpenes, and essential oils, making it important in both traditional and modern medicine. The plant is widely distributed across India, Sri Lanka, Australia, Malaysia, China, and Africa. Traditionally, the entire plant has been used to treat various ailments. In folk medicine, it has been reported to treat hemicrania, epilepsy, and mental disorders It is also utilized as a tonic, laxative, digestive, and anthelmintic, as well as for treating conditions such as insanity, tuberculosis, spleen disorders, anemia, bronchitis, elephantiasis, and leukoderma.1 The leaves of S. indicus are known for their anxiolytic, macro-filaricidal, and antimicrobial activities.2–4 Additionally, a paste made from the herb is used to treat pruritus, edema, arthritis, gout, cervical adenopathy, piles, and hepatitis.5 Previous studies of aerial parts of the S. indicus plant indicate that it is rich in various essential oils and contains the alkaloid Sphaeranthine6,7 Other bioactive compounds such as glucosides, eudesminoids, phenolic glycosides, sesquiterpene lactones, sphaerantholide, flavones, and isoflavone glycosides have also been identified.1,2,7
The extracts, fractions, and isolated constituents of S. indicus flowers have been attributed with several medicinal properties, including hypotensive, peripheral antidiabetic, and immunomodulatory activities.2,8 Despite its widespread medicinal use, the plant faces challenges in natural propagation due to low seed set, poor seed viability, and germination. Moreover, overexploitation for its medicinal value has led to a significant decline in its population5,9
However, as a result of overharvesting, habitat destruction, and environmental pressures, there is an urgent need for its conservation and sustainable propagation. There is an urgent need to propagate S. indicus for conservation and to meet the growing demands of pharmaceutical companies. In vitro propagation presents an effective method for conserving germplasm and multiplying this species, allowing for further research into its medicinal compounds. Currently, very few reports exist on organogenesis from leaf in S. indicus.10
In vitro propagation, commonly referred to as plant regeneration of plant through tissue culture of cells, tissues, or organs under sterile conditions on a nutrient medium. For S. indicus, in vitro propagation offers several advantages:
Conservation
The rapid multiplication of plantlets ensures the preservation of endangered or overexploited species.
Mass Production
In vitro techniques can produce large numbers of plants in compared to traditional propagation methods in relatively short time.
Genetic Stability
Direct organogenesis minimizes somaclonal variation, preserving the genetic traits of the mother plant.
Secondary Metabolite Production
Tissue culture can be utilized to enhance the production of valuable secondary metabolites for pharmaceutical purposes.
The high demand for S. indicus in traditional medicine (figure 1.2) has led to overexploitation, resulting in a rapid depletion of wild populations of in recent years.
In nature, S. indicus is propagated via seed and it also faces problem of low seed set, viability and germination rate.10 These factors greatly hinder the natural regeneration of the plant, limiting its population growth and making it more susceptible to decline. According to IUCN Red list data, S. indicus is in “Least Concern” category and IUCN state that “conservation and research action are needed for the plant S. indicus”. However, the IUCN emphasizes that conservation efforts and further research are necessary to ensure the long-term survival of the species. Despite its current status, the declining populations and overharvesting of S. indicus make it clear that urgent measures are required to safeguard this valuable medicinal plant. Therefore, the immediate need is to be propagate the plant for conservation and for commercial use in future to meet the high demand of pharmaceutical industries for their medicinal importance. In vitro propagation can be used effectively for multiplication and phytochemical analysis will open door for future investigation of its medicinally active constituents.
Direct organogenesis from nodal explants has been widely studied for S. indicus. The most common medium used is MS medium, which is supplemented with different concentrations of different plant growth hormone.10,11 Among these, cytokinins, especially 6-Benzylaminopurine (BAP), have been shown to play a crucial role in inducing shoot proliferation. Studies have reported that the concentration of BAP is pivotal in regulating shoot induction.10,12 This research is aimed to developing a reproducible protocol for rapid propagation of S. indicus through the following objectives:
Material and Methods
Plant Materials
The planting material of Sphaeranthus indicus was procured from Rice paddy field in Banka district of Bihar, India (24°44’30.5″N 87°03’20.3″E).
Surface Sterilization
The collected explants were thoroughly cleaned under running tap water to remove surface debris. They were treated with 0.1% Tween-20 for 5 min and rinsed with distilled water followed by 1% carbendazim for 5 minutes. The sterilization process continued with a 1-minute immersion in 70% ethanol and a subsequent 3-minute treatment in 0.1% mercuric chloride under aseptic conditions in a laminar airflow chamber. After each step, the explants were thoroughly rinsed 3–4 times with sterile double-distilled water and dried on sterile paper towels.10,13,14
Culture Media preparation
Murashige and Skoog (MS) medium was prepared with varying concentrations of 6-Benzylaminopurine (BAP: 1–3 mg/L) to assess its effect on multiple shoot induction. The pH of the medium was adjusted to 5.6–5.8 using 1M sodium hydroxide (NaOH) or 1N hydrochloric acid (HCl) before sterilization in an autoclave at 120°C and 15 psi for 15 minutes.
Explant Selection and Inoculation
The explants were chopped using scalpel and forceps and the explants were then inoculated onto MS medium containing different concentrations of BAP (1–3 mg/L) for multiple shoot induction.
Multiple shoot induction
The cultures were maintained in a growth chamber under the light with 16 hours photoperiod 1 provided by white fluorescent lights at a photosynthetic photon flux density (PPFD) of 83.6 μEm−2s−1. Four to five explants were introduced into each culture bottle containing the medium. The observations on frequency of shoot initiation, frequency and other parameters were taken each week.
Rooting induction
With the emergence of elongated shoots from the clumps, 4-5 cm shoots were separated and cultured on MS medium containing 0.5 mg/L of α- naphthaleneacetic acid (NAA) for root induction. After 10 days of culture, the duration and frequency of root induction, as well as the average number of roots per shoot, were recorded.
Acclimatization
The rooted plantlets were separated from the culture bottles, which was then Rinsed thoroughly under running tap water to eliminate agar residues. The plantlets were transplanted into plastic pots containing a 3:1 mixture of soil rite and soil and initially, they were acclimatized in a culture room for one week under high humidity conditions maintained using polythene covers. The covers were removed after one week and the plantlets were maintained in the culture room for an additional week before being relocated to a greenhouse. Subsequently, the plantlets were moved to earthen pots containing a mix of soil and farmyard manure and eventually transplanted to open-field conditions. The survival rate of the plantlets was recorded during this phase.
Result
Nodal explant of S. indicus were cultured on medium containing different concentration (1 mg/mL, 2 mg/mL, and 3 mg/mL) of BAP to evaluate their potential for multiple shoot induction. The initial response was observed within 7 days of culture. And culture responses were monitored and recoded in 7th day (figure 1: a-c), 14th day (figure 2: a-c) and 21st day (figure 3: a-c).
 |
Figure 1: (a-c) Initiation of multiple shoots from the nodular explants after culture in MS media with 1 mg/L BAP for 7 days. |
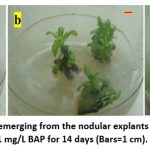 |
Figure 2: (a-c) Multiple shoots emerging from the nodular explants after culture in MS media with 1 mg/L BAP for 14 days (Bars=1 cm). |
 |
Figure 3: (a-c)- Proliferation of multiple shoots in MS media with 1 mg/L BAP for 21 days (Bars=1 cm). |
The emergence of fresh bud from the nodal region marked the beginning of the multiple shoot response. Among the three different concentrations of BAP used; the minimum concentration of BAP (1 mg/L) was the most suitable for multiple shoot induction, with highest frequency of 91.6 % (graph 1). The higher concentration of BAP (2 and 3 mg/L) resulted in inducing callus tissue from the nodal explants and hence not suitable for multiple shoot induction. Response of multiple shoots induction from nodal explant of S. indicus on MS media fortified with BAP were listed in table 01.
Table 1: Response of multiple shoots induction from nodal explant of S. indicus on MS media fortified with BAP.
| BAP (mg/L) | Number of Explantsinoculated | Shoot inductionfrequency (%) | Average No. ofShoots
|
OtherResponse |
| 1 | 48 | 91.6 | 5 | NA |
| 2 | 45 | 55.5 | 5 | Callusing |
| 3 | 48 | 41.6 | 4 | Callusing |
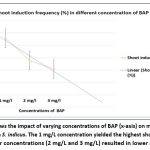 |
Graph 1: The graph shows the impact of varying concentrations of BAP (x-axis) on multiple shoot induction in percentage (y-axis) in S. indicus. |
The elongated multiple shoots were separated and placed on MS medium containing 1 mg/L NAA for root induction (Figure 4a-c). Root induction was successfully achieved at a frequency of 90 % with NAA. Root initiation was observed in 5-6 days on medium with 1 mg/L NAA. About 6-7 numbers of roots per shoot were induced in each shoot after 2 weeks of culture on medium containing NAA (Figure 5a-c). The regenerated plants were acclimatized for 2 weeks in culture room with a survival frequency of 100 % (Figure 6a-c).
 |
Figure 4: (a-c)- Elongation of shoots obtained from the multiple shoots separated and inoculated in root inducing media 1mg/L NAA. |
 |
Figure 5: (a)- Root induction in the rooting medium containing 1mg/L NAA after 10 days of culture; (b-c) Acclimatization of the regenerated plantlets (Bars=1 cm). |
The acclimatized plants were relocated to earthen pots in soil in greenhouse and successfully transplanted in the field. Thus, multiple shoot induction and subsequent plant regeneration was successfully achieved from nodal explants which can be exploited for micro propagation, germplasm conservation, secondary metabolites production etc.
 |
Figure 6: (a-c)- Regenerated plants transferred to soil and established in plastic pots ready for transfer in the green house after 2 weeks of acclimatization (Bars=1 cm). |
Discussion
The present study successfully demonstrated the in vitro regeneration of Sphaeranthus indicus through direct shoot organogenesis from nodal explants. The results indicate that the concentration of 6-Benzylaminopurine (BAP) plays a critical role in shoot induction, with the most favorable outcome observed at the lowest concentration of 1 mg/L BAP. This concentration resulted in a high shoot induction frequency of 91.6%, with multiple shoots emerging from the nodal region within 7 days. The ability to initiate shoots at such a high frequency highlights the suitability of this protocol for efficient micropropagation of S. indicus.
Interestingly, higher concentrations of BAP (2 and 3 mg/L) induced callus formation instead of promoting multiple shoot induction. This observation aligns with findings in other plant species, where elevated levels of cytokinins, such as BAP, tend to promote callogenesis rather than direct organogenesis. 10,15,16 The balance between cytokinin concentration and explant type is crucial, as excess BAP can lead to unwanted tissue responses. In the present study , 1 mg/L BAP provided the optimal conditions for multiple shoot induction without triggering callus formation, making it the ideal concentration for shoot proliferation in S. indicus.
Rooting of the elongated shoots was achieved with 1 mg/L α-naphthaleneacetic acid (NAA), leading to a 90% root induction frequency.17 Root initiation occurred rapidly, within 5-6 days, and the shoots developed 6-7 roots each after two weeks in culture. This demonstrates the efficiency of NAA in stimulating root development, which is critical for plantlet establishment.17 The ability to produce healthy, well-developed roots in a short period highlights the effectiveness of this protocol for producing vigorous plantlets suitable for transplantation.
The high survival rate of 100% during the acclimatization phase further underscores the robustness of the protocol. The plantlets successfully adapted to ex vitro conditions after being transferred from culture room to greenhouse, and eventually to the field. This seamless transition from in vitro conditions to open-field environments is essential for large-scale propagation efforts and for the conservation of this medicinally important species.
Overall, the results of this study provide a reliable and efficient method for the regeneration of S. indicus using nodal explants. The high-frequency shoot induction, rapid root formation, and successful acclimatization make this protocol suitable for various applications, including micropropagation, germplasm conservation, and potentially for the production of secondary metabolites. Given the medicinal importance of S. indicus, the established protocol can contribute to its large-scale cultivation and help meet the demand for this valuable plant in the pharmaceutical industry.
This study successfully established a protocol for the in vitro regeneration of S. indicus through direct shoot organogenesis from nodal explants. In vitro propagation of S. indicus offers a promising tool for its large-scale multiplication, conservation, and sustainable utilization. The protocols developed so far have demonstrated high efficiency in shoot and root induction, as well as successful acclimatization of plantlets. With continued research and optimization, tissue culture can make a significant impact on meeting the growing demand for this medicinally important plant, while also contributing to its conservation and further study.18 Future research should focus on refining protocols to further improve efficiency and reduce the time required for plant regeneration. The exploration of somatic embryogenesis,19 protoplast culture, and genetic transformation20 could open new avenues for both conservation and improvement of S. indicus.
Conclusion
The successful establishment of an in vitro regeneration protocol for S. indicus marks a significant step toward its biotechnological applications. The optimized conditions for shoot induction and rooting provide a practical approach for producing healthy plantlets, ensuring their successful adaptation to ex vitro conditions. By enabling the rapid multiplication of S. indicus, this protocol offers a valuable tool for preserving its genetic resources and supporting its sustainable use in traditional and modern medicine. Beyond its role in large-scale propagation, this method lays the groundwork for deeper exploration into the plant’s secondary metabolite production, which could enhance its pharmacological potential. Additionally, integrating molecular approaches, such as genetic fingerprinting and metabolic profiling, could further validate the genetic stability and biochemical consistency of regenerated plants. By bridging conservation efforts with medicinal research, this study contributes to both the sustainable utilization and scientific advancement of S. indicus. Future efforts should also focus on scaling up production in bioreactors and optimizing ex vitro growth conditions to maximize yield and therapeutic value.
Acknowledgements
The authors are grateful to the Patna University and University of Hyderabad, Telangana for providing internet access, a workspace, and instrumentation facilities for this research work.
Funding Sources
The author(s) received no financial support for the research, authorship, and/or publication of this article.
Conflict of Interest
The authors do not have any conflict of interest.
Data Availability Statement
This statement does not apply to this article
Ethics Statement
This research did not involve human participants, animal subjects, or any material that requires ethical approval.
Author Contributions
Gaurav Kumar Pandit: Conceptualization, Methodology, Writing – Original Draft.
Dr. Nyanthanglo Woch: Data Collection, Analysis, Writing – Review & Editing.
Preeti Kumari: Data Collection, Analysis, Writing – Review & Editing.
Ritesh Kumar Tiwari: Analysis, Writing- Review & Editing
Meenakshi Singh: Visualization, Supervision, Project Administration
References
- Adzu B., Amos S., Amizan M.B., Gamaniel K. Evaluation of the antidiarrhoeal effects of Zizyphus spina-christi stem bark in rats. Acta Trop. 2003:87(2):245-250.
CrossRef - Khan R.J., Jha R.K., Amera G.M. Targeting SARS-CoV-2: a systematic drug repurposing approach to identify promising inhibitors against 3C-like proteinase and 2′-O-ribose methyltransferase. J Biomol Struct Dyn. 2021:39(8):2679-2692.
CrossRef - Jain A., Basal E. Inhibition of Propionibacterium acnes-induced mediators of inflammation by Indian herbs. Phytomedicine. 2003:10(1):34-38.
CrossRef - Gururaj G., Isaac M.K., Subbakrishna D.K., Ranjani R. Risk factors for completed suicides: a case-control study from Bangalore, India. Inj Control Saf Promot. 2004:11(3):183-191.
CrossRef - Vidya T.N.C., Fernando P., Melnick D.J., Sukumar R. Population genetic structure and conservation of Asian elephants (Elephas maximus) across India. Anim Conserv. 2005:8(4):377-388.
CrossRef - George J.D., Vehrs P.R., Allsen P.E., Fellingham G.W., Fisher A.G. VO2max estimation from a submaximal 1-mile track jog for fit college-age individuals. Med Sci Sports Exerc. 1993:25(3):401-406.
CrossRef - Ramachandran S. Review on Sphaeranthus indicus (Koaikkarantai). Pharmacogn Rev. 2013:7(14):157-169.
CrossRef - Nanra J.S., Buitrago S.M., Crawford S. Capsular polysaccharides are an important immune evasion mechanism for Staphylococcus aureus. Hum Vaccin Immunother. 2013:9(3):480-487.
CrossRef - Fernando P., Vidya T.N.C., Payne J. DNA analysis indicates that Asian elephants are native to Borneo and are therefore a high priority for conservation. PLoS Biol. 2003:1(1).
CrossRef - Yarra R., Aileni M., Kumar V. A., Kokkirala R., Umate P., Abbagani S. Direct Shoot Regeneration from Mature Leaf Explants of Sphaeranthus indicus, a Multipurpose Medicinal Plant. Journal of Phytology. 2010:1(5):5-11.
- Harathi K., Naidu C. V. Influence of Ethylene Inhibitor Silver Nitrate on Direct Shoot Regeneration from Raised Shoot Tip Explants of Sphaeranthus indicus —An Important Antijaundice Medicinal Plant. Am J Plant Sci. 2016:7(03):525-532.
CrossRef - Mishra A.K., Tiwari K.N., Mishra P., Tiwari S.K., Mishra S.K., Saini R. Effect of cytokinin and MS medium composition on efficient shoot proliferation of Nyctanthes arbor-tristis through cotyledonary node explant and evaluation of genetic fidelity and antioxidant capacity of regenerants. South African Journal of Botany. 2019:12(7):284-292.
CrossRef - Haque M.I., Singh P.K., Ghuge S. A general introduction to and background of plant tissue culture: Past, current, and future aspects. Advances in Plant Tissue Culture: Current Developments and Future Trends. 2022:1-30.
CrossRef - Sharma S., Reddy M.S., Kumar A. Direct shoot organogenesis from leaf explants of Populus deltoides and changes in selected enzymatic activities. Physiology and Molecular Biology of Plants. 2020:26(2):399.
CrossRef - Nakasha J.J., Sinniah U.R., Kemat N., Mallappa K.S. Induction, Subculture Cycle, and Regeneration of Callus in Safed Musli (Chlorophytum borivilianum) using Different Types of Phytohormones. Pharmacogn Mag. 2016:12(4):460.
CrossRef - Kaur K., Dolker D., Behera S., Pati P.K. Critical factors influencing in vitro propagation and modulation of important secondary metabolites in Withania somnifera (L.) dunal. Plant Cell Tissue Organ Cult. 2022:149(41): 1-2.
CrossRef - Yan H., Li J.N., Zhang X.Q., Effect of Naphthalene Acetic Acid on Adventitious Root Development and Associated Physiological Changes in Stem Cutting of Hemarthria compressa. PLoS One. 2014:9(3):e90700.
CrossRef - Janarthanam B., and Sumathi E. In vitro Plant Regeneration from Nodal Explants of Coleus forskohlii – An Important Medicinal Plant. Plant Tissue Cult. & Biotech.2020: 30(1): 143-148
CrossRef - Martínez T., and Corredoira E. Recent Advances in Plant Somatic Embryogenesis: Where We Stand and Where to Go?. J. Mol. Sci. 2024: (25): 8912.
CrossRef - Mohammadi M.A., Wai1 M.H., Rizwan H.M., Qarluq A.Q., Xu M., Wang L., Cheng Y., Aslam M., Zheng P., Wang X., Zhang W. and Qin Y. Advances in Micropropagation, somatic embryogenesis, somatic hybridizations, genetic transformation and cryopreservation for Passifora Plant Methods. 2023: (19):50
CrossRef

