Introduction
The second most important cultivated vegetable crop globally is tomato, around 115.95 million tons per year, as it is rich with vitamins A and C 1-4. Tomatowilt is caused by Fusarium oxysporum. Lycopersici is one of the most destructive and economically damaging diseases, and it is responsible for reducing the quality and quantity of tomato production. F. oxysporum. Sp. lycopersiciis is a common soil-borne fungus causing severe injury through all phases of tomato growth as it is found in infested agricultural soils, leading to economic losses with characteristic symptoms including wilting, chlorosis, stunted seedlings 3, 5-11. The usage of chemicals is neither economical nor environmentally friendly, and excessive misuse of fungicides has led to the development of resistance against fungicides 12,13. Fusarium wilt damage has stimulated researchers for biological control using fungi or bacteria, which are antagonistic non-pathogenic microorganisms that have the potency to reduce harmful effects of wilt diseases, which is an alternative to chemical control 14-17.
Endophytes are powerful biostimulants in inhibiting pathogens, enhancing environmental stress tolerance through an antioxidant system and promoting plant growth, nutrient availability and yield of the crop through the production of indole acetic acid, siderophore, volatile compounds, inorganic phosphate solubilization17,18-20. Plant growth-promoting fungi effectively control fungal phytopathogens, including F. oxysporum, by producing antioxidants and phytohormones and minimising mycotoxin production 18,21-23.
Utilising microbes to enhance plant growth and combat plant pathogens is an increasingly recognised concept, offering new insights into sustainable agriculture and reducing the reliance on chemical fertilisers and pesticides. In the past few years, the development of microbial bioinoculants to promote plant growth and eliminate pathogens has emerged as an alternative. Bioformulation research focuses on microorganisms with multiple potentials to inhibit plant pathogens, encourage plant growth, and help to enhance the fertility of soil as an environment-friendly approach.
Hence, this research aims to characterise an endophytic fungus, Aspergillus oryzae AVNF4, out of four fungi isolated from the rhizome of Curcuma longa, its potential in fungal antagonism against Fusarium wilt, and the development of liquid bioformulation to promote the growth of tomato seedlings in pot experiments under greenhouse conditions.
Materials and Methods
Isolation of Curcuma endophytic fungi
The endophytic fungus was isolated from rhizomes of Curcuma longa collected from the local agricultural field of Nuthakki(16.4158° N, 80.6507° E), village Guntur, A.P. Rhizome was washed to remove dust, debris and soil or clay particles adhered to it with tap water and distilled water. 1g of sterilised rhizome was smashed with 1 ml of 0.1% NaCl, and then 9 ml of 0.1% NaCl was added. Fungus was isolated on Sabroud’s medium by serial dilution, and the isolate was confirmed as the endophyte described earlier 24. The last rinsed aliquot (100 µl) was added to Sabroud’s Petri plate for control.
Identification of Endophytic fungal strain
The physiological characterisation was done by a qualitative screening of hydrolytic enzymes, i.e., amylase, cellulase, protease, lipase, urease, gelatinase, and catalase25followed by18srRNA gene amplification using a universal primer NS1(5′ GTA GTC ATA TGC TTG TCT C 3′), and NS24 (5′ TCC GCA GGT TCA CCT ACG GA) 3′, and NS24 5′ (TCC GCA GGT TCA CCT ACG GA 3′). A commercial company, Macrogen, sequenced the amplified genes. Seoul, South Korea. Based on 100% sequence similarity, a phylogenetic tree was constructed using the neighbour-joining method. After identification, AVNF4 was deposited at NCBI, India.
Antifungal activity
The antifungal activity of the endophytic fungal isolate was screened against F. oxysporum using a dual culture assay. F. oxysporum mycelium of 1 mm length was spot inoculated at the centre of Sabroud’s dextrose agar (SDA) plate, and the endophytic fungal isolate was streaked on either side of the F. oxysporum spot at a distance of 3 cm and incubated at 35±2 ℃ for 7 days. SDA plate spot inoculated with the F. oxysporum alone was served as control26. The per cent inhibition of the growth of pathogen was determined by using the formula:
Inhibition of mycelial growth (%) = [X/(X-Y)] X100
Where X and Y are radial growth of mycelia of the pathogen in the absence of an antagonist and the presence of an Antagonist
plant growth-promoting traits
Plant growth-promoting traits (PGP) such as Indole-3-acetic acid (IAA), Ammonia (NH4+) production and Inorganic PO4–solubilisation (IPS) were screened both qualitatively and quantitatively.
IAA production was qualitatively determined by the Salkowski reagent method using SDA and L-Tryptophan (1 µg ml-1) 27 after 7 days of incubation. The culture supernatant was separated by centrifugation at 10,000 rpm for 15-20 min. The supernatant(1ml)was mixed with one drop of orthophosphoric acid and 2 ml of Salkowski reagent (150 ml H2SO4, 7.5 ml FeCl3.6H2O 0.5 M in 250 ml distilled H2O)and incubated for 30 min. The appearance of pink determined IAA and measured the quantity spectroscopically at 530nm using the IAA standard curve28.
IPS was qualitatively determined using a Nutrient agar medium supplemented with tricalcium phosphate. A loop full of culture was placed on the centre of agar plates and incubated at 30±10℃ for 5 days. Inorganic phosphate solubilisation was confirmed as a clear zone around the fungal colony 29and was determined quantitatively using Bartons reagent30
NH4+ production was tested in peptone agar media using Nessler’s reagent (0.5 ml) on the plate. Ammonia production was confirmed by the development of a brown to yellow colour, and quantitative production was determined using 10 ml of peptone water incubated for seven days at 35 ± 2°C by the Nesslerization method31.
Optimization studies
Analyzed growth optimization at different pH ranging 5,6,7,8 and different temperatures (25℃, 30℃, 35℃, 40℃, 45℃), 1% carbon sources (starch, sucrose, fructose, mannitol, dextrose, lactose, glucose, glycerol and maltose) and 0.5% of different nitrogen sources (peptone, yeast extract, beef extract, urea, ammonium chloride, ammonium sulphate, potassium nitrate, and sodium nitrate) in culture broth. Growth was measured in terms of biomass after seven days of incubation24.
Development of Liquid bioformulation
A liquid bioformulation of A. oryzae AVNF4 was developed in the optimised SD media on a shaker at 180 rpm and 35 °C for 7 days32.
Greenhouse studies
Preparation of bacterial cell suspension
Royal variety seeds of tomato (Lycopersicon esculentum L.)were selected for greenhouse studies. Seeds were surface sterilised with 5% sodium hypochlorite and 70% ethanol, followed by distilled water rinse for 5times 33. Seed bacterisation was done by soaking 50 ml of freshly prepared AVNF4 bioformulation for 48 h. The SD broth without fungal inoculation was used as a control treatment. The seeds were sowed in plastic pot trays containing 98 cavities filled with well-sterilised coco peat. Seedling vigour index or germination percentage (Gp), speed of seed germination (S), germination rate (GR), and germination index (GI) were analysed after the first week of germination.
Pot experiment
The study was carried out in an open greenhouse condition. Weekly foliar application of the liquid bioformulation was administrated from the second week. This pot experiment was done using the randomised method, ensuring seven replicates of each treatment. The plants were uprooted from AVNF4 liquid bioformulation treated plants and rinsed with water to remove adhering soil. Various growth parameters, including shoot length, root length, fresh weight and number of leaves, were checked at 42 days and 84 days after sowing (DAS) from each replication 34. The biochemical traits, such as protein and carbohydrate of leaf extracts, were determined at 42 days and 84 days DAS of inoculated and uninoculated plants. The data was statistically analyzed by using one way ANOVA followed by Tukey’s HSD post hoc test.
Metabolite Profile Fingerprint of Endophytic Fungi
Fourier transforms infrared spectroscopy (FT-IR), and GC-MS were used to analyse secondary metabolite fingerprinting in ethyl acetate extract of endophytic fungi35,36,37
Results
Four morphologically distinct endophytic isolates were isolated from Curcuma longa rhizome. AVNF4 was selected for the study.
Identification of AVNF4
Based on physiological characteristics, AVNF4 is positive for amylase, cellulase, lipase, protease, urease, gelatinase, and catalase activity (Table 1). 1658 bp of 18s rRNA gene sequence was amplified from AVNF4. The pairwise sequence similarity search of the AVNF4 strain blast in ex-taxon showed that the 18s rRNA gene sequence of AVNF4 has 100% similarity with the partial 18srRNA sequence of the Aspergillus strain (gi: MK371712.1). Phylogenetic analysis based on 18s rRNA partial gene sequence revealed that AVNF4 was closely related to Aspergillus oryzae (Fig. 1). Based on consistent results of sequence analysis of the 18s rRNA partial gene, AVNF4 was deposited in NCBI as Aspergillus oryzae with Gen bank accession number PP962402.
Table 1: Physiological characterization of Fungal endophytes
|
Isolate |
Amylase |
Cellulase |
Lipase |
Protease |
Urease |
Gelatinase |
Catalase |
|
AVNF4 |
+++ |
++ |
++ |
+++ |
++ |
++ |
+++ |
+++ – High; ++ – Moderate; + – Low
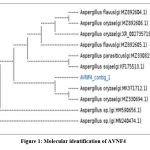 |
Figure 1: Molecular identification of AVNF4 |
Fig 1. Phylogenetic tree constructed by neighbour-joining method showing that AVNF4 has 99% sequence similarity with Aspergillus oryzae
Fungal antagonism and PGP traits
Aspergillus oryzaeAVNF4, screened for antagonistic activity, significantly inhibited Fusarium oxysporum compared to the control by reducing the hyphal growth of F. oxysporum, completely covering the pathogen, by overgrowing the isolate. A. oryzae is positive for qualitatively screening PGP traits like indole acetic acid, ammonia production, and inorganic phosphate solubilisation activities.
Effect of Physical and Chemical Parameters on the Growth of Aspergillus oryzae
Aspergillus oryzae AVNF4 showed maximum growth in terms of biomass (gm/ml) at 35℃, pH 7, 1% fructose and 0.5% peptone (figure 2). A. oryzae showed higher values of biomass at standard culture conditions of temperature (35℃) pH 7 and nitrogen source (peptone), except carbon source where the biomass reported high in fructose. In the optimised medium, the production of IAA, ammonia and phosphate solubilisation was increased by 175%, 150% and 493%, respectively (Table 2).
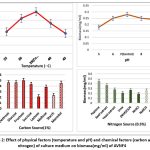 |
Figure 2: Effect of physical factors (temperature and pH) and chemical factors (carbon and nitrogen) of culture medium on biomass(mg/ml) of AVNF4 |
Fig 2: A. oryzae showing higher values of biomass at standard culture conditions of temperature (35℃) pH 7 and nitrogen source (peptone), carbon source (fructose).
Table 2: Quantitative analysis of PGP traits before and after optimization
|
IAA (µg/ml) |
PO4–solubilisation(ppm) |
Ammonia (µg/ml) |
||||
|
|
Before optimization |
After optimization |
Before optimization |
After optimization |
Before optimization |
After optimization |
|
Control |
0.2±0.03 |
0.5±0.03 |
2±0.45 |
4.3±0.35 |
0.2±0.03 |
0.5±0.04 |
|
AVNF4 |
2.4±0.45 |
6.6±0.35⁕ |
125±0.45 |
741±3.6⁕ |
3.2±0.3 |
8.0±0.4⁕ |
Values are the mean of three replicates ± SE.
Table 2: ⁕ Significantly increased after optimisation, a significant increase in IAA, ammonia and phosphate solubilisation by 175%, 150% and 493%
Greenhouse studies
The seedling vigour index results of 48-h bacterised tomato seeds were as follows: The seedling vigour index enhanced the germination percentage (Gp), germination rate (GR), and germination index (Gi) by 70.5%, 37 %, and 76.2%, respectively, compared to the control (Table 3).
Table 3: Seedling vigour index of tomato seeds treated with Aspergillus oryzae AVNF4
|
Growth parameters |
Control |
Aspergillus.oryzae |
% increase |
|
|
Germination percentage (Gp) |
Gp= Ni/NX100 |
31.83% |
54.28% |
70.5% |
|
Speed of seed germination (S) |
S=ni/di |
Day 1=70 Day 2=20 Day 3=8.6 Day 4=5 |
Day 1= 50 Day 2= 60 Day 3= 32 |
|
|
Germination rate (GR) |
(GR)= seed/1st day=……seed/nth day |
103.6 |
142 |
37% |
|
Germination index (GI) |
GI=∑G/T |
30.8 |
54.27 |
76.2% |
The values are the mean of three replicates. ± SE, Germination percentage = the number of seeds germinated per time duration, vigour index calculated during the first week. The value in parenthesis is the percentage increase of growth parameters compared to the control.
Plant growth promotion
The activity of A. oryzae AVNF4 liquid bioformulation on plant growth promotion of tomatoes was studied using a pot experiment under greenhouse conditions. Growth parameters of root and shoot length and height, fresh weight, root/shoot ratio, number of leaves, and metabolites are determined in seedlings treated with A. oryzae liquid formulation. Readings were recorded at intervals of one week (7 days) after sowing until 12 weeks. Liquid bioformulation showed that the growth parameters are significantly increased and also showed progressive growth from the 6th to12th week(Table4)
Table 4: Plant growth promotion of tomato seedlings treated with Aspergillus oryzae AVNF4
|
|
Biomass |
Total |
Shoot |
Root |
Roo/shoot |
No. of |
||||||
|
|
6th |
12th |
6th |
12th |
6th |
12th |
6th |
12th |
6th |
12th |
6th |
12th |
|
Control |
0.08± |
0.18± |
10.6± |
12.4 |
9.2 |
10.4 |
1.4± |
2.0± |
0.15± |
0.186± |
2± |
3± |
|
AVNF4 |
0.15± (87.5%) |
0.85± (372.2%) |
14.3 (34.9%) |
23.8 (91.9%) |
12.3± (33.7%) |
20.1± (93.2%) |
2± (42.8%) |
3.7± (85%) |
0.16± (6.6%) |
0.18± (-3.2%) |
7± (100%) |
9± (200%) |
Values are mean ± SE of three replicates
Table 4: In A. oryzae AVNF4 bioformulation treated plants the percentage increase in biomass is by 87.5% and 372%, plant height by 34.9% and 91.9%, shoot length by 33.7% and 93.2%, root length by 42.8% and 85%, number of leaves by 100% and 200% compared to bioformulation untreated control plant during 6th and 12th week of treatment.
Nutrient metabolites
Nutrient metabolites such as reducing sugars and proteins were also increased in AVNF4treated seedlings after 12 weeks of the treatment (Table 5). Reducing sugars has increased in the order of (100% and 233%) of AVNF4 treated plants, and protein content has increased from (77.1% and 150%) compared to untreated control.
Table 5: Primary metabolites
|
|
Reduced Sugars |
Protein |
||
|
|
6th week |
12thweek |
6thweek |
12thweek |
|
Control |
0.025±0.005 |
0.045±0.005 |
0.035±0.05 |
0.08±0.003 |
|
A. oryzae AVNF4 |
0.05±0.005 (100%) |
0.15±0.05 (233%) |
0.062±0.004 (77.1%) |
0.2±0.04 (150%) |
Table 5: Reducing sugar content is increased by 100% and 233% during the 6th week and 12th week in A. oryzaebioformulation-treated tomato plants compared to bioformulation untreated control plants. The protein content increased by 77.1% and 150% during the 6th and 12th week in A. oryzae bioformulation-treated tomato plants compared to bioformulation untreated control plants.
The FT-IR spectrum of the A. oryzae AVNF4 showed8 absorption peaks at 3322.45 cm-1, 2943.98 cm-1, 2831.94 cm-1, 1659.17 cm-1, 1450.33 cm-1, 1413.25 cm-1, 1113.61 cm-1, 1019.84 cm-1, respectively, with characteristic functional groups at peak at 2943.98 cm−1and 2831.94 cm−1 to alkyl C–H stretch, C=N bonds (1659 cm−1), C-H(1413 cm−1 ), C-O bonds ( 1113 cm−1) andC-F stretch at 1010cm−1respectively(Fig 3; Table 6).
 |
Figure 3: Interpretation of bioactive metabolites of Aspergillus oryzae AVNF4 by FTIR spectral analysis |
Fig 3: FT-IR Spectra of A. oryzaewith peaks3322.45 cm-1, 2943.98 cm-1, 2831.94 cm-1, 1659.17 cm-1, 1450.33 cm-1, 1413.25 cm-1, 1113.61 cm-1, 1019.84 cm-1, respectively.
Table 6: Data Analysis by FTIR Spectra
|
Wavelength range: |
Bond stretch and Functional groups |
|
3322.45 |
Imino compounds, =N-H stretch (medium, blunt) |
|
2943.98 |
C-H, (medium) |
|
2831.94 |
C-H ,(weak, sharp) |
|
1659.17 |
Open-chain imino (-C=N) (short) |
|
1450.33 |
Methylene C-H bend (medium, sharp) |
|
1413.25 |
vinyl C-H (medium) |
|
1113.61 |
Alkyl-substituted ether, C-O ,(short) |
|
1019.84 |
Aliphatic fluoro compounds, C-F ,(strong) |
Table 6: FT-IR spectra of A. oryzae with corresponding functional groups.
In GC MS chromatogram, Metabolic Profile of Fungal extract of A. oryzae AVNF4 17 bioactive metabolites “1,3-Dioxolane, 2-(1-propenyl)-, L-Prolinamide, 5-oxo-L-prolyl-L-phenylalanyl-4-hydroxy-, Pyrrolo[1,2-a]pyrazine-1,4-dione, hexahydro-, dl-Mevalonic acid lactone, Hydro cinnamic acid, Oleic acid, 9-Octadecenoic acid (Z)-, Pyrrolo[1,2-a]pyrazine-1,4-dione, hexahydro-3-(phenylmethyl)-, 1-Allylazetidine, 4H-Pyran-4-one, 2,3-dihydro-3,5-dihydroxy-6-methyl-, 5-Pyrrolidino-2-pyrrolidone, Pyrrolo[1,2-a]pyrazine-1,4-dione, hexahydro-3-(2-methyl propyl)-, 9-Octadecenoic acid (Z)-, hexyl ester, Dehydromevalonic lactone, Acetaldehyde, (3,3-dimethyl cyclohexylidene)-, (Z)-“ were observedas per the NIST Database(fig 4; Table 7)
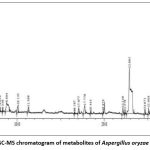 |
Figure 4: GC-MS chromatogram of metabolites of Aspergillus oryzae AVNF4 |
Fig 4: GC-MS spectrum of Aspergillus oryzae AVNF4 crude ethyl acetate extract.
Table 7: Metabolic Profile of Fungal extract of Aspergillus oryzae AVNF4
|
S. No |
RT |
Height (%) |
Compound |
Formula |
Mol wt |
Area(%) |
|
1 |
22.905 |
31.72 |
Phenol,2,4’-isopropylidenedi |
C15H16O2 |
228 |
55.32 |
|
2 |
8.805 |
6.14 |
1,3-Dioxolane, 2-(1-propenyl)- |
C6H10O2 |
114 |
10.59 |
|
3 |
27.678 |
11.20 |
L-Prolinamide, 5-oxo-L-prolyl-L-phenylalanyl-4-hydroxy- |
C19H24N4O5 |
388 |
6.86 |
|
4 |
27.316 |
6.45 |
L-Prolinamide, 5-oxo-L-prolyl-L-phenylalanyl-4-hydroxy- |
C19H24N4O5 |
388 |
5.64 |
|
5 |
17.770 |
5.67 |
Pyrrolo[1,2-a]pyrazine-1,4-dione, hexahydro- |
C7H10N2O2 |
154 |
4.69 |
|
6 |
10.133 |
5.47 |
dl-Mevalonic acid lactone |
C6H10O3 |
130 |
3.74 |
|
7 |
11.309 |
3.66 |
Hydro cinnamic acid |
C9H10O2 |
150 |
2.38 |
|
8 |
22.149 |
4.41 |
Oleic acid, 9-Octadecenoic acid(Z)- |
C18H34O2 |
282 |
2.16 |
|
9 |
25.088 |
4.70 |
Pyrrolo[1,2-a]pyrazine-1,4-dione, hexahydro-3-(phenylmethyl)- |
C14H16N2O2 |
244 |
1.86 |
|
10 |
17.077 |
4.01 |
1-Allylazetidine |
C6H11N |
97 |
1.83 |
|
11 |
8.024 |
4.89 |
4H-Pyran-4-one, 2,3-dihydro-3,5-dihydroxy-6-methyl- |
C6H8O4 |
144 |
1.49 |
|
12 |
24.671 |
3.52 |
Pyrrolo[1,2-a]pyrazine-1,4-dione, hexahydro-3-(phenylmethyl)- |
C14H16N2O2 |
244 |
1.46 |
|
13 |
18.443 |
1.72 |
5-Pyrrolidino-2-pyrrolidone |
C8H14N2O |
154 |
0.53 |
|
14 |
19.878 |
1.74 |
Pyrrolo[1,2-a]pyrazine-1,4-dione, hexahydro-3-(2-methyl propyl)- |
C11H18N2O2 |
210 |
0.46 |
|
15 |
22.376 |
1.79 |
9-Octadecenoic acid (Z)-, hexyl ester |
C24H46O2 |
366 |
0.37 |
|
16 |
8.336 |
1.72 |
Dehydromevalonic lactone |
C6H8O2 |
112 |
0.35 |
|
17 |
16.587 |
1.19 |
Acetaldehyde, (3,3-dimethyl cyclohexylidene)-, (Z)- |
C9H14O |
138 |
0.28 |
Table 7: GC-MS compounds Aspergillus oryzae AVNF4 ethyl acetate crude extract with RT values
 |
Figure 5: Spectrum ofActive metabolites of A. oryzae AVNF4 identified in GC-MS |
Figure 5: GC-MS chromatogram of active metabolites of A. oryzae AVNF4, Oleic acid with molecular formula C18H34O2, RT value 22.1, 9-Octadecenoic acid (Z)-, hexyl ester with molecular formula C24H46O2, RT value 22.3 and Pyrrolo[1,2-a]pyrazine-1,4-dione, hexahydro-3-(phenylmethyl)- with molecular formula C14H16N2O2 and RT value 24.6 respectively.
Discussion
Due to its medicinal properties and wide range of applications, the scope of research on Curcuma longa is leading to discoveries at the molecular and genetic levels. Endophytes help plant growth and produce bioactive secondary metabolites in disease management and agrochemical industries. Hence, A. oryzaeAVNF4, an endophytic fungus of Curcuma longa, was explored to understand its impact in providing biostimulants to the growth of tomato seedlings as a biofertiliser and nutrient for sustainable tomato growth.
By producing hydrolytic enzymes such as cellulases, proteases, and pectinases that break down cell walls or plant tissue, endophytes colonise themselves inside the host plant 38–43. Endophytic fungi isolated from oil seeds and medicinal plants exhibited significant cellulase and pectinase activity levels. Hydrolytic extracellular enzymes, specifically cellulase, protease, and pectinase, are produced by 72% of endophytes isolated from medicinal plants. These enzymes serve as bioactivity to extract nutrients from hosts, bio-resistance during pathogen-host interactions to prevent microbial pathogenic infection, and, on the other hand, to enhance the nutritional status of plants 44. Similarly, the production of hydrolytic enzymes like amylases, cellulase, pectinases and gelatinases (Table 1) by A. oryzae AVNF4 is responsible for degrading the hyphal cell wall of phytopathogen. It may also be the driving force for the positive interaction of A. oryzae AVNF4 with the root system of tomato seedlings, resulting in a significant enhancement in the growth of tomato seedlings (Table 4). Fungal endophytes belonging to the genera Trichoderma, Fusarium, Pestalopsis and Kocuriaroceawere were reported to be isolated from rhizomes of Curcuma longa45.
Endophytic fungi, A. favus, A. fumigatus, A. nidulans, and R. oryzae isolated from Ocimumbasilicumplantandrhizospheric soil inhibited the growth of F. oxysporum19, 6. Metabolic products produced from fungi have properties of antibacterial, antifungal, antidiabetic, antioxidant, and immunosuppressive. They are proven to inhibit the growth of different pathogenic fungi and bacteria46.A.oryzae AVNF4 arrested growth of F. oxysporum in dual culture assay. Similar to our results, hyphal parasitism of P.aphanidermatum by Trichoderma spp. Isolated from soil was reported47. Earlier studies45 reported that endophyte T. harzianumTharDOB-31, isolated from the rhizome of Curcuma, inhibited the mycelial growth of R. solani and P. aphanidermatumby76.9% and 76%. Volatile compounds of endophytes were also reported to control the adverse effects of drought and salinity stresses and influence plant growth 48,49.
Endophytes promote plant growth directly by producing hormones, including indole-3-acetic acid (IAA), gibberellins, cytokinins, phosphate solubilisation, N2 fixation or indirectly by producing antibiotics, and siderophores49,50. Fungal endophytes like Aspergillus spp., P. citrinum and P. oxalicum isolated from seaweeds can phosphorus solubilising activity 51. A. oryzaeAVNF4 is favourable to PGP traits like IAA by producing a pink colour upon the addition of Salkowski reagent, ammonia production by the Nesslerization method and inorganic Phosphate solubilisation by forming a clear halo zone around the culture when supplemented with tricalcium phosphate. Based on the results, A. oryzaeAVNF4 can be considered a potential isolate with multiple PGP traits.
The optimisation of physical and chemical factors in growth reveals that A. oryzae AVNF4 was a low-cost maintenance culture that exploited higher biomass (Fig 2). Production of PGP traits has enhanced by 175%, 150% and 493% of IAA, NH3 production and PO4–solubilization after optimisation (Table 2), indicating that enhancement primarily relies on carbon source rather than pH, temperature and nitrogen source. Hence, a 1% fructose-enriched medium could have enhanced the growth and PGP traits of A. oryzae (Fig 2). Therefore, optimised media can be considered as promising liquid bioformulation. Further investigation is needed to enhance shelf life.
A. oryzae AVNF4-treated tomato seeds showed a good seed vigour index compared to A. oryzae untreated control seeds (Table 3). Earlier studies52 showed that endophyte Aspergillus oryzaecolonisedR. sativus seedlings through seed inoculation and acted as plant growth promoters.
Administration of A. oryzae AVNF4 liquid formulation to tomato seedlings in pot experiments showed progressive enhancement of growth parameters of tomato from the sixth week to the 12th with statistically significant values. Also, it is evidenced that the A. oryzae AVNF4 formulation has a high potential for tomato seedling growth (Table 4). Similar to our results, it was reported that A. oryzae treated cacao (Theobroma cacao), the plant synthesised kojic acid, making the plant more tolerant to insects and pathogens 53. Endophytes A. tubingensis, A. alabamensis and A. oryzae act as plant growth promoters and are commercially used as eco-friendly agents for the defence of pepper seedlings against Fusarium wilt disease 54,55.
Among different microbial groups, fungi have been reported to be more efficient IAA production and phosphate solubilisers in comparison to bacteria influencing overall plant growth and root development, which is a direct mechanism by which biocontrol agents promote shoot and root growth and leaf area in plants56, 57,58. Similarly, A. oryzae (AVNF4) reported high amounts of IAA, ammonia production and inorganic phosphate solubilisation (Table 2). Production of growth-promoting metabolites by fungi is one of the reasons to help host plants survive under stress conditions by secreting favourable secondary metabolites. Fungal endophytes like Alternaria, Aspergillus, Cladosporium, Colletotrichum, Fusarium, Talaromyces, Trichoderma and Penicillium promote plant growth by improving soil structure and by providing resistance against biotic and abiotic stresses 59,60. Liquid bioformulation without a carrier can be handled easily and has the properties of longer shelf life without any contamination 61. Significant enhancement of root growth of a plant when inoculated with fungal endophytes differs with different endophytes, as endophytes have different abilities for IAA production62
When compared with the liquid bioformulation uninoculated control plants, there was a significant increase in fresh weight, root and shoot length, and number of leaves. Primary metabolite content, like protein and carbohydrate content, was also higher in tomato plants treated with A. oryzaeAVNF4 liquid bioformulation, progressively from the 6th week to the 12th week (Table 4).The results revealed that liquid bioformulation of A. oryzae AVNF4 was promising in retaining the ability to promote tomato growth.
Plant growth-promoting bacteria like Bacillus, Brevi bacillus, B. subtilis, and Paenibacillus are used as biofertilisers for Brassica napus, Cajanus cajan, Cicer arietinum, Vigna radiata, and rice 63-67. Our results provide evidence that A. oryzae could act as an endophyte, work as a plant growth promoter, and provide some protection against F. oxysporum.
In the GC MS chromatogram, a significant peak was observed at RT 22.9 with the molecular weight of 228 g/mol with the chemical formula C15H16O2. Compared to the NIST library, a significant peak with a molecular weight of 228 g/mol and the chemical formula C15H16O2 was seen at RT 22.9 in the GC MS chromatogram. The main compound was determined to be Phenol2,4′-isopropylidene, a BPA (Bisphenol A) aberration brought on by analysis compared to the NIST library. The following significant peak, with a 114 g/mol molecular weight and the chemical formula C6H10O2, was detected at RT 8.805. The FTIR data revealed that 1,3-Dioxolane, 2-(1-propenyl)- was the main compound found in the crude extract of A. oryzae compared to the NIST library. Similarly, it has been verified that the compounds produced by A. oryzae identified in the GC-MS study and those found in the FTIR analysis are the same. According to GC-MS data, the A. oryzae AVNF4 crude extract includes volatile organic compounds and secondary metabolites that effectively suppress phytopathogens, promote plant growth, and induce systemic resistance36,68, 37.
Metabolic profile of fungal extract of A. oryzae AVNF4 as per the NIST Database, bioactive metabolites such as 1,3-Dioxolane, 2-(1-propenyl)-, L-Prolinamide, 5-oxo-L-prolyl-L-phenylalanyl-4-hydroxy-, Pyrrolo[1,2-a]pyrazine-1,4-dione, hexahydro-, dl-Mevalonic acid lactone, Hydro cinnamic acid, Oleic acid, 9-Octadecenoic acid (Z)-, Pyrrolo[1,2-a]pyrazine-1,4-dione, hexahydro-3-(phenylmethyl)-, 1-Allylazetidine, 4H-Pyran-4-one, 2,3-dihydro-3,5-dihydroxy-6-methyl-, 5-Pyrrolidino-2-pyrrolidone, Pyrrolo[1,2-a]pyrazine-1,4-dione, hexahydro-3-(2-methyl propyl)-, 9-Octadecenoic acid (Z)-, hexyl ester, Dehydromevalonic lactone, Acetaldehyde, (3,3 dimethyl cyclohexylidene)-, (Z)- showed bioactivities like antifungal, root colonisation, anti-inflammatory, plant defence mechanisms and promotes plant growth and metabolism69-77. Pyrrolo [1,2- a] pyrazine-1,4-dione, hexahydro-3-(phenylmethyl) with a molecular weight of 244.2 g/mol was previously isolated from endophytic Neotyphodium spp. and Epichloe species function against phytopathogens and worms and also observed during plant-microbe relationships (Fig 5). It is also related to pyrazine derivatives and other diketopiperazine-mycotoxins, such as aspergillic acid, produced by the Aspergillus and Candida species 78-81
In earlier studies, an endophytic fungus, Aspergillus oryzae YRA3, and A. flavus, isolated from the wild plant Atractyliscarduus (Forssk.) C.Chr, E. genicu late reported the presence of pyrrolo[1,2-a]pyrazine-1,4-dione, hexahydro, Oleic acid and Octadeconoic acid (fig 5)involved in plant growth promotion of sorghum plants when inoculated with A. oryzae YRA and having biocontrol activity against phytopathogens likeFusariumoxysporum, Eupenicilliumbrefeldianum, Alternaria phragmospora and A. alternate. 54,21
The presented data indicate the possibility of using A. oryzae as a biocontrol agent against the phytopathogenic fungi F. oxysporum. However, this requires further screening of many A. oryzae from different regions of India.
Conclusion
An endophytic fungus of Curcuma longa, A. oryzae AVNF4, showed positive interaction with the root system of tomato due to its physiological characteristics (production of hydrolytic enzymes), resulting in the significant enhancement in the growth of tomato seedlings. For the first time, an attempt was made to develop a liquid bioformulation of A. oryzae, an endophyte of turmeric. Growth optimisation of A. oryzae has also enhanced PGP traits after optimisation of culture conditions and responded positively to stimulate the growth of tomato seedlings. The growth promotion of tomato seedlings in pot experiments has confirmed the potential of liquid formulation as a promising biofertiliser and bio-nutrient for the sustainable growth of tomatoes. Hence, this liquid formulation will be a step in the initiation of the development of an environmentally safe and non-deleterious liquid biofertiliser for vegetable crops.
Acknowledgement
The author would like to thankthe Department of Botany and Microbiology, Acharya Nagarjuna University, Guntur, Andhra Pradesh, for granting the PhD research work, which is highly appreciated by the UGC-SAP laboratory.
Funding Sources
The authors are thankful to CIIPR-ANU (ANU/CIIPR/Project proposal/Finance support/2023)for financial assistance.
Conflict of Interest
Authors do not have any conflict of Interest
Data Availability Statement
This statement does not apply to this article
Ethics Statement:
This research did not involve human participants, animal subjects, or any material that requires ethical approval
Authors contribution:
(S. Narmada): Data Collection, Methodology, Writing – Original Draft.
(Amrutha V Audipudi): Conceptualization, Analysis, Writing,Review & Editing.
References
- Pramanik, K.; Mohapatra, P.P. Role of auxin on growth, yield and quality of tomato-A review. J. Curr. Microbiol. Appl. Sci.2017, 6, 1624–1636.DOI: https://doi.org/10.20546/ijcmas.2017.611.195
CrossRef - Boccia, F.; Boccia, F.; Di Donato, P.; Covino, D.; Poli, A. Food waste and bio-economy: A scenario for the Italian tomato market. Clean. Prod. 2019, 227, 424–433.https://doi.org/10.1016/j.jclepro. 2019.04.180
CrossRef - O’Connell, S., et al. High tunnel and field production of organic heirloom tomatoes: Yield, fruit quality, disease, and microclimate. HortScience, 2012, 47(9), 1283–1290.DOI: https://doi.org/ 10.21273/HORTSCI.47.9.1283
CrossRef - Ramakrishna, W., Yadav, R., & Li, K. Plant growth-promoting bacteria in agriculture: Two sides of a coin. Applied Soil Ecology, 2019, 138, 10–18.https://doi.org/10.1016/j.apsoil.2019.02.019
CrossRef - Elmholt S. Microbial activity, fungal abundance, and distribution of Penicillium and Fusarium as bioindicators of temporal development of organically cultivated soils. Biol Agric Hortic, 1996, 13(2):123–140. https:// doi.org/ 10. 1080/ 01448 765. 1996. 97547 72
CrossRef - Attia MS, Abdelaziz AM, Al-Askar AA, Arishi AA, Abdelhakim AM, Hashem AH. Plant growth-promoting fungi as biocontrol tool against Fusarium wilt disease of tomato plant. J Fungi. 2022a, 8(8):775.
CrossRef - Attia MS, El-Wakil DA, Hashem AH, Abdelaziz AM. Antagonistic effect of plant growth-promoting fungi against Fusarium wilt disease in tomato: in vitro and in vivo study. Appl BiochemBiotechnol. 2022b, 194:5100–5118. https:// doi. org/ 10. 1007/ s12010- 022- 03975-9
CrossRef - Attia MS, Hashem AH, Badawy AA, Abdelaziz AM. Biocontrol of early blight disease of eggplant using endophytic Aspergillus terreus: improving plant immunological, physiological and antifungal activities. Bot Stud. 2022c, 63(1):1–14. https:// doi. org/ 10. 1186/ s40529- 022- 00357-6
CrossRef - Agrios GN. Plant Pathology.Academic Press, San Diego.1997, The 4th edition.
- Abdelaziz AM, El-Wakil DA, Attia MS, Ali OM, AbdElgawad H, Hashem AH. Inhibition of Aspergillus flavus growth and aflatoxin production in Zea mays L. using endophytic Aspergillus fumigatus. J Fungi. 2022b, 8(5):482. https:// doi. org/ 10. 3390/ jof80 50482
CrossRef - Jones JB, Joned JP, Stall RF, Zitter TA. Compendium of tomato Diseases, American phytopathological Society, St.Paul, MN, 1991.
- Ristaino JB, Thomas W. Agriculture, methyl bromide and the ozone hole. Plant Dis. 1997; 81:964-977.DOI: 10.1094/PDIS.1997.81.9.964
CrossRef - Özgönen H, Biçici M, Erkiliç A. The effect of salicyclic acid and endomycorrhizal fungus Glomus etunicatumon plant development of tomatoes and Fusarium wilt caused by Fusarium oxysporumf.sp. lycopersici. Turk. J. Agric. For. 2001, 25: 25-29.https://journals.tubitak.gov.tr/agriculture/vol25/iss1/4
- Caron M, Fortin JA, Richard C. Effect of Glomus intraradiceson infection by Fusarium oxysporum.spp.radicis-lycopersici in tomatoes over a 12-weeks period. Can. J Bot. 1985; 64:552-556.DOI:10.1139/b86-070
CrossRef - Linderman RG. Vesicular-arbuscular mycorrhizae and soil microbial interactions. In: Linderman RG, Bethlenfalvey PF (eds), Mycorrhizae in sustainable agriculture, Madison Wisconsin USA. ASA special publication. 1992, 45-71.https://doi.org/10.2134/asaspecpub54.c3
CrossRef - Mohammed, B.L.; Hussein, R.A.; Toama, F.N. Biological control of Fusarium wilt in tomato by endophytic rhizobacteria. Energy Procedia. 2019, 157, 171–179.DOI:10.1016/j.egypro.2018.11.178
CrossRef - Glick, B.R. Plant Growth-Promoting Bacteria: Mechanisms and Applications. Scientifica. 2012, 963401.DOI: 10.6064/2012/963401
CrossRef - Kour, D.; Rana, K.L.; Yadav, A.N.; Yadav, N.; Kumar, M.; Kumar, V.; Vyas, P.; Dhaliwal, H.S.; Saxena, A.K. Microbial biofertilizers: Bioresources and eco-friendly technologies for agricultural and environmental sustainability. Biocatal. Agric. Biotechnol. 2019, 23, 101487.https://doi.org/10.1016/j.bcab.2019.101487
CrossRef - Abdelaziz, A.M. Inhibition of Aspergillus flavus Growth and Aflatoxin Production in Zea mays L. Using Endophytic Aspergillus fumigatus. J. Fungi.2022, 8, 482.DOI: 10.3390/jof8050482
CrossRef - Mohammed, B.L.; Hussein, R.A.; Toama, F.N. Biological control of Fusarium wilt in tomato by endophytic rhizobactria. Energy Procedia.2019, 157, 171–179.DOI:10.1016/j.egypro.2018.11.178
CrossRef - Abdel-Motaal, F.; Kamel, N.; El-Zayat, S.; Abou-Ellail, M. Early blight suppression and plant growth promotion potential of the endophyte Aspergillus flavus in tomato plant. Ann. Agric. Sci. 2020, 65, 117–123.https://doi.org/10.1016/j.aoas.2020.07.001
CrossRef - Yaldiz, G.; Camlica, M. Impact of Various Environmental Stress Factors on Productivity, Quality, and Secondary Metabolites of Fenugreek (Trigonella foenum-graecum L.). Fenugreek. 2021, 301–326.DOI:10.1007/978-981-16-1197-1_14
CrossRef - Koorapati, R. Comparison of Vegetable and Animal Peptone-Based Culture Media for Detection of Salmonella in Poultry. Ph.D. Thesis, 2010, UNSW Sydney, Sydney, Australia.
- Mani, P. G., &Audipudi, A. V. Penicillium citrinum AVGE1 an endophyte of Acorus calamus its role in biocontrol and PGP in chilli seedlings. J. Curr. Microbiol. Appl. Sci. 2016, 5, 657-667.DOI: http://dx.doi.org/10.20546/ijcmas.2016.505.066
CrossRef - Aneja, K. R. Experiments in Microbiology, Plant Pathology and Biotechnology.4th Edition. New Delhi, 2006, pp. 245-275.
- Kumar NR, Arasu VT, Gunasekaran P. Genotyping of antifungal compounds producing plant promoting rhizobacteria, Pseudomonas fluorescens. Sci. 2002, 82: 1463-1466
- Patten, C. L., & Glick, B. R. Role of Pseudomonas putida indoleacetic acid in development of the host plant root system. Applied and environmental microbiology. 2002, 68(8), 3795–3801. https://doi.org/10.1128/AEM.68.8.3795-3801.2002
CrossRef - Gordon SA, Weber RP. COLORIMETRIC ESTIMATION OF INDOLEACETIC ACID. Plant Physiol. 1951 Jan;26(1):192-5. doi: 10.1104/pp.26.1.192. PMID: 16654351; PMCID: PMC437633.
CrossRef - Gupta, R., SINGAL, R., SHANKAR, A., KUHAD, R. C., & SAXENA, R. K. A modified plate assay for screening phosphate solubilizing microorganisms. The Journal of General and Applied Microbiology. 1994, 40(3), 255-260.DOI https://doi.org/10.2323/jgam.40.255
CrossRef - Mehta, S. and Nautiyal, C.S. An Efficient Method for Qualitative Screening of Phosphate Solubilizing Bacteria. Current Microbiology. 2001, 43, 51-56.https://doi.org/10.1007/s002840010259
CrossRef - J. C. Cappucino and N. Sherman, “Microbiology: A Laboratory Manual,” 3rd Edition, Benjamin/Cumming Pub. Co., New York, 1992.
- Fouda A.H., Hassan S.E.D., Eid A.M., Ewais E.E.D. Biotechnological applications of fungal endophytes associated with medicinal plant Asclepias sinaica (Bioss.) Ann Agric Sci. 2015;60(1):95–104. https://doi.org/10.1016/j.aoas.2015.04.001
CrossRef - Oyebanji, O.B., Nweke, O., Odebunmi, O., Galadima, N.B., Idris, M.S., Nnodi, U.N., Afolabi, A.S., Ogbadu, G.H. Simple, effective and economical explant-surface sterilization protocol for cowpea, rice and sorghum seeds. J. Biotechnol. 2009, 8 (20).https://doi.org/10.5897/AJB09.923
- Allu, S., Kumar, N.P., &Audipudi, A.V. Isolation, Biochemical and PGP characterization of endophytic Pseudomonas aeruginosa isolated from chilli red fruit antagonistic against chilli anthracnose disease.J.Curr.Microbiol.App.Sci.2014, 3(2): 318-329. http://www.ijcmas.com
- Maity J.P., Kar S., Lin C.L., Chen Y.C., Chang Y.C., Jean S.J., and KulpT.K. Identification and Discrimination of Bacteria Using Fourier Transform Infrared Spectroscopy. Spectrochimica Acta Part A: Molecular And Biomolecular Spectroscopy. 2013;116:478- 484.Doi: Org/10.1016/J.Saa.2013.07.062
CrossRef - Ramzan M., Raza A., Musharaff S.G.,UnNisaZ.Recent Studies On Advance Spectroscopic Techniques For Identifying Microorganisms: A Review. Arabian Journal Of Chemistry.2023; 16 (3):104521. Doi.Org/10.1016/J. Arabjc.2022.104521
CrossRef - Moldoveanu, S.C., and David V. Derivatization Methods in GC And GC/MS. 2018; DOI:10.5772/Intechopen.81954
CrossRef - Carrim, A. J. I., Barbosa, E. C., & Vieira, J. D. G. Enzymatic activity of endophytic bacterial isolates of Jacaranda decurrens Cham. (Carobinha-do-campo). Brazilian Archives of Biology and Technology. 2006, 49, 353-359. https://doi.org/10.1590/S1516-89132006000400001
CrossRef - Jalgaonwala, R. E., & Mahajan, R. T. Evaluation of hydrolytic enzyme activities of endophytes from some indigenous medicinal plants. Journal of Agricultural Technology. 2011, 7(6), 1733-1741.http://www.ijat-aatsea.com
- Hallmann, J., Quadt-Hallmann, A., Mahaffee, W. F., & Kloepper, J. W. Bacterial endophytes in agricultural crops. Canadian journal of microbiology. 1997, 43(10), 895-914.https://doi.org/10.1139/m97-131
CrossRef - Reinhold-Hurek, B., & Hurek, T. Life in grasses: diazotrophic endophytes. Trends in microbiology. 1998, 6(4), 139-144.doi: 10.1016/s0966-842x(98)01229-3.
CrossRef - Venkatesagowda, B., Ponugupaty, E., Barbosa, A. M., & Dekker, R. F. Diversity of plant oil seed-associated fungi isolated from seven oil-bearing seeds and their potential for the production of lipolytic enzymes. World Journal of Microbiology and Biotechnology. 2012, 28, 71-80.doi: 10.1007/s11274-011-0793-4.
CrossRef - Sunitha, V. H., Devi, D. N., & Srinivas, C. Extracellular enzymatic activity of endophytic fungal strains isolated from medicinal plants. World Journal of Agricultural Sciences. 2013, 9(1), 01-09.DOI: 10.5829/idosi.wjas.2013.9.1.72148
- Choi, Y. W., Hodgkiss, I. J., & Hyde, K. D. Enzyme production by endophytes of Brucea javanica. J Agric Technol. 2005, 1, 55-66.
- Vinayarani, G., & Prakash, H. S. Fungal endophytes of Turmeric (Curcuma longa L.) and their biocontrol potential against pathogens Pythium aphanidermatum and Rhizoctonia solani. World journal of microbiology & biotechnology. 2018, 34(3), 49. https://doi.org/10.1007/s11274-018-2431-x
CrossRef - Christina, A., Christapher, V., &Bhore, S. J. Endophytic bacteria as a source of novel antibiotics: An overview. Pharmacognosy reviews. 2013, 7(13), 11–16. https://doi.org/10.4103/0973-7847.112833
CrossRef - Chet, I., Harman, G. E., & Baker, R. Trichoderma hamatum: Its hyphal interactions with Rhizoctonia solani and Pythium spp. Microbial Ecology. 1981, 7, 29-38.DOI: 1007/BF02010476
CrossRef - Lodewyckx, C., Vangronsveld, J., Porteous, F., Moore, E. R., Taghavi, S., Mezgeay, M., & der Lelie, D. V. Endophytic bacteria and their potential applications. Critical reviews in plant sciences. 2002, 21(6), 583-606.https://doi.org/10.1080/0735-260291044377
CrossRef - Kumar, A., Singh, R., Yadav, A., Giri, D. D., Singh, P. K., & Pandey, K. D. Isolation and characterization of bacterial endophytes of Curcuma longa L. 3 Biotech. 2016, 6, 1-8.DOI: 1007/s13205-016-0393-y
CrossRef - Kumar, V., Kumar, A., Pandey, K. D., & Roy, B. K. Isolation and characterization of bacterial endophytes from the roots of Cassia tora L. Annals of microbiology. 2015, 65, 1391-1399.https://doi.org/10.1007/s13213-014-0977-x
CrossRef - Noorjahan A., Aiyamperumal B., Anantharaman P. Isolation and characterization of seaweed endophytic fungi as an efficient phosphate solubiizers. BiosciBiotechnol Res Asia. 2019;16(1):33–39. doi: 10.13005/bbra/2718.
CrossRef - Sun, BT., Akutse, K.S., Xia, XF. et al. Endophytic effects of Aspergillus oryzae on radish (Raphanus sativus) and its herbivore, Plutellaxylostella. Planta. 2018, 248,705–714. https://doi.org/10.1007/ s00425-018-2928-4
CrossRef - Chaves, F.C., Gianfagna, T.J., Aneja, M. et al. Aspergillus oryzae NRRL 35191 from coffee, a non-toxigenic endophyte with the ability to synthesize kojic acid. Mycol Progress. 2012, 11, 263–267. https://doi.org/10.1007/s11557-011-0745-2
CrossRef - Rashad, Y.M., Al Tami, M.S. & Abdalla, S.A. Eliciting transcriptomic and antioxidant defensive responses against Rhizoctonia root rot of sorghum using the endophyte Aspergillus oryzae YRA3. Sci Rep. 2023, 13,19823. https://doi.org/10.1038/s41598-023-46696-7
CrossRef - Attia, M.S., Salem, M.S. & Abdelaziz, A.M. Endophytic fungi Aspergillus spp. reduce fusarial wilt disease severity, enhance growth, metabolism and stimulate the plant defense system in pepper plants. Biomass Conv. Bioref. 2024, 14, 16603–16613. https://doi.org/10.1007/s13399-022-03607-6
CrossRef - Saikkonen, K., S.H. Faeth, M. Helander, 51. Sun, X., L.D. Guo and K.D. Hyde, Community T.J. Sullivan. A continuum of interactions with composition of endophytic fungi in Acer truncatum the host plants. Annual Review of Ecology and and their role in decomposition. Fungal Divers. Systematic. 1998, 29: 319-343. 47: 85-95.
CrossRef - Ahmad, F., Ahmad, I., Khan, M.S. Screening of free-living rhizospheric bacteria for their multiple plant growth-promoting activities. Microbiol. Res. 2008, 163: 173–181.DOI: 10.1016/j.micres. 2006.04.001
CrossRef - Jones, D.L., P.R. Darrah. Role of root derived organic acids in the mobilization of nutrients from the rhizosphere. Plant Soil.1994, 166: 247-257.https://doi.org/10.1007/BF00008338
CrossRef - Khalil A.M.A., Hassan S.E.D., Alsharif S.M., Eid A.M., Ewais E.E.D., Azab E., Gobouri A.A., Elkelish A., Fouda A. Isolation and characterization of fungal endophytes isolated from medicinal plant Ephedra pachyclada as plant growth-promoting. Biomolecules. 2021;11(2) doi: 10.3390/biom11020140.
CrossRef - Grabka R., d’Entremont T.W., Adams S.J., Walker A.K., Tanney J.B., Abbasi P.A., Ali S. Fungal endophytes and their role in agricultural plant protection against pests and pathogens. Plants. 2022;11(3):384. doi: 10.3390/plants11030384.
CrossRef - Dhir, B. Biofertilizers and Biopesticides: Eco-friendly Biological Agents. In: Kumar, R., Sharma, A., Ahluwalia, S. (eds) Advances in Environmental Biotechnology.2017, Springer, Singapore. https://doi.org/10.1007/978-981-10-4041-2_10
CrossRef - Zhang, Y. F., He, L. Y., Chen, Z. J., Wang, Q. Y., Qian, M., & Sheng, X. F. Characterization of ACC deaminase-producing endophytic bacteria isolated from copper-tolerant plants and their potential in promoting the growth and copper accumulation of Brassica napus. Chemosphere. 2011, 83(1), 57-62.DOI: 10.1016/j.chemosphere.2011.01.041
CrossRef - Al Kahtani M.D., Fouda A., Attia K.A., Al-Otaibi F., Eid A.M., Ewais E.E.D., Hijri M., St-Arnaud M., Hassan S.E.D., Khan N., Hafez Y.M., Abdelaal K.A. Isolation and characterization of plant growth promoting endophytic bacteria from desert plants and their application as bioinoculants for sustainableagriculture. 2020;10(9):1325. doi: 10.3390/agronomy10091325.
CrossRef - Martínez-Hidalgo P., Flores-Félix J.D., Sánchez-Juanes F., Rivas R., Mateos P.F., Santa Regina I., Velázquez E. Identification of canola roots endophytic bacteria and analysis of their potential as biofertilizers for canola crops with special emphasis on sporulating bacteria. Agro Sur. 2021;11(9):1796. doi: 10.3390/agronomy11091796.
CrossRef - Mukherjee A., Gaurav A.K., Patel A.K., Singh S., Chouhan G.K., Lepcha A., Pereira A.P.A., Verma J.P. Unlocking the potential plant growth‐promoting properties of chickpea (Cicer arietinum L.) seed endophytes bio‐inoculants for improving soil health and crop production. Land Degrad. Dev. 2021;32(15):4362–4374. doi: 10.1002/ldr.4042.
CrossRef - Bhutani N., Maheshwari R., Kumar P., Suneja P. Bioprospecting of endophytic bacteria from nodules and roots of Vigna radiata, Vigna unguiculata and Cajanus cajan for their potential use as bioinoculants. Plant Gene. 2021;28 doi: 10.1016/j.plgene.2021.100326.
CrossRef - Sundaramoorthy, S., Raguchander, T., Ragupathi, N., Samiyappan, R. Combinatorial effect of endophytic and plant growth promoting rhizobacteria against wilt disease of Capsicum annum L. caused by Fusarium solani. Biol. Control. 2012, 60 (1), 59-67.https://doi.org/10.1016/j.biocontrol. 2011.10.002
CrossRef - Haron F.K., Shah M.D.,Yong Y.S., Tan J.K., Lal M.T.M., And Maran B.A.V. Antiparasitic Potential of Methanol Extract of Brown Alga Sargassum polycystum(Phaeophyceae) and its LC-MS/MS Metabolite Profiling. 2022; 14(10) 796; 1-12. Doi.Org/10.3390/ D14100796
CrossRef - Balamurugan M., Selvam G.G., Thinakaran T., Sivakumar K. Biochemical Study and GC-MS Analysis of HypneaMusciformis (Wulf.) Lamouroux. Am. Eurasian J. Sci. Res. 2013;8:117–123. DOI:5829/idosi.aejsr.2013.8.3.12071
- E. Jenisha, E., Rani Juneius, C., & Vinoth, V. Studies on the Effects of L-Prolinamide, 5-OXO-L-Prolyl-L-Phenylanyl-4-Hydroxy Compound Produced byPseudomonas Fluorescence against Cell Wall Protein (3GNU Receptor) of Pythium SPP MTCC 10247. IRA-International Journal of Applied Sciences. 2016, (ISSN 2455-4499), 3(3). doi:http://dx.doi.org/10.21013/jas.v3.n3.p9
CrossRef - Lui, L., Vikram, A., Hamzehzarghani, H. et al.Discrimination of three fungal diseases of potato tubers based on volatile metabolic profiles developed using GC/MS. Potato Res. 2005, 48, 85–96. https://doi.org/10.1007/BF02733684
CrossRef - Kiran GS, Priyadharsini S, Sajayan A, Ravindran A and Selvin J, An antibiotic agent pyrrolo [1, 2-a] pyrazine-1, 4-dione, hexahydro isolated from a marine bacteria bacillus tequilensis MSI45 effectively controls multi-drug resistant Staphylococcus aureus. RSC Adv. 2018, 8: 17837–17846. DOI https://doi.org/10.1039/C8RA00820E
CrossRef - Kannabiran DK. Bioactivity of Pyrrolo[1,2-a]pyrazine-1,4-dione,hexahydro-3-(phenylmethyl)- Extracted from Streptomyces sp. VITPK9 Isolated from the Salt Spring Habitat of Manipur, India. Asian J Pharm Free full text Artic from Asian J Pharm. 2016; 10: 265. https://doi.org/10.22377/AJP.V10I04.865
CrossRef - Zaman NR, Chowdhury UF, Reza RN, Chowdhury FT, Sarker M, Hossain MM, et al.Plant growth promoting endophyte BurkholderiacontaminansNZ antagonizes phytopathogen Macrophominaphaseolinathrough melanin synthesis and pyrrolnitrin inhibition. PLoS ONE. 2021, 16(9): e0257863. https://doi.org/10.1371/pone.0257863
CrossRef - Teoh YP, Don MM, Ujang S. Media selection for mycelia growth, antifungal activity against wood degrading fungi, and GC-MS study by Pycnoporus sanguineus. BioResources. 2011; 6: 2719–2731.https://doi.org/10.15376/biores.6.3.2719–2731
CrossRef - Jimtha, J.C, Jishma, P, Arathy, G.B, Anisha, C, & Radhakrishnan, E.K. Identification of plant growth promoting Rhizosphere Bacillus sp. WG4 antagonistic to Pythium myriotylum and its enhanced antifungal effect in association withTrichoderma. Journal of soil science and plant nutrition. 2016, 16(3), 578-590. Epub July 00.https://dx.doi.org/10.4067/S0718-95162016005000026
CrossRef - Nedved, E.L.; Kalatskaja, J.N.; Ovchinnikov, I.A.; Rybinskaya, E.I.; Kraskouski, A.N.; Nikalaichuk, V.V.; Hileuskaya, K.S.; Kulikouskaya, V.I.; Agabekov, V.E.; Laman, N.A. Growth Parameters and Antioxidant Activity in Cucumber Seedlings with the Application of Chitosan and Hydroxycinnamic Acids Conjugates under Salt Stress. Biochem. Microbiol. 2022, 58, 69–76.https://doi.org/10.1134/ s0003683822010069
CrossRef - Faeth, S.H. Fungal Endophytes: Common Host Plant Symbionts butUncommon Mutualists. Integrative and Comparative Biology. 2002, 42, 360-368.https://doi.org/10.1093/icb/42.2.360
CrossRef - Leuchtmann, A., Schardl, C. The Epichloë Endophytes of Grasses and the Symbiotic Continuum. 2005, 0554, 475-503.DOI:1201/9781420027891.ch24
CrossRef - Malinowski, D.P., Belesky, D.P, Lewis, G.C. Abiotic Stresses in Endophytic Grasses. 2005, 187-199.https://doi.org/10.1002/9780470384916.ch8
CrossRef - Zhang, X., Li, C., Nan, Z. Effects of cadmium stress on seed germination and seedling growth of Elymus dahuricus infected with the Neotyphodium endophyte. Science China Life Sciences. 2012, 55, 793-799.https://doi.org/10.1007/s11427-012-4359-y
CrossRef

