Introduction
The genus Curcuma consists of more than 80 species and is known for its versatile uses, viz. culinary, pharmaceutical, cosmetic, dye, as tropical ornamentals, etc.1 Curcuma mangga Val. et Zijp. (mango ginger) is a perennial herb indigenous to Java and is widely distributed in the Andaman and Nicobar Islands (India), Malaysia, Thailand, and Indonesia.2-4 Its rhizomes are fleshy, yellowish-brown from the outside and citron yellow from the inside. Rhizomes impart a raw mango-like aroma when cut, and hence, it is popularly known as mango ginger. Rhizomes are primarily used as a spice and salad in fresh form,5 apart from their use as a vegetable and raw material for the preparation of pickles, candies, and sauces.
Traditionally, the rhizomes have stomachic properties and are used against chest pain, gastric ulcers, fever, and general debility.6-7 Essential oils are found to be present in the primary, secondary, and mother rhizomes of C. mangga5 and are known to possess antimicrobial activity.8 Beta-Myrcene and Cyclofenchene have been identified as the prime constituents of the essential oils of this species.5 This makes them a suitable candidate for flavouring of culinary preparations. Further, curcumin- an important, versatile bioactive molecule occurring in Curcuma species, has also been reported to be present in the rhizomes in small quantities.5 Fresh rhizomes of C. mangga are perishable in nature. Postharvest shriveling and sprouting of rhizomes are the major concerns during prolonged storage. Despite its high medicinal and nutritional value, no reports are available describing the processing of this species as a ready-to-use paste for prolonging its storage. Therefore, the aim of the present study was to determine the storage stability of its culinary paste at two different conditions: ambient (26 ± 2 °C) and refrigerated (4 ± 2 °C).
Materials and Methods
Experimental material
Rhizomes of Curcuma mangga were procured from the experimental fields of ICAR – Central Island Agricultural Research Institute, Sri Vijaya Puram, India and were used after curing.
Glassware, Chemicals, and Reagents
Glassware were procured from Borosil Glass Works Limited, Mumbai, India. Chemicals (AR grade) were procured from HiMedia Laboratories Pvt. Ltd., Mumbai, India, and Sisco Research Laboratories Pvt. Ltd., Mumbai, India.
Preparation and Storage
Rhizomes were washed under running tap water, followed by peeling and slicing using a stainless steel knife to ca. 6-8 mm thickness. Sliced rhizomes were blanched and ground into a thin paste using a laboratory electric grinder. The paste was mixed with sodium chloride (10%, w/w), citric acid (0.02%, w/w) and sodium benzoate (0.015%, w/w) followed by packing in autoclaved glass jars (8 cm × 8 cm × 8 cm, 200 ml). These glass jars were sealed and stored at room temperature (RT; 26 ± 2 °C), and low temperature (LT; 4 ± 2 °C) in a refrigerator (Whirlpool, FP 263D Protton Roy, India) for three months.
Biochemical Analysis
The product was analyzed at monthly intervals for various biochemical parameters. The TSS was measured using a hand refractometer (Optics Technology, Delhi, India), while pH was determined using a benchtop digital pH meter (Hanna Instruments, HI 2211). For determination of moisture content, the samples were accurately weighed using an analytical balance (CY-104, Citizen, India) and dried in a hot air oven at 105 °C (Deep Vision, India) until the weight became constant.9 For ash content determination, pre-weighed samples were kept in a muffle furnace (Omron, India) at 550 °C till white ash was obtained. They were then shifted to a desiccator before recording the final weights.9 For determination of essential oil content, 50 g of paste was added to 200 ml of deionized water, and hydro-distillation was carried out for 6 h using Clevenger apparatus. Collected oil was dried using anhydrous sodium sulphate and content (v/w) was calculated.5 Ascorbic acid content was determined using the dichlorophenolindophenol titration method.10 Total phenolic content was determined using the Folin-Ciocalteau (FC) method with gallic acid as a standard.11
Microbial Analysis
Microbiological evaluations of the samples were carried out for total bacterial and total fungal count. Different concentrations (10-1, 10-2, and 10-3) were made from each treatment, and the sample was spread over Petri dishes containing NA and PDA media. Samples were then incubated for 48 h at 37 ± 2 °C before recording observations.12 For determination of psychrophiles in the LT-stored samples, incubation in NA medium was done at 7 °C for 7 days.
Statistical Analysis
The experimental design used was completely randomized design. Statistical analysis of the data collected from various experiments was carried out by one-way ANOVA using the Web Agri Stat Package Ver. 2.0 (ICAR-CCARI, Ela, India) with the least significant difference (p ≤ 0.05). All the experiments were performed in triplicates, whereas essential oil estimation was performed in duplicate.
Results and Discussion
Value addition not only provides an opportunity for improving the availability of the produce during off-season in the local markets, but also paves way for increasing the reach of the product to non-native areas for better marketing. Mango ginger in the present study was processed into ready-to-use culinary paste, and results of the storage study have been presented hereunder.
pH and Total Soluble Solids
pH is an important factor that influences the food quality mainly through various chemical reactions.13 The fresh product had a pH of 5.08. It remained stable for two months and increased slightly after three months of storage at LT. However, at RT, a continuous increase in pH from 5.08 to 5.56 was observed (Fig. 1). An increment in temperature might have resulted in increased pH due to dissociation of molecules.
Total soluble solids content of fresh culinary paste was found to be 12 °Brix. There was no significant difference in TSS of product stored at LT (Fig. 2). However, an increment was observed after three months of storage at RT. The increase in TSS may be due to depolymerization or cleavage of polysaccharides (starch and soluble pectin) into their simplest forms. Moreover, non-carbohydrate compounds such as organic acids and salts might have also contributed in increased TSS content. A similar increasing trend in TSS was observed in ginger paste stored in different packaging materials at ambient as well as low temperature.12
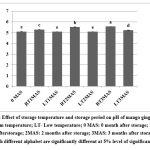 |
Figure 1: Effect of storage temperature and storage period on pH of mango ginger paste. RT: Room temperature; LT- Low temperature; 0 MAS: 0 month after storage; 1MAS: 1 month after storage; 2MAS: 2 months after storage; 3MAS: 3 months after storage.Click here to view Figure |
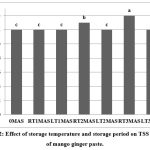 |
Figure 2: Effect of storage temperature and storage period on TSS content of mango ginger paste.Click here to view Figure |
Moisture and Ash Content
Moisture content of a product influences its texture, taste, colour, freshness, microbial safety, etc.14 The initial moisture content of paste was 76.57%, which did not differ significantly during three months of storage at LT (Table 1). However, after three months of storage at RT, significant decrease in moisture content (74.20%) was reported. The present findings are in conformity with those reported by Gamli and Hayoglu15, who observed that pistachio nut paste stored at 4°C retained higher moisture content than that stored at 20°C. Ash content determination is important in nutritional evaluation of food, as ash contains the inorganic residues, mainly minerals. The ash content of fresh product was 9.71%, which did not differ statistically at LT and RT during three months of storage (Table 1). On the contrary, garlic paste stored at 25°C had significantly higher ash content (4.06%) than that stored at 40°C (3.82%).16
Table 1: Effect of storage temperature and storage period on moisture and ash content of mango ginger paste
| Particulars | Moisture (%) | Ash (%) |
| 0 MAS: 0 month after storage | 76.57 a | 9.714 a |
| RT1MAS: Room temperature; 1 month after storage | 76.54 a | 9.690 a |
| LT1MAS: Low temperature; 1 month after storage | 76.11 a | 9.702 a |
| RT2MAS: Room temperature; 2 months after storage | 76.46 a | 9.709 a |
| LT2MAS: Low temperature; 2 months after storage | 76.30 a | 9.697 a |
| RT3MAS: Room temperature; 3 months after storage | 74.20 b | 9.686 a |
| LT3MAS: Low temperature; 3 months after storage | 76.17 a | 9.695 a |
Values followed by similar alphabets in a column are statistically similar
Essential Oil Content
Essential oil of the genus Curcuma is of great importance because of its uses in cosmetics, pharmaceutical industries, and in food as additives. Essential oil obtained from the genus is categorized as ‘Generally Regarded as Safe’. Essential oil content of fresh product in the present investigation was 0.4%. No significant differences were observed at RT and LT after three months of storage (Fig. 3). Several authors have reported the essential oil of C. mangga to possess antioxidant, anti-allergic, and antimicrobial properties.8,17 Further, retention of essential oil content in mango ginger paste during storage revealed its potential to impart the characteristic aroma of the species in the cuisines.
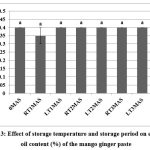 |
Figure 3: Effect of storage temperature and storage period on essential oil content (%) of the mango ginger pasteClick here to view Figure |
Ascorbic Acid Content
Ascorbic acid content of freshly prepared product was 52.57 mg/100 g (Fig. 4). Progressive decrease in vitamin C content was observed with increasing storage duration. Ascorbic acid content decreased gradually from 52.57 to 49.01 mg/100 g after three months of storage at RT. In tomato paste stored at room temperature, degradation of ascorbic acid content with time was evident, and about 68% ascorbic acid remained in the product after three months of storage.18 The decrease in degradation of vitamin C content at RT compared to LT in processed mango ginger paste might be due to increased TSS of samples stored at RT for three months. Robertson and Samaniego-Esguerra19 reported that at constant temperature, an increase in TSS in lime juice could reduce the ascorbic acid degradation process. Rapid deterioration of vitamin C content was observed from 52.57 to 41.38 mg/100 g after one month of storage at LT. It further degraded in a slow manner from 41.38 mg/ 100 g to 39.21 mg/ 100 g. Several studies have reported that aerobic degradation of vitamin C was more rapid during the initial period of product storage and this loss might be due to trapped air within the storage container.19,20 This noticeable difference in ascorbic acid degradation at the studied temperatures is a matter of further investigation.
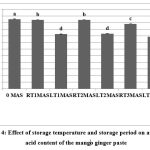 |
Figure 4: Effect of storage temperature and storage period on ascorbic acid content of the mango ginger pasteClick here to view Figure |
Total Phenolic Content
Phenolic compounds are known to contribute to the sensory and nutritional quality of fresh and processed plant foods. Several studies have reported that phenolic compounds act as natural antioxidants.21-23 Various phenolic compounds found in C. mangga are gallic acid, epigallocatechin, gallocatechingallate, epigallocatechingallate, catechin, and epicatechin. In the present study, the total phenolic content of fresh product was 3.211 mg GAE/ 100 g, which decreased to 2.786 mg GAE/ 100 g in product stored at RT for three months (Fig. 5). The decrease might be due to the non-enzymatic browning reaction of phenolic compounds.21 Friedman and Jurgens24 reported that degradation of polyphenolic compounds was higher when exposed to high pH. Low temperature induced a dual effect on the total phenolic content of product with an initial decrease to 3.015 mg GAE/ 100 g after one month of storage, which increased to 3.335 mg GAE/ 100 g three months after storage. The increase might be due to the conversion of polyphenolic compounds into simple phenols.25
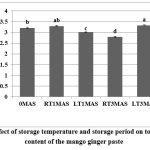 |
Figure 5: Effect of storage temperature and storage period on total phenolic content of the mango ginger pasteClick here to view Figure |
Microbial Evaluation
In the present study, microbiological assessment showed that the product was safe, irrespective of the storage temperatures and period. Thermal processing of mango ginger paste might have proved to be effective against the growth of food spoilage bacteria, yeasts, and molds. The addition of citric acid helped in lowering the pH of the product, thereby inhibiting the growth of food spoilage microorganisms. Further, the addition of common salt (NaCl) reduces the water activity of the food and retards the growth of microorganisms by causing osmotic shock, limiting oxygen solubility, or interfering with cellular enzymes. Yigit and Korukluoglu26 reported that usage of 10% NaCl as a preservative reduced the growth of Aspergillus niger. Moreover,27 reported that the addition of sodium benzoate (200 ppm) in ginger-garlic paste helped in the eradication of microbial load completely. In the case of ginger paste, thermal treatment was found to be effective against microbial counts after six months of storage in refrigerated condition.28 Similar positive results of the combined effect of thermal treatment and added preservatives were also recorded in the present study. Additionally, the essential oil retained in the product throughout the storage duration could have also contributed to avoiding the spoilage.
Sensory Evaluation
Sensory evaluation is important for studying the consumer behavior and attitude towards a new product. The seven-point hedonic scale has been used for testing the consumer preference of processed products.29-30 Scores of sensory parameters of mango ginger paste after storage for 3 months at different temperatures are presented in Table 2. In general, mean sensory scores of product stored at low temperature were higher than those of product stored at room temperature. However, the differences were not significant and hence, both the products were equally acceptable. Aroma was a key characteristic of the product. Unchanged concentration of essential oil content in mango ginger paste, both at RT and LT, might be the reason of good scores of aroma even after three months of storage. A product is said to have market potential if the acceptability scores are more than 70%31 and thus, the present product (both stored at LT and RT) could be considered as a candidate product for commercialization.
Table 2: Mean sensory scores of mango ginger paste after three months of storage at different temperatures
| Treatments | Color | Flavor | Aroma | Overallacceptability |
| RT | 5.85 ± 0.279 | 6.10 ± 0.179 | 6.15 ± 0.258 | 6.15 ± 0.211 |
| LT | 6.10 ± 0.179 | 6.35 ± 0.211 | 6.45 ± 0.216 | 6.35 ± 0.150 |
Conclusion
The present report was an attempt to popularize an underutilized medicinal spice through value addition. The product was shelf-stable and acceptable after three months of storage at ambient as well as low temperature. The product would be of great use in restaurants, institutional catering, and in processing industries to impart the characteristic mango ginger aroma to the cuisines.
Acknowledgement
The presented work is a part of M.Sc. thesis submitted by the first author to the Central University of Haryana. Authors are thankful to the Director, ICAR-CIARI for providing the support for conducting the study.
Funding Sources
The author(s) received no financial support for the research, authorship, and/or publication of this article.
Conflict of Interest
The authors do not have any conflict of interest.
Data Availability Statement
All the relevant data has been presented in the manuscript.
Ethics Statement
This research did not involve human participants, animal subjects, or any material that requires ethical approval.
Informed Consent Statement
This study did not involve human participants, and therefore, informed consent was not required.
Permission to Reproduce Material from other Sources
Not applicable
Clinical Trial Registration
This research does not involve any clinical trials.
Author Contributions
Susanskriti Gupta: methodology, writing first draft;
Pooja Bohra: conceptualization, methodology, supervision, final review and editing;
Ajit Arun Waman: methodology and editing of draft during different stages of reviewing;
Savita Budhwar: editing first draft;
References
- Sasikumar B. Genetic resources of Curcuma: diversity, characterization and utilization. Plant Genet. Resour.: C. 2005; 3(2): 230–251
CrossRef - Leong-Škorničková J., Šída O., Marhold K. Back to types! Towards stability of names in Indian Curcuma (Zingiberaceae). Taxon, 2010; 59(1): 269-282
CrossRef - Singh S., Waman A.A., Bohra P., Gautam R.K., Roy S.D. Conservation and sustainable utilization of horticultural biodiversity in tropical Andaman and Nicobar Islands, India. Resour. Crop Evol. 2016; 63(8): 1431-1445
CrossRef - Lim T.K. Curcuma mangga. Edible Medicinal and Non-Medicinal Plants, vol. 12, 2016, pp. 363-373
CrossRef - Waman A.A., Bohra P., Sounderarajan A. Propagule size affects yield and quality of Curcuma mangga et Zijp.: An important medicinal spice. Ind. Crops Prod. 2018; 124: 36-43
CrossRef - Abas F., Lajis N.H., Shaari K., Israf D.A., Stanslas J., Yusuf U.K. A labdane diterpene glucoside from the rhizomes of Curcuma mangga. Nat. Prod. 2005; 68: 1090-1093
CrossRef - Wahab I.R., Blagojević P.D., Radulović N.S., Boylan F. Volatiles of Curcuma mangga & Zijp (Zingiberaceae) from Malaysia. Chem. Biodiv. 2011; 8(11): 2005–2014
CrossRef - Kamazeri T.S.A.T., Samah O.A., Taher M., Susanti D., Qaralleh H. Antimicrobial activity and essential oils of Curcuma aeruginosa, Curcuma mangga, and Zingiber cassumunar from Malaysia. Asian Pac. J. Trop. Med. 2012; 5(3): 202– 209
CrossRef - Manual of methods of analysis of foods: fruit and vegetable products. Food Safety and Standards Authority of India, Ministry of Health and Family Welfare, Government of India, 2013, p. 57
- Sadasivam S., Manickam A. Biochemical Methods. New Age International Publishers, New Delhi, India, 2008
- Shivashankara K.S., Upreti K.K., Rao, V.K. Compendium of Lectures and Practicals of the ICAR Sponsored Short Course on Metabolite Profiling as a Selection Tool for Abiotic and Biotic Stress Tolerance in Horticultural Crops. ICAR-Indian Institute of Horticultural Research, Bengaluru, India, 2017, pp. 1–170
- Devi T.B., Dash S.K., Bal L.M., Sahoo, N.R. Physicochemical and microbiological characteristics of ginger paste (cv. Suprabha) during storage in different packaging and temperature conditions. Cogent Food Agric. 2016; 2(1): 1223261
CrossRef - Andrés-Bello A., Barreto-Palacios V., García-Segovia P., Mir-Bel J., Martínez-Monzó J. Effect of pH on color and texture of food products. Food Eng. Rev. 2013; 5(3): 158-170
CrossRef - Ray S., Saha S.K., Raychaudhuri U., Chakraborty R. Preparation of okra- incorporated dhokla and subsequent analysis of nutrition, antioxidant, color, moisture and sensory profile. J. Food Meas. Charact. 2016; 11(2): 639–650
CrossRef - Gamlı F.Ö., Hayoğlu, İ. The effect of the different packaging and storage conditions on the quality of pistachio nut paste. J. Food Eng. 2007; 78(2): 443-448
CrossRef - Mutasim Z.A., Elgasim A.E. Proximate analysis of garlic (Allium sativum) paste treated with ascorbic and citric acids. J. Food Process. Technol. 2016; 7: doi:10.4172/2157-7110.1000550
CrossRef - Waman A.A., Kadbhane K.P., Karanjalker G.R. Mango Ginger: Prospects for Domestication and Utilization. In: Medicinal Plants. Domestication, Biotechnology and Regional Importance, Ekiert, H.M., Ramawat, K.G. and Arora, J. (eds.), Springer-Nature, Singapore, 2021; pp. 293-313
CrossRef - Koh E., Charoenprasert S., Mitchell A.E. Effects of industrial tomato paste processing on ascorbic acid, flavonoids and carotenoids and their stability over one-year storage. Sci. Food Agric. 2012; 92: 23–28
CrossRef - Robertson G.L., Samaniego-Esguerra C.M. Effect of soluble solids and temperature on ascorbic acid degradation in lemon juice stored in glass bottles. Food Qual. 1990; 13(5): 361-374
CrossRef - Kefford J.F., McKenzie H.A., Thompson P.C.O. Effects of oxygen on quality and ascorbic acid retention in canned and frozen orange juices. Sci. Food Agric. 1959; 10(1): 51-63
CrossRef - Ho C.-T. Phenolic compounds in food – An overview. In: Huang M-T, Ho CT and Lee CY (eds), Phenolic Compounds in Food and Their Effects on Health II, ACS Symposium Series, vol. 507, American Chemical Society, Washington, 1992, pp. 2-7
CrossRef - Robards K., Prenzler P.D., Tucker G., Swatsitang P., Glover, W. Phenolic compounds and their role in oxidative processes in fruits. Food Chem. 1999; 66(4): 401-436
CrossRef - Velioglu Y.S., Mazza G., Gao L., Oomah B.D. Antioxidant activity and total phenolics in selected fruits, vegetables, and grain products. Agric. Food Chem. 1998; 46(10): 4113–4117
CrossRef - Friedman M., Jürgens H.S. Effect of pH on the stability of plant phenolic compounds. Agric. Food Chem. 2000; 48(6): 2101-2110
CrossRef - Pujimulyani D., Raharjo S., Marsono Y., Santoso, U. The effects of blanching treatment on the radical scavenging activity of white saffron (Curcuma mangga). Intl. Food Res. J. 2010; 17(3): 615-621
- Yigit A., Korukluoglu M. The effect of potassium sorbate, NaCl and pH on the growth of food spoilage fungi. Microbiol. 2007; 57(2): 209–215
CrossRef - Topno P.N., Vinothini, Jayaprakash S.H., Varadaiah V., Sheshagiri S.H., Srinivas P.M., Naidu M.M. Ginger-garlic paste in retort pouches and its quality. Food Process. Engg. 2011; 36(1): 1-8
CrossRef - Unni L.E., Chauhan O.P., Raju P.S. Quality changes in high pressure processed ginger paste under refrigerated storage. Food Biosci. 2015; 10: 18-25
CrossRef - Granato D., De Castro I.A., Ellendersen L.S.N., Masson M.L. Physical stability assessment and sensory optimization of a dairy-free emulsion using response surface methodology. J. Food Sci. 2010; 73: 149-155
CrossRef - Granato D., Masson M.L., Ribeiro J.C.B. Sensory acceptability and physical stability evaluation of a prebiotic soy-based dessert developed with passion fruit juice. Ciência e Tecnologia de Alimentos 2012; 32(1): 119-125
CrossRef - Dutcosky, S.D. Análise sensorial de alimentos, 4th edition. Champagnat, Curitiba, 2013, p. 531



0 Replies to “Storage Studies in Culinary Paste Prepared from Underutilized Medicinal Spice, Mango Ginger (Curcuma mangga Val. et Zijp.)”