Introduction
Global food security is a highly challenging task that initiates the agriculture sector to be revolutionized on time. The overgrowing world population and limited arable land have forced new agro-technological approaches to sustain agricultural production. The use of fertilizers to increase crop production is a common agricultural practice since past periods. However, conventional fertilizers are now less preferable due to their major drawbacks as lesser efficiency of nutrient delivery to soil and plant systems, major contents (50-70%) are washed away from the soil through leaching before being used.1 Overuse of these fertilizers resulted in environmental pollution and disruption of soil quality and agro-ecosystem.2 The adoption of sustainable agricultural approaches are more viable solution to ensure food security without compromising the soil fertility as well as preserving environment and natural resources. Inecent eras, nanotechnologyased fertilization processing has shown high efficiency to resolve the food scarcity problem.3ertilizers in theno-dimension () can be efficiently utilized by soil and plants to deliver adequate nutrient requirements in ow and sustained way, improving in vivo nutrient delivery and sure the distribution of nutrients precisely.4,5,6 Nanofertilizers improve soil properties viz. optimized availability of macro and micronutrients in soil, longerm nutrient accessibility in soil and avoiding nutrient loss due to leaching.3 Several studies reported on novel nano- assisted fertilizer developments, such as chitosan-based nanofertilizer 7, urea–formaldehyde polymer nanocomposite 8, Urea-Kaolinite Nanocomposite thermoplastic starch/urea (TPSUr) 9, nanozeolite/Hydroxyapatite 10, chitosan-montmorillonite (MMT) nano-composite hydrogel. 11
Among different nanomaterials, nanozeolite and Hydroxyapetite based fertilizers are considered high potential nanofertilzer forptimized delivery of essential nutrients to the soil and plant system in low and sustained way. Its nano pore size, high ionic exchange capacity, high rehydration capacityhigh nutrient absorbance capacity make them highly applicable as nanofertilizer.12, 13, 14 Zeolites, aluminosilicate compounds are one ofost promising materials for slowelease nano-fertilizer (SRF) because of its high specific surface areas, ion-exchange abilitiesnano-porous structure.12 Zeolite NaP1 is a synthetic form of nanozeolite, synthesized from various wastes using an alkaline hydrothermal treatment.15 It acts efficiently in enhancing the nutrient retention capacity of soil up to longer periods time because of its slow disintegration and decomposition rate in soil.16 Zeolite NaP1 actively mobilizes the ammonium and potassium ions in plantvailable form, due to its higher affinity with cationic groups. However, the immobilization of phosphate ions is difficult for zeolite structures due to its lesser affinity for anionic groups12. To resolve this problem, various studies suggested the fabrication of zeolite with hydroxyapetite nanoparticles (HAP, [Ca10 (PO4) 6 H2O] crystal calcium phosphate. HAP aids the better efficacy for phosphorus ion mobilization in soil and plant system.17, 18, 19 Hybrid nanocomposite zeolite NaP1/hydroxyapatite(ZHNC) is proven as high potential nano-fertilizer havingtrong affinity to bind both cationic and anionic nutrients and released over a long time.9, 12 However inast, studiesainly concern with its synthesis and physico-chemical characterization of zeolite NaP1/hydroxyapatite(ZHNC). 12, 15 For sustainable applicability of ZHNC asano-fertilizer ineld it is important to examine its accessibility to soil and plant systems
Materials and Methods
Zeolite NaP1/ hydroxyapatite nanocomposite (ZHNC)
The Zeolite NaP1/ hydroxyapatite nanocomposite (ZHNC) used in this study was was purchased from a nano-product company NanoLab, Jamshedpur, Jharkhand, India.
ZHNC is a hybrid nanocomposite of zeolite and nanohydroxyapetite components. Zeolites are three dimensional, crystalline, porous hydrated aluminosilicates compounds containing the constituent SiO44-, AlO45 and various other elements (sodium, potassium, calcium, magnesium). Zeolite NaP1 (Na6Al6Si6O32.12H2O) is a synthetic zeolite form, hydrothermally synthesized from fly ash treating with sodium hydroxide at atmospheric pressure.12 Zeolites are having nanoporous structure, unique physico-chemical properties, high specific surface areas, good ion-exchange abilities and environmental friendly catalytic properties which make them suitable for use in slow-release fertilizers.20 Hydroxyapatite [Ca10 (PO4)6(OH)2] HA is a mineral composed of phosphate ions and calcium ion and found naturally in teeth and bones. Its synthetic nano-form (Nano-hydroxyapatite) has shown high potency as phosphate fertilizer due to its lower solubility and slow-release of phosphate ions.14 Hybrid nanocomposite of zeolite NaP1 and HA nanocomposite are reported to be high potential nanofertilizer for slow-release of multi-nutrients including phosphate ion . 12,14
2Zeolite / hydroxyapetite nanocomposite characterization
The zeolite / hydroxyapetite based nanocomposite was characterized via different techniques i.e., scanning electron microscopy (SEM), energy dispersive x-ray spectrometer (EDX) and Fourier transform infrared spectroscopy (FTIR).
Structural and chemical composition of Zeolite/ hydroxyapetite nanocomposite (ZHNC)
SEM images reveal the surface morphology of Ca2+ –zeolite NaP1/ hydroxyapetite nanocomposite (Fig.1 a, b). As shown in Fig. 1. Ca2+–zeolite NaP1/ hydroxyapetite nanocomposites are amorphous structure showing zeolite NaP1 with needle-like hydroxyapetite crystals on them. Elemental nature of ZHNC nanocompositeonfirmed by EDX spectra , where peaks hikes in regions of 0.25, 0.50, 1.30, 1.50 and 1.75 and 2.0 keV represent the binding energies of Ca, K, O, Fe, Mg, Al, Si, P (Fig. 2). FTIR spectra (Fig. 3) exhibited distinct absorption peaks of ZHNC at 3694.4, 3441.4, 1651, 1032.1, 912.8, 797, 518.2 and 469 cm-1. Major characteristic FT-IR peaks were present at 1032 and 912 cm-1. The other major absorption peaks occur in the regions 3620 –3433 cm-1 and 1640 – 1024 cm-1 and the region below 800 cm-1 (Fig. 3).
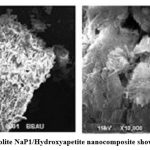 |
Figure 1: SEM image of zeolite NaP1/Hydroxyapetite nanocomposite showing structural morphology |
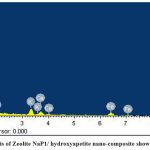 |
Figure 2: EDX analysis of Zeolite NaP1/ hydroxyapetite nano-composite showing elemental nature |
 |
Figure 3: FTIR spectra of ZHNC nanocomposite, control soilZHNF amended soil (ZHNC + soil) showing chemical composition |
Site description and soil properties
The experiment site was Department of Botany, Langat Singh College, Muzaffarpur, located at 25°54′ N and 84°53′ E. Maximum and minimum temperatures during the growing season are 35°C -20 °C. Experimental soil is new alluvium type. Its main characteristics were 0.86% organic matter, 0.49% organic carbon, pH 7.8, 162.84 dS m-1 CEC 54.80 meq Kg-1 soil). The available nitrogen, phosphorus and potassium values were 21.4 mg N Kg-1, 4.63 mg P Kg-1, and 49.4 mg K Kg-1. Other details are mentioned in Table 2.
Experimental design and Treatment conditions
Table 1: Treatment set ups of different fertilizermended soil
|
Treatments |
Soil application with Base Fertilizer |
|
|
i). |
C |
Control soil (without any treatment) |
|
ii). |
F |
inorganic NPK fertilizer |
|
iii). |
NZF |
Nanozeolite based fertilizer |
|
iv). |
ZHNF |
Nano Zeolite/ Hydroxyapetite based nanofertilizer |
Inontrol set up, no fertilizer treatments were provided tooil. For F treatment (ii) recommended dose of NPK as per Agriculture Research Institute, Pusa was amended to soil
In Treatments (iii & iv); 5% of nutrients (N, P, K) inrm of their salts were embedded with aespective dose of 2% (v/w) of NZ and ZHNF aqueous suspension res. The plant experiment was conducted in the soil pot culture, grown under the recommended doses of fertilizers amended soil.
Test for Soil physico-chemical property
Control and different fertilizer amended soil samples were subjected to following physico-chemical analyses by the methods indicated below:-
Soil pH
The pH of the soil was determined electrometrically in aquous suspension of soil using electrode on a digital pH meter. 21
Electrical conductivity
Electrical conductivity of the soil sample was measured in aquous soil suspension at 250 C using conductivity meter. 22
Bulk density
Soil samples were oven dried and weighed and bulk density (g cm-3) was calculated from the known weight and volume of the soil mass as determined by the Black (1965) method.23
The formula for the calculation of Bulk density (B.D) is as follows;

Organic carbon
Organic carbon in the soil samples was determined according to Walkely-Black chromic acid wet oxidation method. 24
Water holding capacity of soil:
To describe the water status in a soil,ater holding capacity (WHC) of the soil was analyzed by methods of Keen box method.25 To determine the water holding capacity by mass, the following equation was used:
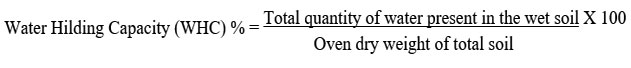
Soil N, P,K analysis
Available nitrogen in soil was estimated using the alkaline potassium permanganate by the Kjeldahl method.26 Available phosphorus by the method of Olsen et al. (1954)27 and soluble potassium was also estimated by the method described by Jackson (1958)28.
Plant Analysis
Experimental Plant
Eggplant (Solanum melongena L.; Solanaceae family) is chosen asperimental plant to evaluate the impacts of different fertilization conditions on plant systems. Eggplant fruits are highiber contents, vitamins and minerals and rich sources of iron and manganese). It is an agronomical and economically valuable vegetable crop.
Parameters assessed
Eggplants grown under different fertilizermended soil were examined for its morphological traits and biomass productivity. Different parameters i.e., seed germination %, Plant height, thickness, plant weight, fruit length and fruit weight was assessed atarvest stage of plants (60 DAG)
Statistical analysis
The data was analyzed using Microsoft Excel and Sigma Plot 12.5 software. All the readings are reported asverage of three repeats.
Results
Effect of ZHNF on soil physicochemical characteristics
Soil analysis results interpret that experimental soil is sandy loam in texture withH range .2-8.4, organic matter (SOM) approx. 0.86 %, total organic carbon (SOC) 0.43%, total N (8.6 g Kg-1), available N (21.41 mg Kg-1), available P (4.63 mg Kg-1), total K (59.4 mg Kg-1) dry weight. Amendments of different fertilization in soil resulted in elevated values for these parameters (Table 2). Total Organic carbon (TOC) and organic matter (OM) showed a significant hike of approx. 2 to 2.5 fold after NZ & ZHNF relatively when compared with untreated (control) soil. Next on, soil bulk density found to decreaserom 1.28 to 0.64 g cm-3, whereas poring density in reversal increased from 2.43 to 5.76 g cm-3), cation exchange ability (CEC); from 54.80 meq Kg-1 to 112.68 meq Kg-1 after ZHNF treatments in respective to control (Table 2). Among soil nutrients, ignificantlyighest increase in N, P, K values were observed in ZHNF treatment with respect to both other treatments (NZ & F), when compared with untreated control soil. NZ and ZHNF by induced 2.6 & 3 fold increments inoil available N. Potassium availability inoil also maintained at a higher level with relative increase9% and 84.5% in NZ and ZHNF treated soil in respective to control. Mean comparison for soil available phosphorous content also follows a similar trend withmaximum increase (almost 4.3 fold) in ZHNF, followed by NZ (2 times).
Table 2: Comparative change in physicochemical characteristics of soil after ZHNC and other fertilizer amendment after 14 days incubation period
|
Soil parameters |
Control soil
|
Inorganic fertilizer amended soil |
Nanozeolite amended soil |
Nanozeolite/ Hydroxyapetite amended soil |
|
pH |
7.84 ± 0.59 |
8.19±0.43 |
7.23±0.58 |
7.52±0.63 |
|
Bulk density (g cm-3) |
1.28 ± 0.11 |
0.98±0.04 |
0.69±0.03 |
0.64±0.03 |
|
Pore density (g cm-3) |
2.43 ± 0.11 |
3.61± 0.21 |
4.58± 0.34 |
5.76± 0.43 |
|
Water-soluble salts (meq Kg-1 soil) |
47.85 ± 2.36 |
57.04 ± 4.38 |
72.31 ± 5.23 |
69.58 ± 4.87 |
|
Water holding capacity of soil (%) |
42.62± 3.97 |
59.17± 4.3 |
68.23±4.7 |
73.76±5.1 |
|
EC (µS cm-1 ) |
162.84 ± 9.8 |
217.23±23.4 |
286.23±17.6 |
314.23±15.9 |
|
Cation exchange capacity (meq Kg-1 soil) |
54.80 ± 0.36 |
72.68±3.28 |
94.73±6.82 |
112±10.5 |
|
Soil Organic carbon (%) |
0.49 ± 0.015 |
0.73±0.02 |
0.95±0.03 |
1.13±0.45 |
|
Organic matter (%) |
0.86 ± 0.25 |
1.37±0.13 |
1.84±0.61 |
2.17±0.83 |
|
Total Nitrogen (g kg–1) |
8.6±1.1 |
12.3±0.92 |
15.8±1.0 |
18.2±0.83 |
|
Nitrogen (avail) (mg/ Kg) |
21.41±0.12 |
38.26±0.21 |
56.61±0.98 |
64.87±0.86 |
|
Phosphorous (avail) (mg/Kg) |
4.63 ± 0.25 |
7.06±0.28 |
15.9±1.1 |
19.85±2.34 |
|
Potassium |
59.4 ± 15.3 |
78.6±0.11 |
94.8±0.36 |
109.6±0.42 |
Values are mean ± S.E. of three replicates.
ZHNF effect on water holding capacity of soil
Inresent study, the ddition of different fertilizer treatments significantly increased the WHC of the soil. Importantly, the addition of ZHNF shows the maximum increase in value of WHC of soil (73%), followed by NZ (60%), whereas F treatment showed only up to 20% hike when compared with untreated control soil (Fig 4).
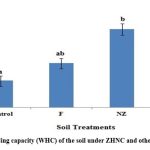 |
Figure 4: Water holding capacity (WHC) of the soil under ZHNC and other fertilizer treatments |
Effects of ZHNF on plant growth traits and productivity
At the stage of harvest (60 DAG), eggplants grown in ZHNF- amended soil are significantly taller (91.3 cm) and thicker (2.86 cm) when compared with control and other treatments (Table 3). In addition, the plant biomass and fruit weight also increased significantly (p < 0.05) by 1.5 and 2.4 fold (Table 3, 4). Nutrient analysis results in fruit parts also follow the trends of maximal increase in N, P and K values (86, 148 & 74% respectively) in ZHNFreated ones, followed by NZ and F respectively (Table 3). The significant (p < 0.05) higher increase in organic phosphorus (almost 2.5 fold) in plants grown under ZHNCreated soil.
Table 3: Comparative change in seed germination potency, plant morphologyplant biomass in purple eggplant Solanum melongena grown under ZHNC and other fertilizeramended soil
|
Soil treatments |
Plant observation |
|||
|
Seed germination (%) |
Plant height (cm) |
Stem diameter (cm) |
Plant dry wt. (g) |
|
|
Control |
45% |
65.6±9.6 |
0.85 ±0.05 |
24.32±0.11 |
|
Inorganic fertilizer |
63% |
73.4±8.4 |
1.27 ±0.12 |
31.6±0.13 |
|
Nanozeolite |
85% |
83.7±8.7 |
2.58 ±0.14 |
52.9±0.12 |
|
Nanozeolite/ Hydroxyapetite |
89% |
91.3±10.1 |
2.86 ±0.11 |
60.2±0.14 |
Results are mean ± S.E. of three replicates
Table 4: Comparative analysis of eggplant fruit size, biomass and nutrient contents in plants grown under ZHNF and other treatment soils at stage of 60 DAG.
|
Parameters |
Treatments |
||||
|
Control (C) |
NPK fertilizer (F) |
Nanozeolite (NF) |
Nanozeolite/ Hydroxyapetite (ZHNF) |
||
|
Eggplant fruit |
fruit height (cm) |
16.29±1.1 a |
19.13±1.ab |
21.21±2.1b |
21.99±1.6b |
|
observation |
Fruit diameter (cm) |
3.26±0.2a |
4.2±.0.2b |
5.16±0.3c |
5.93±0.3bc |
|
No. of fruit per plant |
5.2±0.2a |
7.6±0.4b |
10.8±0.9b |
11.5±1.0b |
|
|
fruit dry weight (g) |
9. 29±8.6a |
12.8±1.1b |
14.35±9.8b |
17.8±12.2b |
|
|
Macro-nutrients |
Nitrogen |
3.6±0.12a |
5.8±0.26b |
6.1±0.18b |
6.7±0.34b |
|
in fruit (% DW) |
Phosphorus |
0.29±0.02a |
0.35±0.01ab |
0.42±0.03b |
0.72±0.03c |
|
|
Potassium |
4.7±0.23a |
6.3±0.27b |
7.8±0.32c |
8.2±0.28c |
Results are mean ± S.E. of three replicates. Values with different letters in the same column differ significantly at p<0.05.
Discussion
Structural morphology of Zeolite NaP1/ hydroxyapetite nanocomposite detects the presence of needle-like hydroxyapetite crystals on amorphous zeolite matrix. Structure well supported with previous related research publications.29, 30, 31 EDX analysis of ZHNC nanocomposite detects the presence of elements Fe, Mg, Al, Si, P. FT-IR peaks assigned to the hydroxyl groups, vibrations of Al-SiO oxide bonds and the PO42- group. 30, 31 Characteristic peaks at 1032 and 912 cm-1 assigned to the asymmetric stretching vibration of the AlO and SieO tetrahedral.32 Absorption spectra for control and ZHNC amended soil were very different, which refers to hange inhemical composition of soil after ZHNC nano-composite amendment. The major absorption peaks are attributed to specific chemical constituents including sand, clay, and organic components. The weak broad band at 1640 and 1428 cm-1 may be due to the C=O and C-H bending and starching O-H deformation.31 IR peaks at 1025–800 cm-1 and 700–400 cm-1 refers to silicate (Si-O) stretching, a major soil component.33 The region below 1025 cm-1 is the fingerprint region of soil, where the absorptions are mainly due to O-H bending N-H bending in the lattice of clay, siltsand.34
Soil analysis results significantly revealed the prominent beneficial aspects of ZHNC treatment in improving soil quality and soil nutrient availability. An increase in soil organic matter and carbon content relates to improved soil health and biological functioning of soils.35 Whereas ZHNC decreased bulk density, high porosity, CEC of soil consequently interpret to loose, well aerated, porous soil texture.36 The higher retention of the nutrient elements (especially nitrogen and Phosphorus) in soil upon the nanocomposite treatment may refer to optimized nutrient uptake by soil particles and lesser loss through mineral leaching. Water holding capacity (WHC) of the soil is also one of the most important physical soil factors for sustainable agriculture management. In view, soil analysis results also show a significant enhancement in water holding capacity of the soil indicating the continued water availability in soil for plant requirement during replenishment. Herein, ZHNF acts significantly in improving soil moisture content vis avoids drought tolerance of plants.
Significant positive impacts of ZHNC in improving soil quality in turn results in to increase in plant growth and productivity grown under ZHNC amended soil along with high nutrient assimilation potency. Highest increase in organic P level in ZHNF treated may be due to facilitation in P solubility in plants by hydroxyapetite as supported by previous research literatures.10
Conclusions
The results of resent study well interpret that hybrid nanozeolite/hydroxyapetite base fertilizer amendment in soil acts positively in improving the soil physico-chemical properties, its water retention potency and overall sustaining the water reservoir for soil-plant system. Further on, plant observation well suggests their positive impact on lant system, boosting its growth and productivity. ZHNF significantly increased the fruit biomass and the content of bioavailable phosphorus along with Nitrogen and Potassium at higher levels in respective to other treatments, indicating the holistic perspective on the potential high benefits of this nanocomposite as a nanofertilizer.
Inverall remark, Zeolite/ Hydroxyapetite acts as highalue nanofertilizer, helps to facilitate nutrient uptake in soil and water, and accelerates its mobilization, transportationabsorption in plant systems. It also proved its high efficiency in the absorption of phosphate ions along with other nutrients, which ifficult to mobilize in se of singly zeolite or other fertilizers. Phosphate is nutrient that contributes significantly to crop productivity. So on, ZHNF proven as a better alternative as multi-nutrients fertilizer (highly efficient N & P) with obtention of high crop yields and productivity.
Acknowledgement
Author acknowledges the Head, Department of Environmental Science and Director, USIC, BBAU, Lucknow, for providing lab facilities to examine nanocomposite characterization. Author also acknowledges Principal, Langat Singh College, Muzaffarpur, Bihar for academic support and facility. This work is a part of CSIR project and author acknowledges financial support from CSIR, New Delhi for providing CSIR-SRA fellowship for this project in year 2017 (Grant no. 13 (8946-A) /2017-Pool.).
Funding Source
At the time of manuscript preparation there is no funding source
Conflict of Interest
The author declares no competing interests
Authors’ contributions
As a sole author R. Kumari solely conceptualize the experiment, methodology standardization, and data preparation, experimental analyses, writing the original manuscript and review.
Informed consent statement
As Rima Kumari is a sole Author of this manuscript and Principal Investigator of concerned research project, informed consent statement is not applicable.
References
- Cui N., Cai M., Zhang X., Abdelhafez A. A., Zhou L., Sun H., Chen G., Zou G., Zhou S.Runoff loss of nitrogen and phosphorus from a rice paddy field in the east of China: effects of long-term chemical N fertilizer and organic manure applications. Global Ecol and Conser. 2020; 22: e01011,
- Ashitha , Rakhimol K.R., Mathew J. “Chapter 2 ─ fate of the conventional fertilizers in environment,” in Controlled release fertilizers for sustainable (Amsterdam, Netherlands). Elsevier. 2021; 25–39.
- Hafez M., Khalil H.F. Nanoparticles in Sustainable Ariculture: Recent Advances, Challenges and Future Prospects. Communications in Soil Science and Plant analysis. 2024; 55(14): 2181-2196.
- Naderi M.R., Danesh-Shahraki A. Nanofertilizers and their roles in sustainable agriculture. Int J Agri Crop Sci. 2013; 5: 2229-2232.
- Raliya R., Saharan V., Dimkpa C., Biswas P. Nanofertilizer for Precision and Sustainable Agriculture: Curr State Future Persp. 2017; 65 (39): 8552-8559.
- Verma K.K., Song X.P., Joshi A., Rajput V.D., Singh M., Sharma A., Singh R.K., Li D.M., Arora J., Minkina T. et al. Nanofertilizer Possibilities for Healthy Soil, Water, and Food in Future: An Overview. Plant Sci. 2022; 13: 865048.
- Corradini E. A preliminary study of the incorporation of NPK fertilizer into chitosan nanoparticles. eXPRESS polymer Letters.2010; 4(8):509-515
- Yamamoto C.F., Pereira E.I., Mattoso L.H.C., Matsunaka T., Ribeiro C. Slow release fertilizers based on urea/urea-formaldehyde polymer nanocomposites. Chem Engineering J. 2016; 287: 390-397.
- Giroto A.S., Guimaraes G.G.F., Foschini M., Ribeiro C. Role of Slow-Release Nanocomposite Fertilizers on Nitrogen and Phosphate Availability in Soil Scientific Reports. 2017; 7: 46032.
- Ahmed A.A. Elsayed., Ahmed EL-Gohary., Zeinab K.T., Hend M.F., Mohamed S.H., Khaled AA. Hydroxyapatite nanoparticles as novel nano-fertilizer for production of rosemary plants. Sci Horticult. 2022; 295: 110851.
- Dou , Bini Farias M.V., Che W. et al. Highly degradable chitosan-montmorillonite (MMT) nano-composite hydrogel for controlled fertilizer release. Front. Environ. Sci. Eng. 2023; 17: 53 .
- Watanabe Y., Yamada H., Ikoma T., Tanaka J., Stevensd G.W., Komatsua Y. Preparation of a zeolite NaP1/hydroxyapatite nanocomposite and study of its behavior as inorganic fertilizer. J Chem Technol Biotechnol. 2014; 89: 963–968.
- Lateef A., Nazir R., Zamil N., Saleem M. Synthesis and characterization of zeolite based nano–composite: An environment friendly slow release fertilizer. Micropor and Mesopor Mat. 2016; 232: 174-183
- Mikhak A., Sohrabi A., Kassaee M.Z., Feizian M. Synthetic nanozeolite/nanohydroxyapatite as a phosphorus fertilizer for German chamomile (Matricaria chamomilla). Industrial Crops and Products 2017; 95: 444–452
- Watanabe Y., Taya N., Fujinaga K., Oshima S., Yamada H., Ikoma T., Tanaka J., Moriyoshi Y., Komatsu Y. Synthesis of zeolite NaP1 from coal fly ash using alkaline hydrothermal treatment and its application as an environmental purification material. J Soc Inorg Mater Jpn. 2010; 17:108–116.
- Rai V., Acharya S., Dey N. Implications of nanobiosensors in agriculture, J Biomater Nanobiotech. 2012; 3:
- Szameitat A.E., Sharma A., Minutello F., Pinna A., Er-Rafik M., Hansen T.H., Husted S. Unraveling the interactions between nanohydroxyapatite and the roots of phosphorus deficient barley plants. Environ Sci: Nano. 2021; 8(2): 444-459.
- Ammar M., Ashraf S., Baltrusaitis J. Nutrient-Doped Hydroxyapatite: Structure, Synthesis and Properties. Ceramics. 2023; 6(3): 1799-1825.
- Taskin H., Gunes A. Synthetic nano-hydroxyapatite as an alternative phosphorus source for wheat grown under field conditions Synthetic nano-hydroxyapatite as an alternative phosphorus source for wheat grown under field conditions. J Plant Nutrition 2023; 46 (5).
- Kumar M.M., Jena H. Direct single-step synthesis of phase pure zeolite Na–P1, hydroxy sodalite and analcime from coal fly ash and assessment of their Cs+ and Sr2+ removal efficiencies, Microporous and Mesoporous Materials. 2022; 333: 111738.
- Jackson, M.L. 1967. Soil chemical analysis. Prentice Hall India Pvt. Ltd., New Delhi, pp. 498
- Bower C.A., Wilcox L.V. Soluble salts. In Methods of soil analysis Part 2 (Eds. CA. Black.) 1965: 933-951. (American Society of Agronomy, Inc: Madison, Wisconsin. U.S.A).
- Black C.A. Methods of Soil Analysis: Part I Physical and mineralogical properties. In: American Society of Agronomy, Madison, Wisconsin, USA. 1965.
- Walkley A., Black I.A., An examination of Degtjareff method for determining soil organic matter and a proposed modification of the chromic acid titration method. Soil Science, 1934; 37: 29–37.
- Keen B.A., Raczkowski H. The relation between clay content and certain physical properties of soil. Journal of Agriculture Science. 1921; 11: 441-449.
- Page M.A. Methods of Soil Analysis. Part 2. Academic press, New York. 1982.
- Olsen S.R., Cole C.H., Wantanab F.S., Dean L.A. Estimation of available phosphorus by extraction with sodium carbonate. U.S. Department of Agricultural Circle, Washington D.C., 1954; 93: 1-19,
- Jackson, M.L. 1958. Soil chemical analyses. Prentice Hall, London.
- Moriyoshi Y., Chiba Y., Monma H., Ikegami T. Preparation of hydroxyapatite in a reaction between alite and phosphoric acid. TransMater Res Soc Jpn. 2000; 25:1143–1146.
- Kuwahara Y., Ohmichi T., Kamegawa T., Kohsuke M., Yamashita H. A novel synthetic route to hydroxyapatite–zeolite composite material from steel slag: investigation of synthesis mechanism and evaluation of physicochemical properties. J. Mater. Chem. 2009; 19: 7263–7272.
- Khorasani F., Khavarpour M.M., Lamuk M.S. The study of morphology and grain size of Hydroxyapatite/Zeolite nanocomposite using SEM and TEM analyses. Specialty J Chem. 2019; 4 (1): 1-8
- Prasetyoko D., Ramli Z., Endud S., Hamdan H., Sulikowski B. Conversion of rice husk ash to zeolite beta. Waste Management 2006; 26: 1173-1179.
- Margenot A.J., Calderon F.J., Goyne K.W., Mukome F.N.D., Parikh S.J. IR Spectroscopy, Soil Analysis Applications. Encycl Spectroscopy and Spectrometry, Third Edition, 2017; 2: 448-454.
- Yang H., Mouazen A.M. Vis/near and mid-infrared spectroscopy for predicting soil N and C at a farm scale. Infrared Spectroscopy—Life Biomed Sci. 2012; 185-210.
- Celik I., Gunal H., Budak M., Akpinar C. Effects of long-term organic and mineral fertilizers on bulk density and penetration resistance in semi-arid mediterranean soil condition. Geoderma. 2010; 160: 236–243
- Juma K.N., Nakhone L., Musandu A.A., Nyalala S., Ogendo J.O., Kimaru L. Availability of nitrogen, microbial respiration and bulk density as influenced by faecal matter fertiliser in Acrisol, Andosol and Planosol. J Soil Sci & Plant Health 2018; 2:1

