Introduction
Coffee is the most important raw material traded throughout the world after crude oil 1. Coffee sustains over 100 million people globally and is rated among the largest export commodities in the world 2. Coffee is the fifth largest foreign exchange earner in Kenya after tourism, tea, horticulture and diaspora remittances 3 and has largely contributed to the Kenyan economy through foreign exchange earnings, as well as creating employment opportunities 4.
Despite its importance, coffee production in Kenya faces a major challenge from Coffee Berry Disease (CBD). The disease is caused by Colletotrichum kahawae, an anthracnose of green and ripe berries. The pathogen is an ascomycete that reproduces both sexually and asexually 5. The fungi can infect the plant during any stage from the flower to the berry. Mummified berries, twigs, bark and dead branches are the sources of primary inoculum. Colletotrichum conidium forms within 24 hours under favourite conditions, after which elongation of the germ tube follows 6. The apical section of the germ tube later differentiates to form an appressorium. The coffee fruit is then colonized by infection hyphae that arise from the appressoria. The symptoms then start to appear that includes; dark, necrotic sunken spots and brown, superficial lesions7. The disease causes premature fruit fall and mummified or damaged fruits which leads to a loss of about 75% of coffee 8.
Coffee Berry Diease was first detected in Sotik in Western Kenya in 1922 9. The disease has spread since then to several countries, including Uganda, Tanzania, Angola, Cameroon, Malawi, DRC Congo, and Rwanda among other countries. Rain is almost 100% responsible for transmission of the spores of C. kahawae, from the surface of infected berries to other berries by raindrops. The disease is mainly reported in coffee farms located at an altitude of between 1400 and 2200 m above sea level 9.
Colletotrichum kahawae in Kenya can be managed using resistant coffee cultivars such as Ruiru 11 and Batian 3, 8,10,11 . However, most farmers have retained traditional varieties like SL 28, SL 34, and K7 which are highly susceptible to CBD due to their high cup quality 10. Other management strategies, include cultural practices like use of shade trees to reduce the impact of raindrops, and timely and proper pruning of coffee plants 9,12. Cultural practices are not 100% reliable for management of CBD, and therefore incorporation of chemical control into integrated disease management programmes is critical 6, 13. There are over 200 commercial fungicides tested and registered in Kenya for management of CBD 14. The registered fungicides can be broadly classified into Strobulins, Coppers, Triazoles and biopesticides. Despite having a number of fungicides to use, most coffee farmers in Kenya use fungicides from the Family of strobulins because of their high efficacy. However, the number of registered fungicides in this family is very narrow, an aspect that increases the risk of resistance development 15. It is therefore recommended by the Fungicide Resistance Action Committee (FRAC) for an interchangeable use of fungicides with different modes of action to avoid fungicide resistance 15.
Pyraclostrobin active ingredient has been used for the control of anthracnose disease of sorghum, Septoria tritici, Puccinia spp, Pyrenophora teres and even Colletotrichum kahawae26. A premix of Fluxapyroxad +Pyraclostrobin active ingredients have been used for the control of several plant pathogens including anthragnose and powdery mildew which has resulted to an increase in mango production13. The same combination has controlled several other fungal pathogens including Fusarium virguliforme, Macrophomina phaseolina, Rhizoctonia solani, and Sclerotinia sclerotiorum 25.
This study, aims to evaluate (Pyraclostrobin 150 g/L + Fluxapyroxad 75 g/L) and (Pyraclostrobin 128 g/Kg + Boscalid 252 g/Kg) as alternative fungicides for management of Coffee Berry Disease to be used interchangeably with the available fungicides as recommended by FRAC.
Study Area
The study was conducted in the Plant Pathology laboratory, at the KALRO- Coffee Research Institute, Ruiru, Kenya. The centre lies at an altitude of 1620 m above sea level, on latitude 0° 41′ 0 S and longitude 34° 46′ 0 E , in Kiambu County. It receives a annual bimodal rainfall of 1063 mm with an average temperature of 16 ºC (Min 12.8 ºC, max 25.2 ºC).
Sample Collection
Purposive sampling technique was used 16 whereby, only coffee berries with CBD symptoms were collected to be used for isolation of C. kahawae. A total of 170 premature green berries and partially ripe beans with slightly sunken and dark brown lesions were collected randomly from sprayed and unsprayed plots of SL 28, Geisha and K7 coffee varieties in Rukera farm at KALRO-CRI, for isolation of C. kahawae.
Fungal Isolation
The diseased berries were washed once in sterile distilled water with Teepol® (Teepol GD 18, Shell Chemical Industries Limited, Nairobi, Kenya). Five drops of Teepol® in 200 ml sterile distilled water using a teat pipette was used to remove initial dust 7. The berries were rinsed with sterile distilled water until no foam was formed. Clean coffee berries were spread on a cellulose wadding on a sterile bench and left for 30 minutes to air-dry. After air-drying, the berries were incubated at 24 ºC for 24 hours in sterile lunch boxes containing cellulose wadding to promote sporulation. After sporulation, the lesions were cut carefully and placed in sterile distilled water and serially diluted to 10-3 concentration. Using the spread plate technique, 10-2 and 10-3 dilutions were spread in a previously prepared Potato Dextrose Agar( PDA) (HiMedia Laboratories Pvt. Ltd. India) on 90mm × 15mm Petri plates. The plates were transferred in a sterile room for growth for seven days at a temperature of 24 ± 1 ºC. A mixed culture of several isolates was obtained after seven days. Pure cultures were obtained by subculturing from regions where culture characteristics corresponded with those documented for C. kahawae 7. The C.kahawae obtained was used in subsequent experiments for Fungicide efficacy assessment.
Anti-Fungal Activity Assay using Poisoned Food Technique
Two new proprietary fungicides, CRI 1 and CRI 2 were subjected to anti-fungal activity assay using poisoned food technique 17. CRI 1 has a combination of Pyraclostrobin and Fluxapyroxad while CRI 2 has a combination of Pyraclostrobin and Boscalid. Two commercial fungicides Pyraclostrobin 250 g/L and Tebuconazole 200 g/L + Trifloxystrobin 100 g/L were used as standard controls. The discs on the media devoid of the fungicide acted as negative controls. Potato Dextrose Agar was prepared and sterilized at 121 ºC, and 15 atmospheres pressure, for 15 minutes in an autoclave (Priorclave Ltd).
The PDA was supplemented with test fungicides at ten concentrations of (0.01 %,0.02 %,0.03 %,0.04 %,0.05 %,0.06 %,0.07 %,0.08 %,0.09 %,0.1 %) for CRI 1 and CRI 2 and two commercial standards Pyraclostrobin 250 g/L (0.04%) and Tebuconazole 200 g/L + Trifloxystrobin 100 g/L (0.1%) . The fungicides containing media were poured into the Petri plates under aseptic conditions and allowed to cool and solidify. After complete solidification of the medium, 5mm disc of seven days old culture of C. kahawae was cut together with the PDA media using a sterile cork borer and placed aseptically upside down at the centre of the Petri plates. The treatments were replicated three times. The plates were incubated at 24 ± 1 ºC and the growth of fungus colony was measured after every 24 hours for 13 days. The colony diameter was measured in millimetres using a digital vernier calliper.
Data Analysis
Data on fungal colony diameter was converted into angles corresponding to percentage angles 18, and Analysis of Variance (ANOVA) was done using CoStat software version 6.400 (Cohort software Limited, United Kingdom).
Results
Isolation of Colletotrichum Kahawae from Infected Coffee Berries
Isolated Colletotrichum kahawae fungal pathogen on PDA media is presented in figure 1. The fungal isolates were identified as C. kahawae based on the morphological appearance when grown on PDA media. Isolate 1 of C. kahawae fungal pathogen was obtained from Rukera farm at CRI where fungicides are applied for control of CBD while isolate 2 of C. kahawae was obtained from Coffee Research Institute coffee farm, where fungicides are not applied.
 |
Figure 1: Dark Gray C. kahawae on PDA media. |
Efficacy of CRI 1 and CRI 2 Fungicides Against C. kahawae
The various candidate fungicide rates recorded significantly (P<0.05) lower colony diameters for both isolate 1 and isolate 2 compared with the control treatment (Table 1). Although there was a reduction in colony diameter with the increasing concentration of CRI 1 fungicide, there was no significant difference that was established among the treatment rates and the standard controls (Pyraclostrobin 250 g/L (0.04%) and Tebuconazole 200 g/L+Trifloxystrobin 100 g/L (0.1%) as indicated in Table 1. CRI 1 Fungicide percentage control increased with the increasing concentration from 0.01% to 0.1% (Table 2). The highest colony diameter that differed significantly (P<0.05) from the candidate fungicides was recorded in the untreated control (40.13 mm and 36.85 mm) for isolate 1 and isolate 2 respectively (Table 1 and figure 2).
Table 1: Mean Colony Diameter of C. kahawae Isolate 1 and Isolate 2.
| CRI 1 | CRI 2 | |||
| Colony Diameter(mm) | Colony Diameter(mm) | |||
| Fungicide % rates | Isolate 1 | Isolate 2 | Isolate 1 | Isolate 2 |
| Control | 40.13 a | 36.85 a | 35.96 a | 38.74 a |
| 0.01 | 13.62 b | 13.52 b | 22.63 b | 25.48 b |
| 0.02 | 13.31 b | 13.31 b | 21.51 bc | 23.93 bc |
| 0.03 | 13.22 b | 13.22 b | 21.21 bcd | 23.37 bcd |
| 0.04 | 13.09 b | 13.22 b | 20.52 cde | 19.59 cde |
| 0.05 | 13.09 b | 13.05 b | 20.26 cde | 19.17 de |
| 0.06 | 13.05 b | 13.05 b | 19.85 cdef | 18.64 de |
| 0.07 | 13.05 b | 13.05 b | 19.43 def | 18.21 e |
| 0.08 | 13.05 b | 13.05 b | 18.72 efg | 17.19 ef |
| 0.09 | 13.05 b | 13.05 b | 18.3 fg | 16.81 ef |
| 0.1 | 13.01 b | 13.05 b | 17.29 g | 16.60 ef |
| Pyraclostrobin 250 g/L (0.04%) | 13.05 b | 13.05 b | 13.05 h | 13.05 f |
| Tebuconazole 200 g/L+Trifloxystrobin 100 g/L (0.1%) | 13.31 b | 13.31 b | 13.31 h | 13.31 f |
| LSD (P=0.05) | 1.06 | 1.75 | 0.87 | 2.28 |
| CV (%) | 8.6 | 14.47 | 5.32 | 13.88 |
Means followed by the same letter in the same column are not significantly different. (LSD test P < 0.05)
Table 2: CRI 1 and CRI 2 Fungicide Percentage Rates and Percentage Control.
| % Control | ||
| Fungicide % rates | CRI 1 | CRI 2 |
| 0.01 | 64.74 | 35.6 |
| 0.02 | 65.42 | 39.17 |
| 0.03 | 65.65 | 40.32 |
| 0.04 | 65.82 | 46.31 |
| 0.05 | 66.04 | 47.22 |
| 0.06 | 66.1 | 48.47 |
| 0.07 | 66.1 | 49.61 |
| 0.08 | 66.1 | 51.93 |
| 0.09 | 66.1 | 53 |
| 0.1 | 66.15 | 54.63 |
| Pyraclostrobin 250 g/L (0.04%) | 66.1 | 66.1 |
| Tebuconazole 200 g/L+Trifloxystrobin 100 g/L (0.1%) | 65.76 | 65.76 |
 |
Figure 2: B1-B10 shows Highly Suppressed Colony Diameter on the Ten Rates of CRI 1 Fungicide. B 11 shows the Highest Colony Diameter on the Untreated Control. |
The colony diameter decreased with the increasing concentration of CRI 2 fungicide with the lowest colony diameter recorded in the plates with rate 0.1% for both isolate 1 and isolate 2 (17.29 mm and 16.60 mm respectively). The fungi colony diameter was significantly (P<0.05) reduced by CRI 2 at 0.01% ,0.02%, 0.03%,0.04%, 0.05%,0.06%,0.07%,0.08%,0.09% and 0.1% in relation to the control treatment although the reduction for rate 0.08%,0.09% and 0.1% did not differ significantly (P<0.05) from that of the standard control treatments ( Table 1). The fungicide percentage controls increased from the lowest concentration of 0.01% to the highest concentration of 0.1% (Table 2 and figure 3).
 |
Figure 3: C1-C9 Shows Colony Growth on the Ten Rates of CRI 2 Fungicide. C10 Shows the Highest Colony Diameter on the Untreated Control. |
Table 3: Fungicides, Isolates and their Interaction on the Control of C. kahawae.
| Source | |
| Main Effects | P-value |
| Treatment | 0.0000 *** |
| Fungicide | 0.0000 *** |
| Plot | 0.8268 ns |
| Interaction | |
| Treatment x Fungicide | 0.0000 *** |
| Treatment x Plot | 0.9353 ns |
| Fungicide x Plot | 0.5241 ns |
| Treatment x Fungicide x Plot | 0.4175 ns |
| LSD (P=0.05) | 1.59 |
| CV (%) | 11.12% |
The interaction between the treatments and the fungicides was significant (P<0.05) in controlling the CBD colony diameter (Table 3). There was no significant (P>0.05) difference between the interaction of the treatment and the plot, the fungicide and the plot and the interaction of the treatments, the fungicides and the plot in controlling the CBD colony diameter (Table 3).
The fungal colony diameter decreased with the increasing fungicide concentration. The mean colony diameter of CRI 1 was smaller than that of CRI 2. The mean colony diameter of CRI 1 decreased with the increasing fungicide concentration from 0.01 % upto 0.05 % and then the colony diameter remained constant upto rate 0.1% (Figure 4).
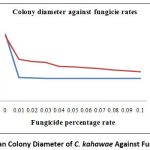 |
Figure 4: Mean Colony Diameter of C. kahawae Against Fungicide Rates. |
Discussion
Colletotrichum kahawae fungus was isolated and identified based on its morphological appearance on PDA media 19,20 ,21. The results of this study have revealed that both CRI 1 and CRI 2 fungicides significantly controlled the growth rate of Colletotrichum kahawae in vitro.
The suppressive active ingredient Pyraclostrobin in the two chemicals hinders spore germination, germ tube elongation, mycelia growth and sporulation 22.
In addition, the study revealed that CRI 1 was more suppressive on the colony diameter compared to CRI 2. This could be attributed to the differences in the chemical ingredients in the two products (CRI 1 and CRI 2). Fluxapyroxad is more effective in suppressing the fungal growth alone 23. Pyraclostrobin has also been found controlling the fungal pathogens independently as in the case of Pyraclostrobin 250 g/L 24.
The mode of actions in the two active ingredients, Fluxapyroxad and Pyraclostrobin in CRI 1 could be the reason for its high efficiency in controlling C. kahawae. The results of this study are in agreement with 13, 25, 26,. In a study on the in vitro evaluation of commercial fungicides against some of the major soil borne pathogens of soybean, Navi concluded that a combination of Pyraclostrobin and Fluxapyroxad controlled several fungal pathogens that included; Fusarium virguliforme, Macrophomina phaseolina, Rhizoctonia solani, and Sclerotinia sclerotiorum 25.
In a study on the evaluation of pre-mix fungicide, fluxapyroxad and pyraclostrobin 500 sc against powdery mildew disease of mango (Oidium mangiferae), Ravikumar confirmed that the fungicide significantly reduced the disease severity and increased the mango productivity 13.
In a study on effectiveness of fungicides and their application timing for the management of sorghum foliar anthracnose in the mid-Atlantic United States, Acharya concluded that Pyraclostrobin and Pyraclostrobin plus Fluxapyroxad controlled anthracnose disease of sorghum 26. These studies reveal that a combination of Pyraclostrobin and Fluxapyroxad is effective in the control of Anthracnose and other fungal pathogens.
Conclusions and Recommendations
All the CRI 1 fungicide rates controlled the growth rate of C. kahawae. All the CRI 2 fungicide rates also controlled the growth rate of C. kahawae. CRI 1 demonstrated to be the best fungicide on the control of C. kahawae even at the lowest rate of 0.01% as compared to Pyraclostrobin 250 g/L and Tebuconazole 200 g/L + Trifloxystrobin 100 g/L at 0.04 % and 0.1 % respectively. CRI 1 is also more suppressive on the control of C. kahawae as compared to CRI 2. CRI 1 is more effective in controlling C. kahawae since it controls the fungus at a rate even lower than Pyraclostrobin 250 g/L and Tebuconazole 200 g/L + Trifloxystrobin 100 g/L. The best rates of CRI 2 for control of C. kahawae are 0.08 %, 0.09%, and 0.1%. This In Vitro study has revealed that CRI 1 and CRI 2 can be used interchangeably as alternative fungicides for management of Coffee Berry Disease with the available fungicides like Pyraclostrobin 250 g/L (0.04%) and Tebuconazole 200 g/L+Trifloxystrobin 100 g/L (0.1%). Further studies on CRI 1 and CRI 2 fungicides should be carried out in the field on commercial farms to assess their effect on CBD and coffee yield.
Acknowledgements
The authors acknowledge Kenya Agricultural and Livestock Research Organization -Coffee Research Institute (KALRO-CRI) and Kenyatta University scientists for reading the paper and suggesting improvements. We also thank the KALRO-CRI plant pathology staff for participating in the study. We also acknowledge Kenya Agricultural and Livestock Research Organization -Coffee Research Institute (KALRO-CRI) for funding the project.
Conflict of Interest
The authors do no have the conflict of interest regarding the work and publication of results for this article.
References
- BLLNR (Billionaire) “A platform for Entrepreneurs, Business Leaders and Creatives in Singapore”. https://www.bllnr.sg/leadership/what-are-commodities-and-what-are-the-top-10-most-traded-commodities-in-the-world#:~:text=With%20over%202.25%20billion%20cups,of%20the%20oldest%20as%20well. Accessed July 14, 2020.
- Mussatto, S. I., Machado, E. M., Martins, S., & Teixeira, J. A., “Production, composition, and application of coffee and its industrial residues”. Food and Bioprocess Technology4(5): 661 (2011).
CrossRef - Gimase, J. M., Thagana, W. M., Omondi, C. O., & Ithiru, J. M., “Evaluation of coffee berry disease resistance (Colletotrichum kahawae) in F2 populations derived from Arabica coffee varieties Rume Sudan and SL 28”. Journal of Plant Breeding and Crop Science11(9): 225-233 (2019).
CrossRef - Thuku, G. K., & Gachanja, P., “Effects of reforms on productivity of coffee in Kenya”. International Journal of Business and Social Science4(15): (2013).
- Alemu, K., Adugna, G., Lemessa, F., & Muleta, D., “Variation among colletotrichum isolates associated with coffee berry disease in Ethiopia”. Cogent Biology6(1): 1740537 (2020).
CrossRef - Kenny, M. K., Galea, V. J., & Price, T. V., “Germination and growth of Colletotrichum acutatum and Colletotrichum gloeosporioides isolates from coffee in Papua New Guinea and their pathogenicity to coffee berries”. Australasian Plant Pathology41(5): 519-528 (2012).
CrossRef - Kenny, M. K., Galea, V. J., & Price, T. V., “Effect of fungicides in vitro and on detached berries on control of coffee berry anthracnose caused by Colletotrichum acutatum and C. gloeosporioides”. Plant Protection Quarterly27(2): 61-63 (2012).
- Alemu, K., Adugna, G., Lemessa, F., & Muleta, D., “Current status of coffee berry disease (Colletotrichum kahawae Waller & Bridge) in Ethiopia”. Archives of Phytopathology and Plant Protection, 49(17-18): 421-433 (2016).
CrossRef - Mouen Bedimo, J. A., Bieysse, D., Nyassé, S., Nottéghem, J. L., & Cilas, C., “Role of rainfall in the development of coffee berry disease in Coffea arabica caused by Colletotrichum kahawae, in Cameroon”. Plant pathology59(2): 324-329 (2010).
CrossRef - Gichimu, B. M., Nyende, A. B., Gichuru, E. K., & Mamati, G. E., “Yield selection within Coffea arabica cv. Ruiru 11”. American Journal of Experimental Agriculture, 3(1): 76-88 (2012).
CrossRef - Kimemia, J., Kasuhi, H. and Kamau, B., “African Coffee Renaissance summit, Nairobi Kenya” (2015).
- Alwora, G. O., & Gichuru, E. K., “Advances in the management of coffee berry disease and coffee leaf rust in Kenya”. Journal of Renewable Agriculture2(1): 5-10 (2014).
CrossRef - Ravikumar, M. R., Navi, V., & Sharma, Y., “Evaluation of Pre-Mix Fungicide, Fluxapyroxad and Pyraclostrobin 500 SC against Powdery Mildew (Oidium mangiferae) Disease of Mango”. J. Curr. Microbiol. App. Sci7(1): 439-446 (2018).
CrossRef - PCPB (Pest Control Products Board). “GoK Regulator For: Importation, Manufacture, Distribution and Safe Use of Pest Control Products/ Promoting Productivity, Safeguarding Human Health and Environment Towards Achieving Vision 2030”. https://www.pcpb.go.ke/ accessed April 2021.
- Fishel, F. M., & Dewdney, M. M., “Fungicide resistance action committee’s (FRAC) classification scheme of fungicides according to mode of action”. University of Florida. 194(7): 2-9 (2012).
- Ames, H., Glenton, C., & Lewin, S., “Purposive sampling in a qualitative evidence synthesis: a worked example from a synthesis on parental perceptions of vaccination communication”. BMC medical research methodology19(1): 26 (2019).
CrossRef - Balamurugan, S., “In vitro anti-fungal activity of Citrus aurantifolia Linn plant extracts against phytopathogenic fungi Macrophomina phaseolina”. International Letters of Natural Sciences8(2): 70-74 (2014).
CrossRef - Bliss, C. I., “The calculation of the dosage‐mortality curve”. Annals of Applied Biology22(1): 134-167 (1935).
CrossRef - Avenot, H. F., & Michailides, T. J., “Progress in understanding molecular mechanisms and evolution of resistance to succinate dehydrogenase inhibiting (SDHI) fungicides in phytopathogenic fungi”. Crop protection29(7): 643-651 (2010).
CrossRef - Cabral, A., Azinheira, H. G., Talhinhas, P., Batista, D., Ramos, A. P., Silva, M. D. C., … & Várzea, V., “Pathological, Morphological, Cytogenomic, Biochemical and Molecular Data Support the Distinction between Colletotrichum cigarro comb. et stat. nov. and Colletotrichum kahawae” . Plants9(4): 502 (2020).
CrossRef - Kilambo, D. L., Guerra-Guimarães, L., Mabagala, R. B., Varzea, V. M. P., Haddad, F., Loureiro, A., & Teri, J. M., “Characterization of Colletotrichum kahawae strains in Tanzania”. International Journal of Microbiology Research5(2): 382 (2013).
CrossRef - Tonin, R. F. B., Avozani, A., Danelli, A. L. D., Reis, E. M., Zoldan, S. M., & Garcés-Fiallos, F. R., In vitro mycelial sensitivity of Macrophomina phaseolina to fungicides. Pesquisa Agropecuária Tropical43(4): 460-466 (2013).
CrossRef - EPA (Environmental Protection Agency) “Registration of New Active Ingredient”. https://www3.epa.gov/pesticides/chem_search/reg_actions/registration/fs_PC-138009_02-May-12.pdf . Accessed May 2012.
- Karadimos, D. A., G. S. Karaoglanidis, and K. Tzavella–Klonari., “Biological activity and physical modes of action of the Qo inhibitor fungicides trifloxystrobin and pyraclostrobin against Cercospora beticola.” Crop Protection24(1): 23-29 (2005).
CrossRef - Navi, S. S., Rajasab, A. H., & Yang, X. B., “In Vitro evaluation of commercial fungicides against some of the major soil borne pathogens of soybean”. Plant Pathol Microbiol, 7, 340-347 (2016).
CrossRef - Acharya, B., O’Quinn, T. N., Everman, W., & Mehl, H. L., “Effectiveness of Fungicides and Their Application Timing for the Management of Sorghum Foliar Anthracnose in the Mid-Atlantic United States”. Plant disease103(11): 2804-2811 (2019).
CrossRef


