Introduction
Bemisia tabaci Gennadius (Hemiptera: Aleyrodidae) is one of the main pests in tomato plants1. B. tabaci is a polyphagous insect that has many host plants such as ornamental plants, vegetables, fruits, and wild plants (weeds)2. This insect make damage directly on plants by sucking the liquid, causing a physiological disturbance on plants, chlorosis on leaves, and disturb ripening of tomatoes3,4. In addition to direct damages, the pest cause indirect damage by the accumulation of honeydew produced by B. tabaci which leads to mold growth on foliage, and as a vector of Tomato yellow leaf curl virus (TYLCV) and more than 100 other begomoviruses5,6. The loss of yields due to B. tabaci attacks and yellow virus ranges from 20 to100%7. This pest was first found in Indonesia in 1938 on a tobacco plant8. The reproduction and spread of these pests are very fast, in fact, in a year, is able to produc till 15 generations9.
Traditionally, synthetic insecticides have been used for controlling B. tabaci, but excessive and irrational use of insecticides has led to adverse effects on the environment. To reduce the use of pesticides, it is important to developing alternative safety control methods such as the entomopathogenic fungus Beauveria bassiana (Bals.) Vuill. B. bassiana is considered the most effective alternative control method of B. tabaci. It is is an entomopathogenic fungus with a wide host range, able to infect various kinds of insects, from pre-adult to adult10,11. B. bassiana infects insect by digestion, respiration, and particularly through the integuments of insect12.
Beauveria bassiana have been used as a biological agent to control several insect pests. In laboratorium condition, B.bassiana was able to kill Crocidolomia pavonana larvae till 80%. The mortality of larvae depends on isolates13. B. bassiana can kill several kinds of vegetable pests such as Spodoptera exigua14, S. litura15, Nezara viridula16, and Eurydema pulchrum17. The dose of B. bassiana had a significant effect on adult of Aphis crassivora and B. tabaci. An increase of B. bassiana concentration markedly decreased adult longevity, period of reproduction, and fecundity of the two insects18. One of the essential criteria in selecting entomopathogenic fungi for commercial development is to have high virulence to target insects. In order to evaluate the importance of virulent strains for an efficient biological control of B. tabaci on tomato, we analyzed under laboratory conditions the effect of five fungal isolates of indigenous B. bassiana on eggs and nymphs of B. tabaci.
Materials and Methods
Beauveria Bassiana Isolates
Five fungal isolates of B. bassiana were obtained from the collection of entomopathogenic fungal culture maintained by the Biological Control Laboratory, Department of Pests and Plant Diseases, Faculty of Agriculture, Andalas University. (Table 1.a). B. bassiana isolates were obtained from plants and pest insects. Isolates were cultivated on the Sabouraud dextrose agar + yeast extract (SDAY) medium.
Table 1.a: List of B. Bassiana Isolates Used in Research.
| Isolates code | Source | Location |
| WS | Leptocorisa oratorius | Duku (Padang Pariaman), West Sumatra, Indonesia |
| TD312 | Wheat stems endophyte | Koto Laweh (Tanah Datar), West Sumatra, Indonesia |
| PB211 | Chili stems endophyte | Parabek (Agam), West Sumatra, Indonesia |
| PD114 | Chili leaves endophyte | Parabek (Agam), West Sumatra, Indonesia |
| PA221 | Chili roots endophyte | Parabek (Agam), West Sumatra, Indonesia |
The conidial suspension of B. bassiana was obtained by adding 10 ml of distilled water and 0.1% Tween 80 to Petri dishes containing the fungus culture, and conidia were harvested by scraping the surface of the plate with a steril spatula. The conidial concentration was determined using an improved Neubauer haemocytometer and adjusted to 108 conidia / mL.
Insects
Bemisia tabaci adults were collected from chili plants in Padang areas, West Sumatra, Indonesia and insects mass-reared on tomato plants. The tomato plants were planted in the polybag and put into large screen cages (60 x 75 x 100 cm). A large colony of B. tabaci adults was introduced into the screen cages for three days. After that, whitefly adults were removed and tomato plants infested by eggs were transferred to other cages. The eggs laid by female adults of B. tabaci were used for B. bassiana bioassay on eggs. Other eggs were also kept until nymphs emergence to evaluate the efficacy of the fungus against nymphal stages of B. tabaci.
Bioassay of B. Bassiana Against B. Tabaci Eggs
The tomato leaf contained 20 eggs selected and treated with the fungus. Eggs were sprayed by conidial suspension of B. bassiana, then eggs were put into Petri dish on a moist filter paper. The assays was repeated five times. As control, the same number of eggs were treated with distilled water. The eggs were reared till their hatching. Eggs mortality and infection of first instar nymphs were assessed and recorded daily for eight days.
Bioassay of B. Bassiana Against B. Tabaci Nymphs
The second instar nymphs of B. tabaci were moved on 30 days old tomato plants using a soft brush. Furthermore, 2 ml of the B. bassiana fungal suspensions, for each unit test, were sprayed on the insects using hand sprayer. For the control, the insects were sprayed with distillate water. The tomato plants were put into a cylindrical tubular-shaped plastic mica cage (high 60 cm, diameter 45 cm) and covered with gauze. The treatments were repeated five times, and every unit of treatment consisted of 10 nymphs. The mortality of nymphs was observed every day by counting the numbers of test insects that died until seven days after application (DAA).
Data Analysis
The data obtained were analyzed by ANOVA and followed by a test of Duncan’s New Multiple Range Test (DNMRT) a significant level of 5%.
Results and Discussion
Eggs Mortality
Results of the virulence test of five B. bassiana isolates against B. tabaci eggs showed that all tested isolates kill B. tabaci eggs, but with very low mortality. Statistical analysis showed a significant effect of B. bassiana isolate on the mortality of B. tabaci eggs (F = 26.15; db = 5, 24; P <0.0001). The mortality of B. tabaci eggs after B. bassiana application reported in Table 1.b.
Table 1.b: Mortality of B. Tabaci Eggs after the Application of B. Bassiana Isolates.
| Isolates | Eggs Mortality±SE |
| WS | 19.00±1.00 a |
| TD312 | 17.00±2.55 a |
| PB211 | 5.00±1.58 b |
| PD114 | 4.00±1.87 bc |
| PA221 | 2.00±1.22 bc |
| Control | 0.00±0.00 |
Means followed by the same letter are not significantly different (P<0.05) by Duncan’s Multiple Range Test.
Treatment of WS isolates on B. tabaci eggs resulted in the highest egg mortality (19%) compared to other isolates. Treatment with PA221 isolate resulted in the lowest egg mortality, namely 2%. In control, there was no death of the eggs. All the eggs hatched into nymphs. Low mortality of B. tabaci eggs after the fungal application is thought to be caused by the eggshell of B. tabaci which has a specific layer that can prevent the conidial tube from penetrating to the inside of the egg. The wax coating on insects could inhibit the germination of B. bassiana conidia. In addition, the failure of the fungus to infect eggs can be related to the presence of anti-fungal compounds found on the surface of the eggshell, which inhibits the germination process of fungal conidia. Eggs of Bemisia argentifolii Bellows & Perring (Homoptera: Aleyrodidae) were resistant to B. bassiana infection. Electron microscopy observations showed that only 13.0% of B. bassiana conidia germinated on eggs11.
The results of this study are similar to the results of Al-Deghairi19 who observed a mortality of B. tabaci eggs after B. bassiana application of only 4,49%. Eggs of B. tabaci was more tolerant to B. bassiana infection and were not easily killed even by the highest conidial concentration. Furthermore, Islam et al20 also reported that applying B. bassiana to B. tabaci eggs with a concentration of 108 conidia/ml resulted in a mortality of 25.2%. and Trizelia et al. 21 reported that B. bassiana could not infect Crocidolomia pavonana eggs. In eggs of Blissus antillus (Leonard) (Hemiptera: Lygaeidae), egg mortality due to B. bassiana infection varied between isolates. Isolate CG24 can cause egg infections by 43.3%, while isolates CG04 and ARSEF792 were only 7.8%. The results of observations with fluorescent microscopy showed that the difference in virulence was due to differences in the ability of the fungal conidia to adhere to the surface of the egg and then penetrate the chorion22.
Based on macroscopic observations, the fungal mycelial covered the eggs. Fungal mycelia on the surface of the eggs were seen four days after the inoculation of the fungus. Al-Deghairi19 also reported that infection symptoms on the eggs of B. tabaci were observed on the 3rd day of inoculation. Within four days from the treatment, the eggs that became subsequently infected by the fungal had little color change but appeared slightly shrunk when observed under the microscope. One week after treatment, most of the unhatched eggs became conspicuously shrunk and had fewer fungal outgrowths on the surface.
Besides being able to infect the eggs, nymphal mortality also occurs in 1st nymphs upon hatching from eggs contaminated by B. bassiana (Table 2). The nymph mortality varied between isolates (F = 3.55; db = 5, 24; P> 0.0152). WS isolates produced the highest nymph mortality, namely 7.43%, while PA221 isolates only produced nymph mortality of 2.05%. In control, there was no mortality in nymphs.
Table 2: Mortality of the First-Instar Nymph of B. Tabaci.
| Isolates | Mortality of First Nymphs ± SE |
| WS | 7.43±1.27 a |
| TD312 | 5.97±1.87 ab |
| PB211 | 4.16±1.96 abc |
| PD114 | 3.10±1.27 abc |
| PA221 | 2.05±1.26 bc |
| Control | 0.00±0.00 c |
Means followed by the same letter are not significantly different (P<0.05) by Duncan’s Multiple Range Test.
The results showed that conidia remained active and can infect nymphs successfully emerging from eggs. Occurrence mortality of 1st instar nymphs is thought due to contact nymphs emerging from eggs with the conidia attached to the surface of the eggshell and on the leaves. Other researchers23 also reported that B. tabaci eggs had low susceptibility to B. bassiana, with >91% nymphs successfully emerging from eggs. However, there is significant mortality of 1st and 2nd instar nymphs originating from the treated eggs. These results indicated that newly hatched nymphs probably acquired conidia from the eggs soon after hatching or from the leaf surface as secondary exposure. Trialeurodes vaporariorum eggs treated with entomopathogenic fungus Aschersonia aleyrodis did not become infected, but larvae that hatched from these eggs were infected24.
Mortality of Bemisia tabaci Nymphs
The results showed that B. bassiana isolate had a significant effect on the mortality of 2nd instar nymph of B. tabaci. WS isolates were the most virulent isolates with the highest mortality namely 70.00% after seven days after the fungal application. PA221 isolate had a low virulence with a mortality of 36.0% (Table 3).
Table 3: Mortality and LT50 Values of 2nd Instar Nymphs of B. TabaciTreated with 108 Conidia/ml of B. Bassiana Isolates at Seven Days Post-Inoculation.
| Isolates | 2nd Instar Nymph Mortality (%) ± SE | LT50 (Day) |
| WS | 70.00 ± 4.47 a | 4.87 (4.41-5.46) |
| TD312 | 64.00 ± 2.45 a | 5.51 (5.03-6.17) |
| PB 211 | 44.00 ± 2.45 b | 7.23 (6.57-8.62) |
| PD114 | 44.00 ± 4.00 b | 7.34 (6.59-8.81) |
| PA221 | 36.00 ± 2.45 b | 7.80 (6.89-9.82) |
| Control | 00.00 ± 0.00 c |
Means followed by the same letter are not significantly different (P<0.05) by Duncan’s Multiple Range Test.
The difference in the ability of B. bassiana isolates in killing B. tabaci nymphs is thought to be due to differences in viability of conidia or the ability to produce enzymes and toxins. The difference in mortality of C. pavonana larvae after B. bassiana application was caused by differences in physiological and genetic characteristics of the isolates25. Several researchers26,27,28 stated that the germination of conidia and the ability to produce enzymes and mycotoxins during the infection process in insects affects insect mortality by B. bassiana.
This research showed that the mortality of nymphs of B. tabaci after application B. bassiana was influenced by isolate source. B. bassiana isolated from insects belonging to the same taxon of the test insect (WS) was more virulent than that isolated from the plants. Based on the observed values, surveys of B. bassiana isolates collected from homopterans seems to be a suitable approach for silverleaf whitefly microbial control programs. Other researchers also reported that mortality of insect was dependent on the fungal isolates. Isolates or strains of entomopathogenic fungi isolated from the same taxon of the whitefly (Order Hemiptera, suborder Homoptera) were more virulent than the isolates from Lepidoptera, Coleoptera, and Hymenoptera 23,29,30,31.
There was a difference in LT50 between isolates (Table 3) and LT50 value related to the virulence isolate. LT50 values ranged from 4.87 to 7.80 days. WS isolate has the shortest LT50 value compared to other isolates (4.87 days), and this means that the time needed to kill 50% of 2nd instar nymphs of B. tabaci is shorter than other isolates. There are difference in the LT50 value between B. bassiana isolates to 2nd instar nymphs of B. tabaci biotype B. Estimated LT50 values showed that most B. bassiana and I. fumosorosea isolates killed whiteflies faster (3–5 d) compared with L. muscarium isolates23.
Mortality of second instar B. tabaci nymph due to B. bassiana infection began on the second day post-inoculation, and nymph mortality increased after three days. The development of mortality of B. tabaci nymphs due to B. bassiana infection can be seen in Figure 1.
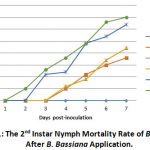 |
Figure 1: The 2nd Instar Nymph Mortality Rate of B. Tabaci After B. Bassiana Application. |
B. tabaci nymphs that die from B. bassiana infection are characterized by the presence of white mycelia or conidia on the surface of the nymph’s body. Nymphs of B. tabaci treated with B. bassiana dried and presented reddish coloration upon death29. Mycelia of B. bassiana emerged on the cuticles of the immature insects (B. tabaci, Bactericera cockerelli, Frankliniella occidentalis) 2-3 days after death, and most conidia were recorded on legs, wings, and thoraces of some adult cadavers32. Chergui et al.33 noted that dead individuals of Ceratitis capitata were covered with a white mycelium characteristic of the fungus B. bassiana.
Adults Emergence
The results showed that B. bassiana applied to 2nd instar nymphs of B. tabaci had a significant effect on the emerged numbers of B. tabaci adults. Percentages of emerged adults under five fungal isolates can be seen in Table 4.
Table 4: Percentages of Emerged Adults of B. Tabaci after Application of B. Bassiana.
| Isolates | Percentage of emerged adults± SE |
| Control | 98.00 ± 9.80 a |
| PA221 | 60.00 ± 3.16 b |
| PB 211 | 52.00 ± 2.00 bc |
| PD114 | 48.00 ± 4.90 c |
| TD312 | 26.00 ± 2.45 d |
| WS | 22.00 ± 2.00 d |
Means followed by the same letter are not significantly different (P<0.05) by Duncan’s Multiple Range Test.
The application of fungus B. bassiana to the 2nd instar nymph showed a significant effect on adult emergence. In control, the percentage of adult emergence was the highest at 98%, while in the treatment of WS isolates only 22% of nymphs became adult. The low rate of adult emergence was because a lot of some nymphs was killed before becoming an adult. B. bassiana applied to C. capitata larvae decreased the percentage of pupae formed and adult emergence33. B. bassiana can also reduce pupation and adult emergence of S. litura. Reduction in pupation and adult emergence is due to phagodepression and difficulty in molting34.
Conclusions
The present study showed that B. bassiana can infect B. tabaci eggs and nymphs. The mortality of B. tabaci eggs is only at 2-19%. Isolate WS had the highest virulence, which caused 70.00% mortality of 2nd instar nymphs, with an LT50 of 4.87 days. Isolates of B. bassiana have significant effect on the mortality of second instar B. tabaci. Nymphs of B. tabaci were highly susceptible to B. bassiana infection compared with eggs. B. bassiana applied to B. tabaci nymphs could decrease the percentage of adult emergence.
Acknowledgement
We thank the Directorate of Research and Community Service of the Directorate General of Strengthening Research and Development of the Ministry of Research, Technology and Higher Education, under Research Contract 034/SP2H/LT/DRPM/2020 the Fiscal Year 2020, for providing us the finance for this research.
References
- Yuliani, Hidayat, P., Sartiami, D. Identifikasi kutukebul (Hemiptera: Aleyrodidade) dari beberapa tanaman inang dan perkembangan populasinya. Jurnal Entomologi Indonesia. 2006; 3(1):41-49.
CrossRef - Hill, D.S. Agriculture insect pests of the tropics and their control. Cambridge University Press., Cambridge. 1987.
- Van de Ven, W.T., LeVesque, C.S., Perring, T.M., Walling, L.L. Local and systemic changes in squash gene expression in response to silverleaf whitefly feeding. The Plant Cell. 2000; 12:1409–1423.
CrossRef - McCollum, T., Stoffella, P., Powell, C., Cantliffe, D., Khan, H. Effects of silverleaf whitefly feeding on tomato fruit ripening. Postharvest Biology and Technology. 2004; 31:183–190.
CrossRef - Byrne, D.N., Bellows, T.S. Whitefly biology. Annual Review of Entomology. 1991; 36:431-457.
CrossRef - Jones, D. Plant viruses transmitted by whiteflies. European Journalof Plant Pathology. 2003; 109:195–219 (2003).
CrossRef - Setiawati, W., Udiarto, B.K., Gunaeni, N. Preferensi beberapa varietas tomat dan pola infestasi hama kutu kebul serta pengaruhnya terhadap intensitas serangan virus kuning. Jurnal Hortikultura. 2007; 17(4): 374-386.
- Kalshoven, L.G.E. The pests of crops in Indonesia. Jakarta: Ichtiar Baru-Van Hoeve. 1981.
- Brown, J.K. Current status of Bemisia tabaci as a plant pest and virus vector in agro-ecosystems worldwide. FAO Plant Protection Bulletin. 1994; 42, 3–32.
- Lord, J.C. Desiccant dusts synergize the effect of Beauveria bassiana (Hyphomycetes: Moniliales) on stored-grain beetles. Journalof Economic Entomology. 2001; 94: 367-372 (2001).
CrossRef - James, R.R, Buckner, J.S., Freeman, T.P. Cuticular lipids and silverleaf whitefly stage affect conidial germination of Beauveria bassiana and Paecilomyces fumosoroseus. Journal of Invertebrate Pathology. 2003; 84:67-74.
CrossRef - Broome, J.R., Sikorowski, P.P., Norment, B.R. A mechanism of pathogenicity of Beauveria bassiana on larvae of the imported fire ant. Solenopsis richteri. Journal of Invertebrate Pathology. 1976; 28:87-91.
CrossRef - Trizelia, Nurdin, F. Virulence of Entomopathogenic Fungus Beauveria bassiana isolates to Crocidolomia pavonana F(Lepidoptera: Crambidae). AGRIVITA Journal of Agricultural Science. 2010; 32(3): 254-260.
- Razak, N.A., Nasir, B., Khasanah, N. Efektifitas Beauveria bassiana terhadap pengendalian Spodoptera exigua Hubner (Lepidoptera : Noctuidae) pada tanaman bawang merah lokal palu (Allium wakegi). eJ. Agrotekbis. 2016; 4 (5) : 565-570.
- Trizelia, Reflin, Ananda, W. Virulensi beberapa isolat cendawan entomopatogen endofit Beauveria bassiana terhadap Spodoptera litura F. (Lepidoptera: Noctuidae). Prosiding Seminar Nasional BKS PTN Wilayah Barat Bidang Ilmu Pertanian. 2016; 409-415.
- Prayogo, Y. Patogenisitas cendawan entomopatogen Beauveria bassiana (Deuteromycotina: Hyphomycetes) pada berbagai stadia kepik hijau (Nezara viridula). Jurnal Hama dan Penyakit Tumbuhan Tropika. 2013; 13 (1):75 – 86.
CrossRef - Trizelia, Yanti, Y., Suhriani. Potensi cendawan entomopatogen Beauveria bassiana (Bals.) untuk pengendalian kepik kubis Eurydema pulchrum Westw. (Hemiptera: Pentatomidae). Prosiding Seminar Nasional Agroteknologi Jurusan Agroteknologi Universitas Islam Negeri Sunan Gunung Djati Bandung. 2019; 346-352.
- Zaki, F.N. Efficiency of the entomopathogenic fungus, Beauveria bassiana (Bals), against Aphis crassivora Koch and Bemesia tabaci Journalof Applied Entomology. 1998; 122: 397-399.
CrossRef - Al-Deghairi, M.A. Bioassay evaluation of the entomophatogenic fungi, Beauveria bassiana Vuellemin against egg and nymphs of Bemisia tabaci Gennadius (Homoptera: Aleyrodidae). Pakistan Journal of Biological Sciences. 2008; 11(12):1551-1560.
CrossRef - Islam, Md. T., Castle, S.J., Ren, S. Compatibility of the insect pathogenic fungus Beauveria bassiana with neem against sweetpotato whitefly, Bemisia tabaci, on eggplant. Entomologia Experimentalis et Applicata. 2010; 134: 28–34.
CrossRef - Trizelia, Santoso, T., Sosromarsono, S., Rauf, A., Sudirman, L. Patogenisitas jamur entomopatogen Beauveria Bassiana (Deuteromycotina; Hyphomycetes) terhadap telur Crocidolomia pavonana (Lepidoptera: Pyralidae). Agrin. 2007; 11(1):52-59.
- Samuels, R.I., Coracini, D.L.A., dos Santos, C.A.M., Gava, C.A.T. Infection of Blissus antillus (Hemiptera: Lygaeidae) eggs by entomopathogenic fungi Metarhizium anisopliae and Beauveria bassiana. Biological Control. 2002; 23:269-273.
CrossRef - Mascarin, G.M., Kobori, N.N., Quintela, E.D., Delalibera, I. The virulence of entomopathogenic fungi against Bemisia tabaci biotype B (Hemiptera: Aleyrodidae) and their conidial production using solid substrate fermentation. Biological Control. 2013; 66: 209–218.
CrossRef - Fransen, J.J., Winkelman, K., van Lenteren, J.C. The differential mortality at various life stages of the greenhouse whitefly, Trialeurodes(Homoptera: Aleyrodidae), by infection with the fungus Aschersonia aleyrodis (Deuteromycotina: Coelomycetes). Journal of Invertebrate Pathology. 1987; 50: 158-165.
CrossRef - Cendawan entomopatogen Beauveria bassiana (Bals) Vuil. (Deuteromycotyna: Hypomycetes). keanekaragaman genetik, karekteristik fisiologi, dan virulensinya terhadap Crocidolomia pavonana (F) [Disertasi]. Institut Pertanian Bogor. Bogor. 2005.
- Geden, C.J, Rutz, D.A., Steinkraus, D.C. Virulence of different isolates and formulations of Beauveria bassiana for house flies and the parasitoid Muscidifurax raptor. Biological Control. 1995; 5:615-621.
CrossRef - Sumikarsih, E., Herlinda,S., Pujiastuti, Y. Conidial density and viability of Beauveria bassiana isolates from Java and Sumatra and their virulence against Nilaparvata lugens at different temperatures. AGRIVITA Journal of Agricultural Science. 2019; 41(2): 335–350.
CrossRef - Tanada, Y., Kaya, H.K., Insect pathology. Academic Press, INC. Harcourt Brace Jovanovich, San Diego.1993.
- Vicentini, S., Faria, M., r.v. de oliveira, M. Screening of Beauveria bassiana (Deuteromycotina: Hyphomycetes) isolates against nymphs of Bemisia tabaci (Genn.) biotype B (Hemiptera: Aleyrodidae) with description of a new bioassay method. Neotropical Entomology. 2001; 30(1): 97-103.
CrossRef - James, R.R., Lighthart, B. Susceptibility of the convergent lady beetle (Coleoptera: Coccinellidae) to four entomogenous fungi. Environmental Entomology. 1994; 23:190-192.
CrossRef - Samuels, R.I, Coracini, D.L.A. Selection of Beauveria bassiana and Metarhizium anisopliae isolates for the control of Blissus antillus (Hemiptera: Lygaeidae). Scientia Agricola (Piracicaba. Braz). 2004; 61(3):271-275.
CrossRef - Rios-Velasco, C., Pérez-Corral, D.A., Salas-Marina, M.A., Berlanga-Reyes, I., Ornelas-Paz,J.J., Acosta Muñiz, C.H., Cambero-Campos, J., Jacobo-Cuellar, J.L. Pathogenicity of the hypocreales fungi Beauveria bassiana and Metarhizium anisopliae against insect pests of tomato. Southwestern Entomologist. 2014; 39(4):739-750.
CrossRef - Chergui, S., Boudjemaa, K., Benzehra, A., Karaca, I. Pathogenicity of indigenous Beauveria bassiana (Balsamo) against Ceratitis capitata Wiedemann (Diptera: Tephritidae) under laboratory conditions. Egyptian Journal of Biological Pest Control. 2020; 30:128.
CrossRef - Malarvannan, S., Murali, P.D., Shanthakumar, S.P., Prabavathy, V.R., Nair, S. Laboratory evaluation of the entomopathogenic fungi, Beauveria bassiana against the Tobacco caterpillar, Spodoptera litura Fabricius (Noctuidae: Lepidoptera). Journal of Biopesticides. 2010; 3: 126 – 131.


