Introduction
Zinc (Zn) is essential for the normal growth and development of plants however its content in soils is low compared with that of other essential nutrient elements.1 The deficiency of zinc is more common in calcareous than in non-calcareous soils, and its deficiency is having a profound economic impact in terms of yield loss.2 Various factors including pH,3 ionic strength as well as clay mineralogy have been shown to affect Zn availability4 however, zinc accessibility is likewise directed by adsorption-desorption procedures and its parcelling between the arrangement and strong phases.5 Most examinations on Zn sorption and desorption by soils have been founded on balance conditions. Be that as it may, because of moderate compound responses, plant take-up, manure expansion and different variables, horticultural soils are once in a while in a balance state concerning Zn sorption and discharge. Adsorption isotherms can be utilized to depict the equilibrium connection between the measures of adsorbed and broke down species at a given temperature6 which considers force, amount and limit factors that are essential for anticipating the measure of soil supplement required for most extreme plant development.
Materials and Methods
Ten Surface soil samples (0-25 cm) were collected from the major agricultural based land uses (paddy, apple, maize, saffron and vegetables) of Kashmir based upon location, elevation, soil complexion, texture and vegetation (Table-1) using Global Positioning System (GPS). Soil was analysed for various physico-chemical properties viz. soil reaction (pH), electrical conductivity (EC), organic carbon (OC), calcium carbonate (CaCO3) content, cation exchange capacity (CEC), and particle size distribution (Figure-1) by following standard laboratory protocols . For Zn adsorption, 2.5 g of processed soil samples in triplicate were equilibrated with 20 mL of 0.01 M KNO3 containing 0, 5, 10, 20, 40, 60, 80, 100, 150, 200 and 250 mg ml-1 of Zn as Zn(NO3)2 for 24 hours at room temperature (25±1°C). After equilibration samples were centrifuged at 2500 rpm for 15 minutes and filtered. The Zn concentration in filtrate was determined by atomic absorption spectrophotometer.7 The amount of adsorbed Zn was calculated from the difference in the initial and final Zn concentrations taking into account the amount of Zn present in supernatant solution in no Zn treatment. The experimental results were fitted to Langmuir and Freundlich equations.
- Langmuir equation: C/(x/m) = 1/b (C) + 1/b K
- Freundlich equation: Log (x/m) =l/n Log C +Log K
Where, C= equilibrium concentration, x/m = adsorbed Zn, b = adsorption maxima, k = a constant related to bonding energy, l/n = slope of the regression line, K and n = empirical constants
Statistical analysis was done by standard statistical procedures. The least square regression equation was used for modelling. Value of coefficient of determination (R2) was chosen as the criteria of goodness of fit. The model/equation with highest value of coefficient of determination (R2) was chosen as the best in describing the zinc adsorption behaviour of soil.
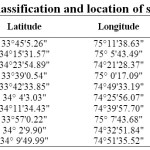 |
Table 1: Classification and location of soil samples Click here to View table |
Result and Discussion
Physico-chemical characteristics
The detailed physical characteristics of the soils are represented in figure-2 which reveals that the sand, silt and clay content are in the range of 18.20 to 25.90, 41.97 to 56.10 and 23.40 percent with mean value of 22.76, 50.80 and 26.71 percent, respectively. In general, the soils were slightly acidic to alkaline in reaction with the pH variation from 5.98 to 8.01. EC of the soils showed non-saline nature and ranged between 0.11 to 0.33 dSm-1. The organic carbon content of these soils showed a conspicuous variation and ranged from 0.59 to 0.94 percent with a mean value of 0.76 percent. The higher values in soils may be due to the continuous organic manuring, addition through vegetation and low mineralization rates in these soils.8 Cation exchange capacity of the soils showed little variation between the samples and varied from13.30 to 17.21 cmol(p+) kg-1 with an average value of 15.08 cmol(p+) kg-1of soil. The calcium carbonate content was present in meagre amounts in most of the soils with values ranging from 0.00 (S9) to a maximum value of 0.72 percent (S5) with a mean value of 0.27 percent in the soil.
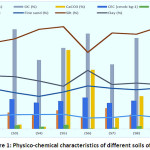 |
Figure 1: Physico-chemical characteristics of different soils of Kashmir. Click here to View Figure |
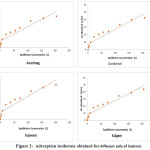 |
Figure 2: Adsorption isotherms obtained for different soils of Kashmir. Click here to View Figure |
Adsorption of zinc by soils
The after effects of zinc adsorption by the soils under examination obviously demonstrated that every one of the soil have proclivity for zinc adsorption. The measure of zinc adsorbed for every one of the soils was plotted against the equilibrium zinc concentration to get the adsorption isotherms (Figure-3), demonstrating the impact of zinc focus on zinc adsorption. The adsorption isotherm demonstrated that however the adsorption of zinc expanded with expanding focus in the balance arrangement, yet the level of adsorbed zinc diminished. This might be a direct result of an expansion in the proportion of adsorbate to adsorbent.9 The adsorption isotherms were L-shaped and showed that more destinations in the substrate were occupied10 and in this way the solute atoms confronted extraordinary trouble to locate any empty site accessible. This prompts a proposal that either the adsorbed atom was not vertically situated or that there is no solid challenge from the solvent.11 The information on zinc adsorption by various soils demonstrated that the adsorption limit of various soils for zinc was extraordinary and the conduct of adsorption of zinc by various soils was not uniform in the entire focus run. Adsorption of zinc was observed to be most extreme in soils of Baramulla locale and this might be credited to the high soil substance and CEC of the soil. The soils of Shopian locale demonstrated least adsorption limit of zinc which is likely because of coarse surface and low CEC.12 From these outcomes, it could be inferred that adsorption of zinc was principally represented by soil substance and CEC of the soils which was affirmed factually since these soils demonstrated positive and critical connection with clay content (r=0.884**) and CEC (r=0.697**).
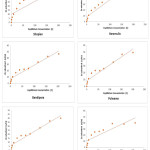 |
Figure 3: Adsorption isotherms obtained for different soils of Kashmir. Click here to View table |
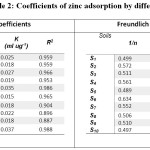 |
Table 2: Coefficients of zinc adsorption by different soils Click here to View table |
Langmuir adsorption isotherm was fitted to the information where a plot of C/(x/m) versus C gave a straight line with incline 1/b and a block of 1/kb. The adsorption maxima (b) esteem went from 41.66 to 76.92 µg Zn g-1 soil (Table-2). Most extreme value was found for the soils of Baramulla locale and least for Shopian region. The bonding energy constant (k) was most extreme (0.0378 ml g-1) in soils of Srinagar region and least of 0.018 ml g-1 in soils of Ganderbal locale.
The plot of zinc adsorbed against balance zinc focus on a log-log scale gave direct relationship in every one of the soils for Freundlich adsorption show. Freundlich k esteems extended broadly among soils, going from 2.22 to 3.55 µg g-1 (Table-2). This model was substantial for more extensive scope of zinc concentrations13 as at higher groupings of connected zinc, multilayer adsorption and additionally precipitation responses seems to have occurred.14 In this way this came about a superior occurrence of information in Freundlich isotherms as contrast with Langmuir models.
Conclusion
Zinc adsorption information was tastefully portrayed by both Langmuir and Freundlich adsorption isotherms over the whole focuses utilized however Freundlich isotherms gave a best fit. Adsorption of zinc was principally represented by the clay content and CEC of soils. An endeavour was made in this examination to know the best fit numerical model to comprehend procedure of zinc adsorption which thusly can helps in advancing appropriate administration practices to defeat weaknesses of zinc in soils. Further research should be done to check the conduct of zinc discharge and alleviation to any issue emerging from abundance zinc adsorption process.
Acknowledgement
We the authors deeply acknowledge all the persons who have provided a helping hand to carry out this work. Moreover, we would like to thank SKUAST-K especially its soil science department for providing financial support and kind guidance.
Conflict of Interest
Authors declare no conflict of interest.
References
- Hafeez B., Khanif Y. M. and Saleem M. Role of Zinc in Plant Nutrition- A Review. American Journal of Experimental Agriculture. 2013. 3(2): 374-391.
CrossRef - Ali S., Riaz K.A., Mairaj G., Arif M., Fida M. and Bibi S. Assessment of different crop nutrient management practices for yield improvement. Australian Journal of Crop Science. 2008. 2: 150-157.
- Stahl R.S. and James B.R. Zinc sorption by B horizon as a function of pH. Soil Science Society of America Journal. 1991. 55:1592-1597.
CrossRef - Alloway B.J. Zinc in Soils and Crop Nutrition. International Zinc Association (IZA) and International Fertilizer Association (IFA). Brussels, Belgium and Paris, France. 2008.
- Gaudalix M.E. and M.T. Pardo. Zinc sorption by acid tropical soil as affected by cultivation. European Journal of Soil Science. 1995. 46: 317-322.
CrossRef - Anyika C.C., Ninyo A. and Kparmwang T. Sorption desorption of Zn by a Rhodic Khandiustult in the northern guinea Savanna of Nigeria. Continental Journal of Agronomy. 2010. 4: 15-22.
- Perkin Elmer A Analyst 100 U.S.A. http://www.perkinelmer.com/category/atomic-absorption-spectroscopy-aa
- Najar G.R. Akhtar F. Singh, S.R. and Wani, J.A. Characterization and classification of some apple growing soil of Kashmir. Journal of the Indian Society of Soil Science. 2009. 57(1): 81-84.
- Narwal R.P. and Singh, B.R. Sorption of cadmium, zinc, copper and lead by soils developed on alun shales and other materials. Norv. J. Agri. Sci. 1995. 9(3-4): 177-179.
- Tiwari R.P., Ramudu P.B., Srivastava, R.K. and Gupta, M.K. Sorption and desorption of metallic Zn on an alluvial soil. Iran journal of Environmental Health Sciences and Engineering. 2007. 4: 139-146.
- Gurpreet-Kaur Sharma, B.D. and Sharma, P. Zinc adsorption as affected by concentration, temperature, and time of contact in the presence of electrolytic and aqueous medium in benchmark Soils of Punjab in Northwest India. Communications in Soil Science and Plant Analysis. 2012. 43: 701-715.
CrossRef - Kabata-Pendias A. Trace Elements in Soils and Plants. 4th edition, CRC Press, Boca Raton FL, USA. 2011.
CrossRef - Shukla L.M. Sorption of Zn and Cd on soil clays. Agrochemica. 2002. 44: 101-106.
- Saha U.K., Tanjguchi S. and Sakurai, S. Adsorption behavior of Cd, Zn, and Pbonhydroxyaluminum-and hydroxyaluminosilicate-montmorillonite complexes. Soil Science Society of American Journal. 2001. 65: 694-703.
CrossRef


