Introduction
Plants are for its highly versatile defenses owing to the immobile nature. They have developed various tiers of immune responsive strategies to withstand biotic and abiotic stresses. Unlike animals, plants rely on their innate immune responsive machinery as they lack specialized immune cells. Therefore, they employ a two-tier immune responsive apparatus.1 The first tier is a surface-localized pattern recognition receptors (PRRs) leading to PRR-triggered immunity (PTI).2 The second tier consists of intracellular immune receptors, mostly NOD type receptor, which directly or indirectly identifies virulence effectors secreted within host cells by pathogens, thereby inducing effector-triggered immunity. First-tier immune response specifically PTI immunity involves, rapid generation of ROS as an immediate response to biotic stress experienced by plants. Reactive Oxygen Species (ROS) is a proven defense regime employed by plants. ROS system further normalizes upon activation of subsequent tiers of plant immunity system.3 ROS and antioxidant enzymes are primary immune responses to the biotic stress induced by Fusarium oxysporum f. sp. lycopersici (FOL) on tomato (Solanum lycopersicum var. Pusa Ruby) plant4. Fusarium wilt disease in tomato may lead to a whopping 10-90% loss in yield.5 Therefore, besides being known as a popular cash crop, it is also treated as a model plant system for plant–pathogen interactions.6 FOL is one such pathogen studied for causing infection in tomato plant. It is a well-known soil borne pathogen which segregated from its non-pathogenic Fusarium oxysporum ancestor6 and is known to produce Fusarium wilt disease in Tomato plant further triggering immune responsive machinery.7,8 The immune response generated in the form of ROS and antioxidant enzyme approach is further alleviated by application of Arbuscular Mycorrhizal Fungus (AMF) Glomus fasciculatum (GF) to the plant under study. AMF symbiosis in cash crops will play a significant role to evaluate the potential of and burden on First-tier immune response. Similar studies are carried out earlier focusing mainly on evaluation of phenotypic characters instead of ROS pathway.9,10 Our study shall answer few fundamental questions, which cater a relationship between first-tier immune responsive machinery i.e. ROS pathway in Tomato with that of GF application, possibly potentiating the plant against FOL induced biotic stress. Moreover, LC-MS of FOL revealed some novel compounds, which can be correlated to the pathogenicity of FOL and its ability to generate high ROS and antioxidants in FOL treated plants. These compounds can be targets for agriculturalists and scientists towards mitigating FOL related infections in future. Therefore, a time-dependent renaissance in various tiers of innate immune responses of AMF-GF treated plants may be evaluated through such studies to tackle fungal infections.
Material and Methods
Plant and Fungal Material
Tomato (Solanum lycopersicum – Pusa Ruby) seeds were obtained from Mahatma Phule Krishi Vidyapeeth (MPKV), Rahuri. Seeds were surface sterilized with commercial bleach (Sodium hypochlorite 5%, v/v) and further treated with 1% HCl to break dormancy and get better germination. The seeds were then inoculated in pots containing wet autoclaved vermiculite. After a period of two weeks, emergence of first true expanded leaf was confirmed and the seedlings were then transferred in cylindrical plastic container (250mL) containing Glomus fasciculatum soil-based inoculum. Glomus fasciculatum was provided by Dr. Bhagyaraj, CNRBCD, Bengaluru.
Mycorrhizal inoculation was carried out by adding 10% of the final volume of a soil/sand-based inoculum enriched in fungal propagules and containing chopped Allium porrum L. roots colonized by AM fungus. Subsequent development of mycorrhizal colonization was periodically assessed. Absence of mycorrhizal colonization was confirmed in control plants as well as plants to be treated with FOL. The pathogen FOL was inoculated in the rhizospheric region of test plantlets which were having more than 40% mycorrhizal (AMF) colonization (usually 4 weeks of potting).
For the germination and proper growth of the tomato plant, the climatic conditions like temperature and humidity were maintained. Plants are grown in between 70 to 80 degree Fahrenheit and the humidity ware maintained 65 to 75% during the night and 80 to 90% during the day.
Preparation of Fusarium Inoculum
Fusarium oxysporum f. sp. lycopersici (Kindly gifted by the Dr. Laxman Prasad, S.V.P. University of Agriculture and Technology, Meerut, India) was inoculated in a conical flask containing 10g of soaked Jowar seed. FOL culture was incubated for three days on shaker at 180 rpm followed by centrifuged at 8000 rpm for a period of 20 mins in order to obtain supernatant containing microconidia. The spore count was adjusted to 2×107 microconidia/ml and this supernatant served as inoculum.
Measurements and Analyses
Plant growth and symbiotic development with GF
Tomato seeds were potted in vermiculite in order to generate seedling with fairly developed root system. The 14 days old healthy plantlets were kept ready for transferring to GF supplemented soil. Two-week-old plantlets were cultivated in soil supplemented with GF to develop natural symbiosis. (Figure 1(a)iii)
Application of FOL Inoculum
At 45th day all plantlets were treated with Fusarium microconidia of a concentration of 2×107 microconidia/ml are inoculated near the rhizosphere of AM colonized and Control plants. Hence, the treatment consisted of control plants, control plant infected with FOL, AMF colonized plants, and AM colonized plant infected with Fusarium. The control Fusarium infected and Mycorrhizal Fusarium infected plants were harvested after the inoculation of 24, 48, 72, 96, 120 hours of time interval.
Mycorrhizal colony Estimation
The root hairs collected in a test tube were incubated with 10% KOH in hot water bath for 10 min. KOH was drained followed by 2% HCl addition and re-suspended in the hot water bath for 5-6 min. HCL was then replaced by the Trypan Blue stain and again kept for 10-15 min in hot water bath. Following this the root hairs were de-stained for 24 hr in milli-Q water for a day with succeeded by microscopic examination of root fragments for mycorrhizal colonization (McGonigle et al., 1990), Arbuscular abundance is noted to calculate below-mentioned parameters using an online platform Mycocalc (http://www2.dijon.inra.fr/mychintec/Mycocalc-prg/download.html):
- F: Frequency of mycorrhiza in RS
- M: Intensity of mycorrhizal colonization in the RS
- m: Intensity of mycorrhizal colonization in the root fragment
- a: Arbuscule abundance in the mycorrhizal root fragment
- A: Arbuscule abundance in the mycorrhizal RS
Reactive oxygen species detection by DCFH-DA
ROS generation was detected by using a cell permeable probe 2, 7-dichlorodihydrofluorescein diacetate (DCFH-DA), which is a precursor of the DCF (Dichlorofluorescein). The DCFH-DA probe is cleaved into a polar molecule acetate and impermeable DCFH2 (2-Dichlorodihydrofluorescein) by cellular esterase enzyme. Subsequently, DCFH2 gets oxidized by ROS, H2O2, or peroxyl radical, or peroxynitrite anion DCF which is detected by its fluorescence intensity11. 500µm concentration of DCFH-DA stock solution was prepared in DMSO (Dimethyl Sulfoxide) and kept -20ºC for further use. 100 mg of root tissue was incubated in the DCFH-DA (final concentration of 5μm) solution for 30 min in the dark condition. The solutions were then decanted and the root tissue frozen in liquid N2, homogenized and thawed. Freeze thawing was repeated at least thrice and then the tissues were suspended in 200μl of 10mm Tris-HCl buffer, pH 7.5. The samples were centrifuged at 1000 rpm for 5 min at 4°C and the supernatant from each sample ware collected Fluorescence measurements were made by using a Spectro-fluorometer at an excitation wavelength of 488 nm and an emission wavelength of 525 nm. The fluorescence values represent arbitrary fluorescence units.
Assay of antioxidant enzyme activities
For antioxidant enzyme assays, 100mg leaf tissue was homogenized in 1mL extraction buffer consisting of 1ml 0.1M Potassium phosphate buffer (pH 7.5), 0.01ml of 0.1M EDTA (pH 7.5), 100mg PVP and 0.2µl β-mercaptoethanol.12 The homogenate was centrifuged at 9000rpm for 15 min at 4oC and the supernatant was collected as crude enzyme extract for enzymatic assays.
Superoxide dismutase (SOD; EC 1.15.1.1) activity was assayed by measuring its ability to inhibit the photochemical reduction of Nitro Blue Tetrazolium (NBT).13 The reaction mixture (3ml) contained 2.54 ml of 0.05M Potassium phosphate buffer (pH 7.8), 0.03 ml of 0.005M EDTA (pH 7.5), 0.3 ml of 0.130M Methionine, 0.03 ml of 0.0075M NBT, 70 µl plant enzyme extract. The reaction was started with 0.03 ml of 0.002M riboflavin.14 Two sets of each sample were prepared; one of which was incubated in uniform light and the other was kept in dark, which served as corresponding blank. The absorbance of each reaction mixture was measured at 560 nm after 15 min. incubation. One unit of SOD activity was defined as the amount of enzyme which causes 50% inhibition of the photochemical reduction of NBT, and was expressed as Units/mg protein.
The activity of catalase (CAT; EC 1.11.1.6) was determined as a decrease in absorbance at 240 nm for 5 min following the decomposition of H2O2. The 3ml reaction mixture contained 2.96 ml of 0.1M Potassium phosphate buffer (pH 7.0) and 20 µl of enzyme extract. The reaction was started by adding 2.5 µl of 30% H2O2.15 Molar extinction coefficient of H2O2 (3.6 x 10 M-1cm-1) was used to calculate enzyme activity in terms of mm/mg of protein.
The activity of Peroxidase (POX; EC 1.11.1.7), was determined as an increase in absorbance at 470nm for 2 min. The 3ml reaction mixture contained 2.47 ml of 0.1M Potassium phosphate buffer (pH 7.0), 20 µl of enzyme extract and 500 µl of 0.03M Guaicol. The reaction was started by adding 5 µl of 30% H2O2.
LC-MS analysis of FOL
The LC-MS analysis was performed as per suggested earlier.16 Briefly, to 5g of dried and milled FOL, 20ml of extract solvent (methanol) was added. The extraction was done for 48 hr followed by filtration (Whatmann no. 1 filter paper). The filtrate was concentrated in Rota evaporator and given for LC-MS analysis. The detection and quantitation of FOL metabolites was performed with a 6200 series Accurate-mass Time-of-flight (TOF) LC-MS (Santa Clara, United States).
Statistical Analysis
The result analysis for statistical significance was done by one-way analysis of variance (ANOVA) test. Entire data was expressed in mean ± Standard deviation. Statistical differences were considered significant at P > 0.05.
Results
Plant growth, symbiotic development and FOL treatment
The plant root mass was observed to be more in control plant as compared to FOL infected ones, however, plants which were initially colonized with GF followed by FOL infection also showed similar root mass as compared to that of the control. The experimental setup of control plant, GF symbiotic and FOL infected is explained in Figure 1a.
Mycorrhizal Colonization
The frequency of GF colonization in the FOL microconidia inoculated root system was obtained similar as mycorrhizal roots not inoculated with pathogen. The colonization intensity and arbuscule abundance in the FOL microconidia inoculated root system was also obtained similar as mycorrhizal roots not inoculated with pathogen (Figure 1b).
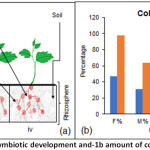 |
Figure 1: Symbiotic development and-1b amount of colonization. Click here to View Figure |
Reactive oxygen species detection by DCFH-DA
An increase in ROS in the roots of plants inoculated with FOL microconidia as compared to GF colonized plants inoculated with FOL (GF+FOL) at each of the time point up to 72 hr. Later, after 72 hr a decrease in amount of ROS generation was obtained in both CF and AM+F (Figure 2a).
Activity of Antioxidant Enzymes
Antioxidant enzymatic machinery was evaluated for all the treatments and at different time points. Overall a similar trend was observed for all the tested enzymes viz. superoxide dismutase, catalase and peroxidase. With high expression in FOL followed by GF+FOL, GF and lastly control. This observation remained constant over a period of 120 mins, which was in line with the non-enzymatic antioxidant response of the plants (Figure 2b, 2c, 2d).
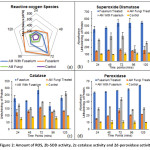 |
Figure 2: Amount of ROS, 2b-SOD activity, 2c-catalase activity and 2d-peroxidase activity. Click here to View Figure |
LC-MS analysis of FOL
The LC-MS profile of FOL shows presence 57 different compounds with some primary metabolites and secondary metabolites (Fig. 3) (Table 1, Supplementary). However, as per the literature reviewed, we can confirm that some of them are reported for the first time in FOL i.e., leukotriene d4, fenbutyramide, anabasamine, 11alpha-acetoxykhivorin and scopoline.
 |
Figure 3: LCMS Chromatogram. Click here to View Figure |
Discussion
Transient ROS production was seen in FOL infected plants, which is in line with the earlier reports.7,17 This oxidative outburst is a part of first tier defenses employed by plants against pathogens. AMF is known to alleviate the oxidative response in tomato plant.18 In our study, tomato roots inoculated with FOL showed marked increase in ROS as compared to the AMF-GF treated plants. A report of AMF Glomus mosseae treated tomato plant have shown significant reduction of the damage caused by plant pathogen Phytopthora.19 Such prior finding was supported by our study, and thus a combinatorial approach of GF treated and FOL infected plants showed prominent reduction in ROS as compared to only FOL infected control plants. AMF-GF treated plants could withstand FOL infection by controlled enhancement of ROS to a certain level. Similar to acquired immunity in animals, ROS generated to a basal level in GF treated plant played a significant role in activating plant’s secondary defense responses. Even ROS scavenging enzymes viz. SOD, CAT and POX where found high in FOL treated plants as compared to GF and GF+FOL treated plants. Certainly, first-tier defenses viz. ROS and antioxidant enzymes activated in GF treated plants could alleviate negative effect of FOL treatment. ROS and antioxidant enzymes are characterized as a plant’s primary immune response and are known to trigger plant’s second tier of defenses, which include activation of defense genes, cell wall strengthening, induced systemic resistance and even cell death.20–22 Though the immune system was significantly boosted by AMF treatment, the time of treatment, genus and species of AMF, density of AMF in the rhizosphere, type of plant and pathogen are going to be the deciding factors before employing AMF for agricultural applications.23
The LC-MS profile of FOL showed some interesting novel compounds. Out of the dominant novel ones, leukotriene D4 is well-known for inducing ROS machinery in plants ultimately leading to programmed cell death.24 The abundance of this compound can be co-related to the pathogenicity of FOL against the tomato plant. Since the pathogenic effect of FOL in AMF-GF treated plants was significantly lesser, we may state that AMF-GF plays a significant role in subduing detrimental effects of such mycotoxins.
Conclusion
Tomato plants growing in symbiosis with AMF-GF clearly indicates its immune boosting potential. Initial inoculation of tomato plant with AMF-GF provide some buffer time for plants to activate their primary defenses. There, use of such AMF-GF priming strategies will help overcome pathogenic infections and enhance the yield of agriculturally important crops. Although, our findings are restricted to FOL, AMF-GF and tomato plant, similar strategies with different fungal phytopathogens, mycorrhizas and plants hosts will give an insight in their synergism and immunity enhancing capacity.
Acknowledgment
The study was supported by Department of Botany, Savitribai Phule Pune University, Ganeshkhind – 411007, India and Centre for Innovation Research and Consultancy, Pimpri, Pune – 411018, India. Ito S, Ihara T, Tamura H, Tanaka S, Ikeda T, Kajihara H, Dissanayake C, Abdel-Motaal FF, El-Sayed MA.
Conflict of Interest
Authors declare no conflict of interest.
References
- Dodds PN, Rathjen JP. Plant immunity: towards an integrated view of plant–pathogen interactions. Nat. Rev. Genet. 2010;11(8):539-548. doi:10.1038/nrg2812
CrossRef - Macho AP, Zipfel C. Plant PRRs and the activation of innate immune signaling. Mol. Cell. 2014;54(2):263-272. doi:10.1016/j.molcel.2014.03.028
CrossRef - Shen Y, Liu N, Li C, Wang X, Xu X, Chen W, Xing G, Zheng W. The early response during the interaction of fungal phytopathogen and host plant. Open Biol. 2017;7(5):170057. doi:10.1098/rsob.170057
CrossRef - Ignjatov M, Milosevic D, Nikolic Z, Gvozdanovic-Varga J, Jovicic D, Zdjelar G. Fusarium oxysporum as causal agent of tomato wilt and fruit rot. Pestic i fitomedicina. 2012;27(1):25-31. doi:10.2298/PIF1201025I
CrossRef - Kumar Singh A, Kamal S. Chemical Control of Wilt in Tomato (Lycopersicon esculentum L.). Int J Hortic. 2012;2(2):5-6. doi:10.5376/ijh.2012.02.0002
CrossRef - Inami K, Kashiwa T, Kawabe M, Onokubo-Okabe A, Ishikawa N, Pérez ER, Hozumi T, Caballero LA, de Baldarrago FC, Roco MJ, Madadi KA, Peever TL, Teraoka T, Kodama M, Arie T. The Tomato Wilt Fungus Fusarium oxysporum f. sp. lycopersici shares Common Ancestors with Nonpathogenic F. oxysporum isolated from Wild Tomatoes in the Peruvian Andes. Microbes Environ. 2014;29(2):200-210. doi:10.1264/jsme2.ME13184
CrossRef - Ito S, Ihara T, Tamura H, Tanaka S, Ikeda T, Kajihara H, Dissanayake C, Abdel-Motaal FF, El-Sayed MA. α-Tomatine, the major saponin in tomato, induces programmed cell death mediated by reactive oxygen species in the fungal pathogen Fusarium oxysporum. FEBS Lett. 2007;581(17):3217-3222. doi:10.1016/j.febslet.2007.06.010
CrossRef - Di X, Cao L, Hughes RK, Tintor N, Banfield MJ, Takken FLW. Structure-function analysis of the Fusarium oxysporum Avr2 effector allows uncoupling of its immune-suppressing activity from recognition. New Phytol. 2017:897-914. doi:10.1111/nph.14733
CrossRef - Akköprü A, Demir S. Biological control of Fusarium wilt in tomato caused by Fusarium oxysporum f. sp. lycopersici by AMF Glomus intraradices and some rhizobacteria. J Phytopathol. 2005;153(9):544-550. doi:10.1111/j.1439-0434.2005.01018.x
CrossRef - Dasgan HY, Kusvuran S, Ortas I. Responses of soilless grown tomato plants to arbuscular mycorrhizal fungal (Glomus fasciculatum) colonization in re-cycling and open systems. African J Biotechnol. 2008;7(20):3606-3613. doi:10.5897/AJB08.367
- Tarpey MM, Fridovich I. Methods of Detection of Vascular Reactive Species. Circ Res. 2001;89(Vitamin C):224-237. doi:10.1161/hh1501.094365
CrossRef - Malan C, Greyling MM, Gressel J. Correlation between CuZn superoxide dismutase and glutathione reductase, and environmental and xenobiotic stress tolerance in maize inbreds. Plant Sci. 1990;69(2):157-166. doi:10.1016/0168-9452(90)90114-4
CrossRef - Stewart RRC, Bewley JD. Lipid Peroxidation Associated with Accelerated Aging of Soybean Axes. Plant Physiol. 1980;65(2):245-248. doi:10.1104/pp.65.2.245
CrossRef - Beauchamp C, Fridovich I. Superoxide dismutase: Improved assays and an assay applicable to acrylamide gels. Anal Biochem. 1971;44(1):276-287. doi:10.1016/0003-2697(71)90370-8
CrossRef - Volk S, Feierabend J. Photoinactivation of catalase at low temperature and its relevance to photosynthetic and peroxide metabolism in leaves. Plant Cell Environ. 1989;12(7):701-712. doi:10.1111/j.1365-3040.1989.tb01630.x
CrossRef - Freitas-Silva O, de Lourdes Mendes de Souza M, Venancio A. Tracing Fungi Secondary Metabolites in Brazil Nuts Using LC-MS/MS. Drug Metab Lett. 2011;5(3):150-155. doi:10.2174/187231211796905053
CrossRef - Mandal S, Mitra A, Mallick N. Biochemical characterization of oxidative burst during interaction between Solanum lycopersicum and Fusarium oxysporum f. sp. lycopersici. Physiol Mol Plant Pathol. 2008;72(1-3):56-61. doi:10.1016/j.pmpp.2008.04.002
CrossRef - Hajiboland R, Aliasgharzad N, Laiegh SF, Poschenrieder C. Colonization with arbuscular mycorrhizal fungi improves salinity tolerance of tomato (Solanum lycopersicum L.) plants. Plant Soil. 2010;331(1):313-327. doi:10.1007/s11104-009-0255-z
CrossRef - Pozo MJ, Cordier C, Dumas‐Gaudot E, Gianinazzi S, Barea JM, Azcón‐Aguilar C. Localized versus systemic effect of arbuscular mycorrhizal fungi on defence responses to Phytophthora infection in tomato plants. J Exp Bot. 2002;53(368):525-534. doi:10.1093/jexbot/53.368.525
CrossRef - Torres MA. Reactive Oxygen Species Signaling in Response to Pathogens. Plant Physiol. 2006;141(2):373-378. doi:10.1104/pp.106.079467
CrossRef - Song Y, Chen D, Lu K, Sun Z, Zeng R. Enhanced tomato disease resistance primed by arbuscular mycorrhizal fungus. Front Plant Sci. 2015;6(September):1-13. doi:10.3389/fpls.2015.00786
CrossRef - Schouteden N, Waele D De, Panis B, Vos CM. Arbuscular mycorrhizal fungi for the biocontrol of plant-parasitic nematodes: A review of the mechanisms involved. Front Microbiol. 2015;6(NOV):1-12. doi:10.3389/fmicb.2015.01280
CrossRef - Borowicz AV. Do Arbuscular Mycorrhizal Fungi Alter Plant–Pathogen Relations? | 10.1890/0012-9658(2001)082[3057:damfap]2.0.co;2.2001;82(11):3057-3068. https://sci-hub.tw/10.1890/0012-9658(2001)082[3057:damfap]2.0.co;2.
CrossRef - Iriti M, Faoro F. Review of innate and specific immunity in plants and animals. Mycopathologia. 2007;164(2):57-64. doi:10.1007/s11046-007-9026-7
CrossRef