Introduction
Biosolids (formerly known as sewage sludge) are highly variable products that can range in composition depending on the treatment process, source, weather and temperature. It is difficult to produce a standard, homogeneous biosolids product to enable the fertilizer potential of similarly treated biosolids to be evaluated. Biosolids contain high concentrations of P and organic matter,1 and most of the fertilizer value of biosolids is due to P.2 Nitrogen is also present in biosolids and contributes N to the soil. Biosolids can be applied to agricultural land as a soil amendment with fertilizer properties to improve the growth of plants and to improve the fertility of the soil. There are many ions in soil that have the potential to react with P, especially Fe, Al and Ca. Precipitation and sorption reactions of P with these elements have the potential to reduce the bioavailability of P.
The published literature contains conflicting reports on the effect of applying biosolids on P availability to plants. One study3 found that P was more available in sludge when it had an iron Fe/P ratio of 1.6 than in NPK fertilizer. Increasing rate of sludge application reduced P recovery and a Fe/P ratio in the sludge of 9.8 reduced P availability to zero.
The long term effects of biosolids application has been documented4 and has been found to increase total and Olson P in 0-50 cm depth soils sampled 13 years after biosolids application. In a glasshouse experiment conducted over 6 weeks5 no difference in wheat yield was found between fertilization with biosolids and superphosphate. When Fe and Al salts were applied to wastewater to remove P from effluent in an earlier experiment6 the Pin the resultant biosolids was virtually unavailable to the plant, although it was acknowledged that the availability was lower than for other biosolids reported in the literature. The addition of municipal sewage sludge to soil has been found to increase Olson extractable P,7 which led to higher canola yield
The major limitation to understanding the effects of biosolids on P availability to plants is that of distinguishing the P in soil from that added in the biosolids. Zapata and Zaharah5 addressed this problem by labeling the soil with 32P. This current protocol extends this approach to deal with the situation where fertilizer is also applied.
In view of the conflicting views on the effects of Fe, Al and Ca treated biosolids on plant growth, P uptake and soil chemistry dual radioisotopes of P were employed to enable separation of the fate of P from biosolids, fertilizer and native soil P.
Materials and Methods
Soil
A bulk surface soil sample was collected from Hill A, Kentgrove South Farm, 8 km east of Goulburn, NSW, Australia from a site used by NSW Agriculture as part of a biosolids trial conducted over 9 years. The Kurosol soil8 has a duplex profile with a sandy A horizon, over a medium to heavy clay B horizon. The soil was collected from a non-treated area of the trial site and had a pH of 7.4, total P of 174 mg/kg and a Colwell bicarbonate P of 3 mg/kg.
Biosolids
The biosolids used were obtained from a sewage treatment plant which treats approximately 32ML of domestic sewage daily which produces 60-80 product tonnes of biosolids. It uses chemical precipitation with Fe and Al for the removal of P from the wastewater. The Fe is added as spent pickle liquorin the ferrous sulfate (FeSO4.7H2O) form which is a waste product from a wire stripping factory. This is then oxidized to ferric sulfate prior to P removal, as ferric Fe is more efficient at removing P. The spent pickle liquor contains 14.5% Fe and is added at 3000-4000L daily. Due to the variations in the volume of sewage, excess spent pickle liquor may sometimes be added, resulting in excess Fe either in the biosolids product or discharged in the effluent. The Al is added as alum (Al2(SO4)3.14H2O) solution containing 600g Al/L at the rate of 600-700L per day. The composition of the biosolids is presented in Table 1.
Table 1: Composition of Biosolids Used in the Study.
| Characteristic | Characteristic | ||
|
pH (1:5 H2O) |
6.6 |
Al-P (µg/g) |
0.3 |
|
Total P (% of oven d.wt.) |
4.1 |
Fe-P (µg/g) |
3.0 |
|
Inorganic P (% of oven d.wt.) |
3.9 |
Ca-P (µg/g) |
0.5 |
|
Organic P (% of oven d.wt.) |
0.1 |
Total Fe (µg/g) |
9.9 |
|
% of Total P in Organic P form |
2.0 |
Total Al (µg/g) |
0.9 |
|
Bicarbonate P (µg/g) |
0.1 |
Total Ca (µg/g) |
1.0 |
|
Total N (µg/g) |
2.0 |
Radioisotope Technique
A dual radioisotope method was used to separate the contributions of soil, fertilizer and biosolids to plant P uptake. The soil was labeled with 32P and fertilizer, added in solution, was labeled with 33P. The reverse dilution technique of Shedley et al., (1979) was used to measure the contribution of native soil P in each soil fraction, and in the plant.
In this procedure the specific radioactivity (SR, Bq/mgP) of the fertilized treatment is measured and this divided by the SR of the unfertilized control. The resulting value is termed the Specific Radioactivity Ratio (SRR) and the proportion of fertilizer P in the measured pool calculated as 1-SRR.
Measurement of 33P from the fertilizer allowed the direct determination of this source. By calculating the total P in the plant and soil pools and subtracting the radioactive fractions the proportion of P derived from the biosolids was determined.
A 1 mL solution of orthophosphoric acid labeled with 925 MBq of 32P (AMRAD catalogue no. NEX-0535) was made to 1L with distilled water containing 10 mg of KH2PO4. 1600 g samples of the soil (previously sieved to < 2 mm) were weighed into plastic bags, water was added to the equivalent of half field capacity and they were mixed thoroughly. 5 mL of the 32P solution was added so that 4.625 MBq of 32P was added to the soil in each bag and the soil remixed and incubated for one week to allow 32P equilibration with native soil P. The soil was mixed every second day.
The amount of unlabeled P added in the 32P solution was only 50 g which would not alter soil P pools. After one week the wet biosolids were applied to the soil at application rates equivalent to 0, 7.5, 15, 30 and 60 dry t/ha, and mixed. Five rates of application were used so that regression analyses could be used over a wide range of application rates instead of fewer rates with replication. The moisture content of the biosolids was 4.9 g/g biosolids.
Fertilizer was added as KH233PO4. A 1 mL solution of 185 MBq of carrier free 33P (i.e. contains insignificant stable 31P isotope) (AMRAD catalogue no. NEZ-080) was added to 22 g KH2PO4 in distilled water and made up to a final volume of 1 L. For the +fertilizer treatments 20 mL aliquots of the KH233PO4 fertilizer containing 3.7 MBq were added to the biosolids/soil mixtures. This was equivalent to 80 kg P/ha. Basal nutrients K and S were added to the soil as K2SO4 at 20 kg K/ha and 8 kg S/ha. The fertilizers were mixed thoroughly with the biosolids/soil mixtures. Additional N was not applied, as it was not common practice to add N fertilizer with biosolids on account of the perceived potential of N to leach into groundwater, or runoff into surface water.
The plastic bags containing the mixtures were placed in 15 cm diameter and 12 cm deep (10 cm soil depth) non-leaching pots. The bulk density of the mixtures was 1200 kg/m3. Eight maize seeds (Zea Mays, HYCORN 83) were planted in each pot. The maize plants were thinned to 3 per pot after one week and the pots watered daily and once a week to field capacity by weight. The plants were harvested after 5 weeks.
Plant and Soil Analyses
Approximately 0.2 g samples of ground plant material were digested in HClO4 (70%) and H2O2 (30%) using the Sealed Container Digestion method.10 Phosphorus in the digests was analysed using an ARL 3560 ICP-AES (Applied Research Laboratories, Waltham, MA, USA.
Soils were analyzed for total, inorganic and organic forms of P11, bicarbonate P12 and Al, Fe and Ca forms of P.13 The bicarbonate P was determined calorimetrically using an ammonium molybdate-sulfuric acid-ascorbic acid reagent on an auto analyzer (Skalar San++ continuous flow analyzer, Breda, The Netherlands).
Radioactivity Counting
Radioactivity was measured using a United Technologies Packard Tri-carb 2000 Liquid Scintillation Analyzerâ (Perkin Elmer, Acron, Ohio).
17mL of scintillation fluid containing toluene, p-terphenyl and POPOP14 was added to 3 mL of the plant or soil extract in a 20mL scintillation vial.
The 32 P and 33P were counted in the separate windows and spillover of 32P into the lower energy 33P window was accounted for.
Terminology and Calculations
P derived from the various sources was determined as follows:
%P derived from fertilizer = (SR of sample/SR of fertilizer added) x 100
% P derived from soil= (1-SRR) x 100
% P derived from biosolids = 100 – % P derived from fertilizer – % P derived from soil
Statistical Analysis
In order to explore a wide range of biosolid/fertilizer ratios true replication was not feasible in the development of this experimental protocol A series of 5 rates of biosolid application rates,with and without fertilizer were made and parametric regressions were fitted to the data points using the regression error as a surrogate for ‘true’ error. All analysis was completed using the R statistical Package15 using the R package “boot” and “nlme”.16 The most appropriate parametric model was selected for each regression series by minimizing the Akaike information criterion. Phosphorus contents, dry weights and P concentration measures for the no fertilizer treatment were best represented by a three parameter asymptotic exponential function. The tissue P concentration for the added fertilizer treatment was better represented by the four parameter logistic equation. The proportion of P derived from the biosolid was best described by the Michaelis-Menten model while the proportion of P derived from the fertilizer and from the soil in the treatment with fertilizer added was best described by the Weibull equation. Finally, the three-parameter logistic growth equation best described the proportion of P derived from the soil where no fertilizer P was added. Residuals for the most appropriate model were calculated against the predicted values and bootstrapped (R =1000) by randomly assigning the residuals to the fitted values and refitting the parametric functions.16, 17, 18 Mean values and confidence intervals (95%) shown in the tables and figures for the applied rates were estimated from the bootstrapped curve parameters and means were deemed significantly different where confidence intervals did not overlap.
Results
Plant Response to Biosolids Application and Sources of P in the Plant
Yields (g/3 plants) and P content of shoots (mg/3 plants) were not significantly different between the with and without fertilizer treatments so mean data are presented. Both shoot P content and yield increased with biosolids increasing application rate (Figure 1). The proportion of P in the maize tops derived from the fertilizer decreased from 55.3 % to 6.3 % between the 0 and 60 dry t of biosolids /ha (Figure 2).
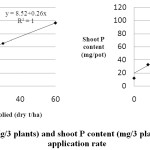 |
Figure 1: Shoot DM Yield (G/3 Plants) and Shoot P Content (Mg/3 Plants) as Affected by Biosolids Application Rate Click here to View figure |
The proportion of P in the maize tops derived from the biosolids increased as the biosolids application rate increased and was significantly lower in the +fertilizer than the – fertiliser treatment (Figure 2). When fertilizer was applied, P in the shoots derived from the biosolids increased from 29.0% at the 7.5 dry t/ha application rate to 69.1% at the 60 dry t/ha rate. When fertilizer was not applied the P in the shoots derived from the biosolids increased from 64.9% at the 7.5 dry t/ha rate to 88.6% at the 60 dry t/ha rate.
The P derived from the soil decreased as the biosolids application rate increased from 7.5 to 60 t/ha (Figure 2). The decrease was from 33.8% to 12.2% in the absence of fertilizer and from 44.3% to 24.6% when fertilizer was applied.
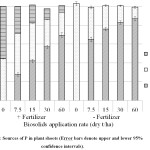 |
Figure 2: Sources of P in Plant Shoots (Error Bars Denote Upper and Lower 95% Confidence Intervals). Click here to View figure |
Effects of Biosolids and Fertilizer on Soil P
The application of biosolids to the soil had a significant effect on the enrichment of P concentration in the different soil pools like total P, inorganic P, organic P, bicarbonate P, Al-P, Fe-P and Ca-P (Table 2).
Biosolids P contributed in excess of 50% of the P in all soil pools when applied at either 7.5 or 60 t/ha and the proportion in each pool remained relatively constant as application rate increased from 7.5 to 60 t/ha except in the Fe-P pool where it increased from 57.6% at 7.5 t/ha to 75.7% at 60 t/ha. Increasing rates of biosolids resulted decreasing proportions of fertilizer P in the measured soil pools (Table 2).
Table 2: P Concentration, Proportion of Fertilizer P, Bio solids P and Soil P in Measured Soil Pools in the Presence of Two Doses of Bio solids With Added P Fertilization (80 Kg P/Ha).
| Total P | Inorganic P | Bicarbonate P | Al-P | Fe-P | Ca-P | |||
|
7.5 t/ha biosolids |
||||||||
|
P conc. (mg/kg) |
185a |
105a |
37a |
42a |
36a |
70a |
||
|
% of P from fertilizer |
10.2b |
12.0b |
18.9a |
15.9b |
4.5a |
8.5b |
||
|
% of P from biosolids |
84.0a |
88.2a |
72.8a |
80.8a |
57.6a |
77.6a |
||
|
% of P from soil |
5.8a |
-0.2a |
8.3a |
3.3a |
37.9b |
14.0a |
||
|
60 t/ha biosolids |
||||||||
|
P conc. (mg/kg) |
1556b |
1282b |
400b |
292b |
860b |
318b |
||
|
% of P from fertilizer |
4.2a |
2.9a |
15.0a |
10.3a |
3.0a |
4.2a |
||
|
% of P from biosolids |
82.8a |
89.7a |
78.9a |
80.8a |
75.7b |
82.2a |
||
|
% of P from soil |
13.1b |
7.4b |
6.1a |
8.9b |
21.3a |
13.6a |
||
Numbers within a column followed by the same letter are not significantly different based on the bootstrapped curve parameters.
Discussion
The use of the two isotopes of P used in this study allowed partitioning of the P in the plant derived from the fertilizer, biosolids and soil. This technique has not been applied before to biosolids research.
Modern scintillation counting equipment has software to take account of the “spillover” of the different particle energy strengths in the 2 counting windows. If dual window isotope counting equipment used does not contain this software then re-counting the samples after the half-life of each isotope (14.3d32 P, 25.3d33 P) will allow “spillover” effects to be calculated by correcting for the decay of each isotope.
The decrease in uptake of soil and fertilizer P with increasing biosolids application can be attributed to the decrease in the proportion of these P sources in the total pool of available P. Biosolids P was the dominant source of P in the plant at all application rates in the absence of fertilizers and at all rates except 7.5 dry t/ha when fertilizer was applied. The reduction in the uptake of the fertilizer P by the plant as biosolids application rate increased is interpreted as competition between the two sources and not due to Fe or Al hydroxides in the biosolids immobilizing P. The biosolids used in this study contained 3.0% Fe-P and 0.3% Al-P (Table 1) so that these components increased with increasing rate of biosolids application.
If the Fe in biosolids was having an adverse effect on the availability of P derived from soil or fertilizer, then P uptake would be expected to decline with increasing application rate. In fact, there was an increase in the P concentration in the bicarbonate extractable P pool with application rate indicating that biosolids P provided an available source of P to plants. Two reports19, 20 have shown an increased amounts of bicarbonate extractable P in soils following biosolids application. In another study21 biosolids application was found to increase the P content in ryegrass.
A study on an Oxisol in Brazil22 found that the predominant fraction of in the soil was Fe-P, followed by Al-P and Ca-P and the concentration of Pin the Fe-P and Al-P fractions was positively correlated with the amounts of P applied in urban sewage sludge.
The present study provides no evidence that the addition of P fertilizer enhances the availability of biosolids P. In an earlier study23 it was found that there was no benefit to Sudan grass from applying fertilizer P with the biosolids as they contained relatively high concentrations of available P.
The uptake of P following biosolids application depends on soil type, crop and year as well as on the forms of P present in the biosolids.21
The use of duel radioisotopes in the present study allowed the source of the P in the system to be traced, which in the case of the particular biosolids used confirms that their addition increased the P in the system that is available to the plant.
Conclusion
The dual radioisotope technique employed here has been shown to be capable of separating the shore term contribution of P from three sources of nutrient. This is important when determining if ions such as Fe and Al, which are often added in the sewage treatment plant, affect the availability of P in the biosolids and if so whether fertilizer P can be used to alleviate the problem.
Acknowledgements
The research was made possible with the funds provided by Sydney Water Corporation, NSW Agriculture, Land and Water Resources Research and Development Corporation (LWRRDC) (now Land and Water Australia) and the University of New England. We thank all these organisations for their support. Expert professional assistance was provided by Dr. Donald McLeod and Dr. Ray Till and technical assistance was provided by Leanne Lisle and Judi Kenny.
References
- Sommers, L.E., Nelson D.W., Yos K.J. Variable nature of chemical composition of sewage sludges. J of Envir Qual. 1976; 5: 303-306.
CrossRef - Furrer, O.J., Gupta S.K., Stauffer W. Sewage sludge as a source of phosphorus and consequences of phosphorus accumulation in soils. In ‘Processing and use of sewage sludge.’ (Ed. L’Hermite P and Ott H.). (D.Reidel Publishing Company, Netherlands). 1984; 279-293.
- Kahiluoto, H.M., Kuisma E., Ketoja T., Salo, Heikkinen, J. Phosphorus in manure and sewage sludge more recyclable than in soluble Inorganic fertilizer. Environ Sci and Tech. 2015; 49: 2115-2122.
CrossRef - Xue, J.M., M.O. Kimberley, C. Ross, G. Gielen, L.A. Tremblay, O. Champeau, J. Horswell, and H.L Wang.. Ecological impacts of long-term application of biosolids to a radiate pine plantation. Sci Tot Environ. 2015; 530: 233-240.
CrossRef - Zapata, F., A.R. Zaharah. Phosphorus availability from phosphate rock and sewage sludge as influenced by the addition of water soluble phosphate fertilizer. Nut Cycl in Agroecosys. 2002; 63: 43-48.
CrossRef - de Haan, S. Sewage sludge as a phosphate fertiliser. In ‘Phosphorus in sewage sludge and animal waste slurries.’ (Eds. Hucker TWG. and Gatroux G.) (D. Reidel Publishing Company, Netherlands). 1981; 149-161.
- Shaheen, S., Tsadilas C. Utilization of biosolids in production of bioenergy crops I: Impact of application rate on Canola biomass, soil properties, and nutrient availability. Comm Soil Sci and Pl Anal. 2013; 44: 243-258.
CrossRef - Isbell, R.F. The Australian soil classification. Revised ed. CSIRO Publishing: Melbourne, Australia.2002.
- Shedley, C.D., Till A.R. Blair G. J. A radio tracer technique for studying the nutrient release from different fertiliser materials and its uptake by plants. Comm Soil Sci and Pl Anal. 1979; 10: 737-745.
CrossRef - Anderson, D.L., Henderson L.J. Sealed chamber digestion for plant nutrient analysis. Agron J. 1986; 78: 937-938.
CrossRef - Walker, T.W., Adams A.F.R. Studies on soil organic matter: I. Influence of phosphorus content of parent materials on accumulation of carbon, nitrogen, sulfur and organic phosphorus in grassland soils. Soil Sci. 1958; 85: 307-318.
CrossRef - Colwell, J.D. 1965. An automatic procedure for the determination of phosphorus in sodium hydrogen carbonate extracts of soil. Chem Ind.1957; 10: 893-895.
- Chang, S.C., Jackson M.L. Fractionation of soil phosphorus. Soil Sci.1957; 84: 133-144.
CrossRef - Till, A.R., McArthur G.S., Rocks R.L. An automated procedure for the simultaneous determination of sulphur and phosphorus and of radioactivity in biological samples. In ‘Proceedings of Sulfur 84’, Alberta, Canada, 3-6 June 1984, (Sulfur Development Institute Canada (SDIC), Calgary, Canada). pp 649-660.
- R Development Core Team. R: A language and environment for statistical computing (Version 2.10.1). Vienna, Austria: R Foundation for Statistical Computing. 2010. Retrieved from http://www.R-project.org/(Accessed March 1, 2108)
- Crawley, M. The R book. Southern Gate, Chichester, West Sussex, England: John Wiley & Sons Ltd; 2007.
CrossRef - Canty, A., Ripley B. D. boot: Bootstrap R (S-Plus) Functions 2017.
- Hinkley, D. V., Davison A. C. Bootstrap Methods and Their Applications. Cambridge: Cambridge University Press.1997.
- Soon, U.K., Bates T.E., Moyer J.R. Land application of chemically treated sewage sludge: II Effects on plant and soil phosphorus, potassium, calcium and magnesium and soil pH. J of Environ Qual. 1978; 7: 269-272.
CrossRef - O’Connor, B.A., Knudtsen K.L., Connell G.A. Phosphorus solubility in sludge -amended calcareous soils. J of Environ Qual. 1986; 15: 308-312.
CrossRef - Koskela, I. Phosphorus in sewage sludge in Finland. In ‘Phosphorus in sewage sludge and animal waste slurries.’ (Eds. Hucker TWG. and Catroux G). (D. Reidel Publishing Company, Netherlands), pp.109-117.1981.
- Alleoni, L.R.F., Fernandes A.R., Correia B.L. Sequential extraction of phosphorus in an Oxisol amended with biosolids in a long-term field experiment in Brazil. Agric Ecosys. Environ 2012; 161:145-151.
CrossRef - Kirkham, M.B. Agricultural use of phosphorus in sewage sludge. Advances in Agronomy 1982; 35: 129-163.
CrossRef


