Introduction
At present a large amount of metals are introduced through human activities into the natural environment. Globally, heavy metal pollution is becoming a serious issue of increasing concern to the survival of living organisms, including fish. Cadmium, mercury and lead are toxic to most aquatic organisms even in low concentrations1 that produce histological alterations including liver damage,2 respiratory dysfunctions,3 testicular and ovarian alterations.4,5,6 From recent few years, lead has gained considerable attention among the scientific community due to its potential human health hazards.7 Popularity of lead is due to its dense ductile, highly malleable and corrosion resistant properties8 as it is used in manufacturing of storage batteries, paints, dyes, pigments, coloured inks, pipes, soldering materials, cement manufacturing, mining, smelting, etc.9 Histopathological studies are being widely used as reliable biomarkers for determining the health of the contaminants exposed fishes.10,11 These pathological studies indicated the damage of cell membranes which increases the injury to more critical targets.12 Lead is also known to accumulate in different tissues in fishes.13 Reproductive impact of lead exposure is spreading worldwide14 almost affecting all characteristic of reproduction.15 Therefore, the present study was carried out to investigate the testicular histopathological alterations in Channa striatus, induced by chronic exposure to lead nitrate.
Materials and Methods
Live specimen of fish, Channa striatus (average length 20-25 cm and weight 50-60 gms) were collected from different fish markets of Bhopal, Madhya Pradesh for experimentation. Fishes were divided into four groups as Group I, Group II, Group III and Group IV, and were exposed to sub lethal concentrations (0, 8, 18 and 28 mg/l, respectively) of lead nitrate (Ranbaxy India Ltd.) for a total period of 90 days. Exposure concentration was decided on the basis of 96 hrs LC50 value of lead nitrate. LC50 values at 95% confidence limits were calculated by using the software “Trimmed Spearman Karber method”, version-1.516 for different exposure period. To maintain apposite lead nitrate concentration throughout the experimentation duration of 90 days, water of each aquarium was changed on every alternate day. At regular interval of 30 days (at the end of 30th, 60th and 90th days of experimentation), fishes from each groups were harvested, and the testis of all the four groups were removed aseptically. Tissues were preserved in aqueous Bouin’s fixative for 48 to 72 hours.17 After fixation, 5-6 μm thick sections were cut with the help of rotatory microtome and stained with Ehrlich’s haematoxylin and eosin (H&E) for histopathological examination.
Results
Histological sections of the testis of the experimental fishes are shown in Fig. 1 to Fig. 10. Histological sections of testis of control fish (Group I) showed typical histological picture similar to that of a normal fish, characterised by a number of seminiferous tubules irregularly arranged inside and spermatozoa in the germinal epithelium (Fig. 1). In case of Group II fishes, damaged walls of seminiferous tubules and indistinguishable leydig’s cells on 30th day (Fig. 2), disorganization of seminiferous tubules, vacuolization and indistinguishable leydig’s cells on 60th day of lead nitrate exposure (Fig. 3), while disorganization of seminiferous tubules, deshaped seminiferous tubules, necrosis and centralization of spermatozoa on the 90th days of exposure (Fig. 4) were observed. Deshaped and damaged seminiferous tubules, clumping of seminiferous tubules, necrosis and indistinguishable leydig’s cells on 30th day (Fig. 5), while deshaped seminiferous tubules and necrosis on 60th day (Fig. 6) and damaged walls of seminiferous tubules, centralization of spermatozoa and disappearance in the number of leydig’s cells on the 90th day of lead nitrate exposure (Fig. 7) were observed in case of Group III fishes. In case of Group IV fishes, the common histoarchitectural alterations observed on the 30th day of lead nitrate exposure were clustering of seminiferous tubules, disorganization of seminiferous tubules, damaged walls of seminiferous tubules and centralization of spermatozoa (Fig. 8). The alterations were more severe as disorganization of seminiferous tubules, deshaped and damaged walls of seminiferous tubules, centralization of spermatozoa, severe reduction in the number of spermatozoa and indistinguishable leydig’s cells on the 60th and 90th days (Fig. 9 & 10, resp.) were observed.
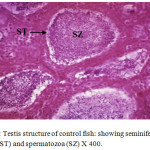 |
Figure 1: Testis structure of control fish: showing seminiferous tubules (ST) and spermatozoa (SZ) X 400. |
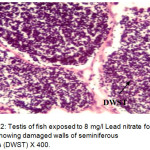 |
Figure 2: Testis of fish exposed to 8 mg/l Lead nitrate for 30 days showing damaged walls of seminiferous tubules (DWST) X 400. Click here to View Figure |
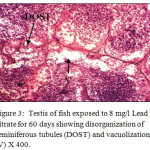 |
Figure 3: Testis of fish exposed to 8 mg/l Lead nitrate for 60 days showing disorganization of seminiferous tubules (DOST) and vacuolization (V) X 400. |
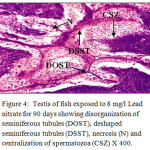 |
Figure 4: Testis of fish exposed to 8 mg/l Lead nitrate for 90 days showing disorganization of seminiferous tubules (DOST), deshaped seminiferous tubules (DSST), necrosis (N) and centralization of spermatozoa (CSZ) X 400. Click here to View Figure |
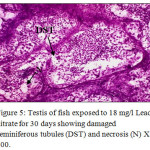 |
Figure 5: Testis of fish exposed to 18 mg/l Lead nitrate for 30 days showing damaged seminiferous tubules (DST) and necrosis (N) X 400. Click here to View Figure |
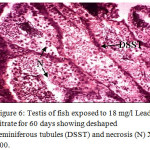 |
Figure 6: Testis of fish exposed to 18 mg/l Lead nitrate for 60 days showing deshaped seminiferous tubules (DSST) and necrosis (N) X 400. Click here to View Figure |
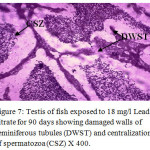 |
Figure 7: Testis of fish exposed to 18 mg/l Lead nitrate for 90 days showing damaged walls of seminiferous tubules (DWST) and centralization of spermatozoa (CSZ) X 400. Click here to View Figure |
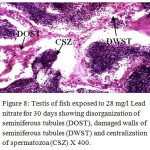 |
Figure 8: Testis of fish exposed to 28 mg/l Lead nitrate for 30 days showing disorganization of seminiferous tubules (DOST), damaged walls of seminiferous tubules (DWST) and centralization of spermatozoa (CSZ) X 400. Click here to View Figure |
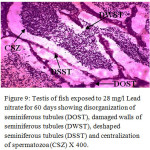 |
Figure 9: Testis of fish exposed to 28 mg/l Lead nitrate for 60 days showing disorganization of seminiferous tubules (DOST), damaged walls of seminiferous tubules (DWST), deshaped seminiferous tubules (DSST) and centralization of spermatozoa (CSZ) X 400. |
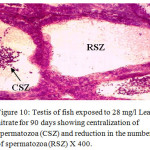 |
Figure 10: Testis of fish exposed to 28 mg/l Lead nitrate for 90 days showing centralization of spermatozoa (CSZ) and reduction in the number of spermatozoa (RSZ) X 400. |
Discussion
Similar results were seen by18 in case of Lepomis macrochirus exposed to endosulfan for 24, 48, 72, 96 hr, 1 and 2 week periods. The histopathological alterations in testes of fish showed connective tissue breakage, dilation of lumens, reduction in the size of primary spermatogonia, breakage of primary spermatocyte walls, greater increase in the size of seminiferous tubules and lumens, damaged sertoli cells and spermatids, damaged primary spermatogonia, decrease in the number of spermatozoa and spermatids, disruption of seminiferous tubule walls and disorganised structure of the testis. Cytolysis, vacuolation in seminiferous tubules, disorganized testicular architecture and extensive damage to germinal epithelium, necrotic cells and connective tissues in the testis of air-breathing fish, Heteropneustes fossilis due to the exposure of cadmium chloride for 45 days was also reported by.19 Loosening and indistinguishable arrangement of tissue, disorganization of the lobules, disintegration of the spermatogonia and interstitial tissue, epithelial vacuolization, disorganization of germ cells, reduction in the number of spermatogonia in the testis of African catfish Clarias gariepinus exposed to 0.05 mg/l of mercuric chloride for 60 days was observed by.20 It is construed from our study that the testicular histopathological alterations increased in proportion to the increase of lead nitrate concentration and length of period of intoxication in Channa striatus.
Conclusion
From the present study it is concluded that the extent of histological changes in the testis of Channa striatus not only depends on the concentration of lead nitrate but also on the duration of exposure.
Acknowledgements
The authors are thankful to Head, Department of Zoology and Applied Aquaculture, Barkatullah University, Bhopal (M.P) India, for providing necessary laboratory facilities to carry out the present work successfully.
References
- Abdul-Hassan J. K., Hashim E. S. Histological changes induced by lead nitrates in the gills of grass carp. Ctenopharyngodon idelia (Val.) juveniles. Bas J Vet Res. 2009; 8(1): 1-10.
- Shaikh Z. A., Vu T. T., Zaman K. Oxidative stress as a mechanism of chronic cadmium induced hepatotoxicity and renal toxicity and protection by antioxidants. Toxicol Appl Pharmacol. 1999; 154(3): 256-263.
CrossRef - Watrin A., Mayer-Gostan N. Simultaneous recognition of ionocytes and mucous cells in the gill epithelium of turbot and in the rat stomach. J Exp Zool. 1996; 276(2): 95-101.
CrossRef - Haubruge E., Petit F., Gage M. J. G. Reduced sperm counts in guppies (Poecilia reticulata) following exposure to low levels of tributyltin and bisphenol A. Proc Biol Sci. 2000; 267(1459): 2333-2337.
CrossRef - Jones M. M., Xu C., Ladd P. A. Selenite suppression of cadmium-induced testicular apoptosis. Toxicol. 1997; 116(1-3): 169-175.
CrossRef - Bayley M., Junge M., Baatrup E. Exposure of juvenile guppies to three antiandrogens causes demasculinization and a reduced sperm count in adult males. Aquat Toxicol. 2002; 56(4): 227-239.
CrossRef - Paul N., Chakraborty S., Sengupta M. Lead toxicity on non-specific immune mechanisms of freshwater fish Channa punctatus. Aquat Toxicol. 2014; 152:105-112.
CrossRef - Sharma S., Singh B. Effects of acute and chronic lead exposure on kidney lipid peroxidation and antioxidant enzyme activities in BALB-C mice (Mus musculus). Int J Sci Res. 2012; 3(9): 1564-1567.
- W. H. O. Inorganic Lead. International programme on chemical safety, Environmental health criteria. 1995; 165.
- Thophon S., Kruatrachue M., Upathan E. S., Pokethitiyook P., Sahaphong S., Jarikhuan S. Histopathological alterations of white sea bass, Lates calcarifer in acute and subchronic cadmium exposure. Environ Pollut. 2003; 121(3): 307-320.
CrossRef - Dar B. A., Jha G. N. Protective effects of dietary Spirulina against Cadmium chloride exposed histoarchitectural changes in the kidney of a freshwater catfish, Clarias batrachus. J Inland Fish Soc India. 2013; 45(2):1-7.
- Bagchi D., Joshi S. S., Bagchi M., Balmoori J., Benner E. J., Kuszynski C. A., Stohs S. J. Cadmium and chromium-induced oxidative stress, DNA damage, and apoptotic cell death in cultured human chronic myelogenous leukemic K562 cells, promyelocytic leukemic HL-60 cells, and normal human peripheral blood mononuclear cells. J Biochem Mol Toxicol. 2000; 14(1): 33-41.
CrossRef - Nussey G., van Vuren J. H. J., du Preez H. H. Bioaccumulation of chromium, manganese, nickel and lead in the tissues of the moggel, Labeo umbratus (Cyprinidae), from Witbank Dam, Mpumalanga. Water Science. 2000; 26:269-284.
- Patrick L. Lead toxicity part II: The role of free radical damage and the use of antioxidants in the pathology and treatment of lead toxicity. Altern Med Rev. 2006; 11:114-127.
- Zheng W., Aschner M., Ghersi-Egea J. F. Brain barrier systems: A new frontier in metal neurotoxicological research. Toxicol Applied Pharmacol. 2003; 192:1-11.
CrossRef - Hamilton M. A., Russo R. C., Thurston R.V. Trimmed Spearman-Karber method for estimating median lethal concentration in toxicity bioassays. Environ Sci Technol. 1977; 11(7):714-719.
CrossRef - Luna L. G. Histopathological methods and color atlas of special stains and tissue artifacts. Gaithersburg, MD: American Histolabs, Inc. 1992.
- Dutta H. M., Misquitta D., Khan S. Effects of endosulfan on the testis of blue gill fish, Leponis macrochirus. Arch Environ Contam Toxicol. 2006; 51(1):149-156.
CrossRef - Sharma S., Vyas V., Tamot S., Manhor S. Histological changes in the testis of air-breathing fish, Heteropnuestes fossilis (Bloch) following Cadmium chloride exposure. Int J Chem Biol Phys Sci. 2013; 3(2):1216-1221.
- Kumar R., Saroch J. D., Shrivastav R., Qureshi T. A., Manohar S. Effect of Spirulina on the histology of testes of mercuric chloride effected cat fish, Clarias gariepinus. Int J Green Herb Chem. 2013; 2(1):154-161.

