Introduction
Pectinase enzyme is one of the important enzymes that find its application in food processing industries.23,24 A variety of pectin in plant material can be degraded by pectinolytic enzyme systems. The breakdown of citrus pectin is brought about by different enzymes, that include pectin methylesterase (EC. 3.1.11.1), endo-polygalacturonase (EC. 3.2.1.15), exopolygalacturonase (EC. 3.2.1.67), pectate lyase (EC. 4.2.2.2) exo-pectate lyase (EC. 4.2.2.9) and endopectin lyase (4.2.2.10).2,7,28,29
The enzymes that break down pectin have gained enormous importance both for industry as well as for commertial purposes.17 Almost 5% of the global enzyme production and sales is shared by pectinases.8 These enzymes are applied in juice processing (extraction and clarification), alcoholic beverage processing, vegetable oil extraction and other food industries.9,23 The biosynthesis of pectinase was studied using different fulgal strains like A.foetidus, A.Niger,30 A.awamori,4 Rhizopus, Trichoderma, Penicillium and Fusarium spp.2,26
Mangrove forests are known to be very rich in organic matter and nutrients. Diversified microorganisms are found in mangrove environment. Microbial isolates from mangrove environments play pivotal role in biodegradation of mangrove plant materials by the microbial pectinase enzymes.31 Polygalacturonase is one of the commercial enzyme preparations which are in use by the food industry. Specifically derived enzymes include pectin esterase and Pectate lyase, which are of fungal strains like Mucor and Aspergillus.27 Various types of enzymes are produced by Aspergillus species, degrade pectin.9 Fermented foods and other food products, derived from Aspergillus are safe and are recognized as GRAS (generally recognized as safe), by regulatory authorities. They include Aspergillus sojae and Aspergillus oryzae.2,9,11
The factors that influence the production of microbial enzymes are incubation time, temperature, pH, and some nutritional sources like carbon and nitrogen. The composition and concentrations of Carbon and Nitrogen sources are of special interest to the scientists and researchers for the design of low-cost media. Growth medium design can decrease the production cost effectively. This shows that optimization of the growth medium plays a vital role in the cost effective production of enzymes.14 Physical parameter, such as incubation time, temperature and pH plays an important role in pectinase production. Apart from these, nutritional parameters complement the production of pectinase.22,13,9 About 10% of the global production of enzymes is occupied by pectinase.25,8 Citrus peel was used as the raw material for the pectinase production from long time, as it contains 24% of pectin.8
The objective of this study is to optimize nutritional compositions (substrate, carbon and nitrogen sources) and Physical conditions like (temperature, pH, and incubation time) for Aspergillus oryzae RR103 producing pectinase under optimum conditions.
Materials and Methods
Isolation and Screening of Fungi for Pectinolytic Activity
Fungal strains were isolated from mangrove soils of Gilakaladhindi and Malakayalanka of Machillipatnam, Krishna district, Andhra Pradesh, India. Czapek-dox agar Medium was used for isolation of fungi. Potent producers of polygalacturonase enzyme were screened by using modified pectin agar medium. The composition of modified pectin agar medium is Citrus Pectin-10g, Di-ammonium orthophosphate-3g, K2HPO4– 2.0, MgSO4-0.1g, Agar-20g, Distilled water-1000ml, pH-5.5.To this medium composition, streptomycin (100mg/ml) was added to restrict bacterial growth.
Screening and Identification of Fungal Isolate For Pectinolytic Activity
Pectin agar medium was used for the growth of isolated fungal strains. They were incubated for a week at room temperature. After incubation, flooded with iodine-potassium iodide solution (Iodine-1.0g, potassium-Iodide-5.0g in 330ml distilled water) on the culture plates and the plates were observed for zone of hydrolysis. One of the positive cultures was selected from these isolates after screening and it was identified as Aspergillus oryzae RR103 by 18SrRNA sequencing and the sequence was deposited in Gene bank with the accession number KU 613361.
Production of Pectinase Enzyme
The enzyme production was studied for 10 days. The sample from each flask was harvested at regular intervals (24hrs) and filtered. The clear extract was qualitatively analysed by standard procedure for polygalacturonase activity.18
Optimization Of Physico-Chemical Parameters
Effect of temperature, pH, substrate concentration, NaCl concentration, Carbon and Nitrogen sources were studied by standard methods.
The Effect Of Temperature On Enzyme Activity
Pectin broth was prepared by adding citrus pectin as substrate and sterilized in autoclave at 1210C temperature and 15 lbs pressure. The fungal culture was plated onto the pectin agar medium plate and after the sufficient growth of the fungal mat; pieces of solid agar medium with fungal culture were taken using 6 mm borer and were inoculated in the sterilized broth flasks in aseptic conditions. Then the inoculated broth flasks were placed in incubators maintaining different temperatures ranging from 25oC to 45oC for ten days. After incubation, the culture broth was filtered with Whatmann number 1 filter paper. The filtrates were assayed for polygalacturonase activity.
The Effect Of Ph On Enzyme Activity
Pectin broth was prepared with different pH concentrations ranging from pH 4 to pH 9. After incubation the culture broth was filtered and was assayed for polygalacturonase activity.
The Effect Of Nacl Concentrations On Enzyme Activity
Effect of salinity on growth and production of enzyme was also studied. Different salt concentrations of pectin broths were prepared ranging from 1% to 3%, culture was inoculated, incubated and assayed for polygalacturonase activity.
The Effect Of Substrate Concentrations On Enzyme Activity
Pectin broth was prepared with the substrate (citrus pectin). Different substrate concentration ranges from 0.5g to 2.5g. After incubation, culture filtrate was assayed.
The Effect Of Various Carbon Sources On Enzyme Activity
Monosaccharide, disaccharide and Polysaccharide carbon sources were used to optimise the growth and production of polygalacturonase enzyme. Glucose, Fructose and Arabinose were the simple sugars used. Disaccharides used were Sucrose, Lactose and Maltose. Moreover, Cellulose and Starch were the polysaccharides used. For each type of sugar used, 1% of sugar was added to the Pectin broth medium. Flasks were incubated and then filtered for further Polygalacturonase enzyme assay.
The Effect Of Various Nitrogen Sources On Enzyme Activity
Effect of different organic inorganic nitrogen and amino acids sources was studied. Organic nitrogen sources include Urea, Tryptone, Malt extract, Yeast extract NaNo3 and Peptone. Apart from these, effect of some essential amino acids was also studied, which include Cystine and Glycine. The effect of Sodium Nitrate as inorganic nitrogen source was also studied. 1 % of different nitrogen source was added to flasks containing Pectin broth medium. After the incubation period, the spent broth was filtered and used for Polygalacturonase enzyme assay.
Data were analyzed statistically by ANOVA method. P-values below 0.05 and were regarded as statistically highly significant.
Results & Discussion
The fungi were isolated from mangrove soil and screened for pectinase activity. The production of pectinase is regulated by physico-chemical and nutritional factors.20 Microbes are the best source of enzymes, useful for human needs. Microbial synthesis of enzymes is affected mainly by concentration of the substrate, days of incubation, temperature, pH, NaCl concentration, Carbon and Nitrogen sources.10 The present study focuses on the impact of various physical and chemical parameters for enzyme production. Fungal isolate which has shown maximum enzyme activity was sent to MACROGEN, SOUTH KOREA for 18S rRNA sequencing and was identified and the sequence was deposited in (NCBI) gene bank. The fungal strain is identified and the accession number is KU 613361 (Aspergillus oryzae RR 103).
Effect of incubation time
In this experiment, enzyme activity was gradually increased and maximum pectinase activity was observed after 192 hrs of incubation. The enzyme activity was almost constant up to 216 hrs and thereafter, the activity decrease slightly. Similar reports were observed in Aspergillus niger5 and Rhizoctonia solani Kuhn (AG2-2).1
Effect of Temperature
The experiments were run at various temperatures between 25 and 45oC. 35oC temperature was the most suitable temperature for the growth and production of polygalacturonase activity. 30oC was the favourable temperature for the growth and production of pectinase by Aspergillus niger.3 In the room temperature it produced 1.17 IU/ml and the enzyme production was increased with temperature. Maximum of the enzyme production 2.04 IU/ml was observed at 35oC. Any increase in temperature above 40oC affects metabolic activities of the microorganism which results in reduced growth and enzyme production. The results obtained in the present investigation are in line of strong agreement with the data reported earlier by Pedrolli et al.,3,21,23 Temperature at 45oC the growth and enzyme production was inhibited.
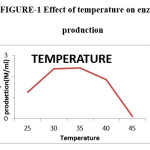 |
Figure 1: Effect of temperature on enzyme production. |
Substrate Concentration (Citrus Pectin)
In the present work, citrus pectin was used as substrate. Different substrate concentrations were studied which vary from 0.5% to 2.0%. The maximum (1.6 IU/ml) enzyme production was observed at 1% of citrus pectin. Slight decrease in the enzyme production was observed till the concentration reached 2%. Citrus pectin concentration result was similar with the reports published by Padmavathi and Raghu ram (2016).19
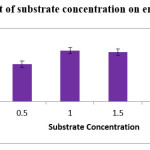 |
Figure 2: Effect of substrate concentration on enzyme production. |
Effect of pH
The optimum pH was studied by using varied pH conditions in different flasks, ranging from pH 4-9 for the production of pectinase by Aspergillus oryzae RR103. The enzyme activity of Aspergillus oryzae RR103 begins at pH 4 and slightly increased at pH 5. The maximum enzyme production (2.07IU/ml) at pH 6 finally stable at pH 6.5. The production of enzyme began to decrease from pH 7 and there was negligible activity at pH 9. The pH conditions needed for enzyme production by Aspergillus terreus strain GA2 and Aspergillus niger strain CFR 1105.16,17
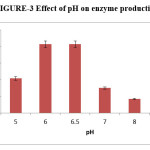 |
Figure 3: Effect of pH on enzyme production. Click here to View figure |
NaCl Concentration
Effect of salinity on growth and production of enzyme was experimented. Different concentrations of NaCl studied were, ranging from 0.5% to3.0%. Gradual increase was observed in enzyme production with increase of NaCl concentration from 0.5% to 2.0%. Thus, enzyme production and growth of the isolate was influenced with the NaCl concentration. Finally it is noticed that 2% NaCl concentration was the suitable for the growth and enzyme production.
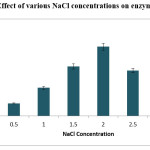 |
Figure 4: 4 Effect of various NaCl concentrations on enzyme production. Click here to View figure |
Effect of Various Carbon Sources
The effects of glucose and sucrose on the production of pectinase have also been previously reported by Patil and Dayanand (2006).20 Maximum (2.22IU/ml) enzyme production was observed when cellulose is used as a carbon source. Very little difference was noticed in starch and sucrose has carbon sources. 1% of cellulose was effective and thus the carbon source for polygalacturonase production was optimised. Naidu and Panda in (1998) reported that high concentrations of carbon may inhibit the process of enzyme synthesis.
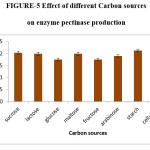 |
Figure 5: Effect of different Carbon sources on enzyme pectinase production. Click here to View figure |
Effect of various nitrogen sources for pectinase production
The source of nitrogen in growth medium has great significance in fungal growth and enzyme production.12 The present study shows the optimal nitrogen source for the highest production of pectinase as malt extract. The maximum enzyme production 2.03 IU/ml was observed when malt extract was added to the pectin agar medium as additional nitrogen source. Pectinases production was also enhanced by the addition of nitrogen sources like yeast extract, peptone and ammonium chloride was inhibited by Glycine.5,6,15
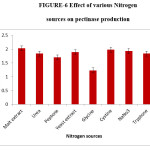 |
Figure 6: Effect of various Nitrogen sources on pectinase production. Click here to View figure |
Acknowledgements
Author would like to acknowledge the support and facilities provided by the department of Botany and Microbiology, Acharya Nagarjuna University and also would like to thank UGC- RGNF for providing financial support to carry out this work.
References
- Al-Rajhi, A.M. Purification and Characterization of an Extracellular Poly-Galacturonase from Rhizoctonia solani Kühn (AG2-2). World Applied Sciences Journal. 2013;21(4):476-484.
- Anand, G., Yadav, S. and Yadav, D. Production, purification and biochemical characterization of an exo-polygalacturonase from Aspergillus niger MTCC 478 suitable for clarification of orange juice. 3 Biotech. 2017;7(2):122.
- Batool, S., Asad, M.J., Mahmood, S.S.N.R.T., Guffar, A., Gulfraz, M. and Hadri, S.H. Production and partial purification of pectin lyase by Aspergillus niger grown on orange peels. African Journal of Microbiology Research. 2013;7(13):1144-1149.
- Blandino, A., Iqbalsyah, T., Pandiella, S., Cantero, D. and Webb, C. Polygalacturonase production by Aspergillus awamori on wheat in solid-state fermentation. Applied Microbiology and Biotechnology. 2002;58(2):164.
CrossRef - Darah, I., Nor-hawani, S., Hong, L., Rosma, A. and Haritharan, W. Pomelo peels as alternative substrate for extracellular pectinase production by Aspergillus niger HFM-8. Malaysian Journal of Microbiology. 2013;9(4):308-316.
- Fagerquist, C.K., Miller, W.G., Harden, L.A., Bates, A.H., Vensel, W.H., Wang, G. and Mandrell, R.E. Genomic and proteomic identification of a DNA-binding protein used in the “fingerprinting” of Campylobacter species and strains by MALDI-TOF-MS protein biomarker analysis. Analytical chemistry. 2005;77(15):4897-4907.
CrossRef - Gummadi, S.N. and Panda, T. Purification and biochemical properties of microbial pectinases—a review. Process biochemistry. 2003;38(7):987-996.
CrossRef - Heerd, D., Diercks-Horn, S. and Fernández-Lahore, M. Efficient polygalacturonase production from agricultural and agro-industrial residues by solid-state culture of Aspergillus sojae under optimized conditions. SpringerPlus. 2014;3(1):742.
CrossRef - Hoa, B.T. and Hung, P.V. Optimization of nutritional composition and fermentation conditions for cellulase and pectinase production by Aspergillus oryzae using response surface methodology. International Food Research Journal. 2013;20(6):3269-3274.
- Jacob, N. and Prema, P. Novel process for the simultaneous extraction and degumming of banana fibers under solid-state cultivation. Brazilian Journal of Microbiology. 2008;39(1):115-121.
CrossRef - Jørgensen, T.R. Identification and toxigenic potential of the industrially important fungi, Aspergillus oryzae and Aspergillus sojae. Journal of food protection. 2007;70(12):2916-2934.
CrossRef - Juwon, A.D. and Emmanuel, O.F., 2012. Experimental investigations on the effects of carbon and nitrogen sources on concomitant amylase and polygalacturonase production by Trichoderma viride BITRS-1001 in submerged fermentation. Biotechnology research international. 2012.
CrossRef - Kathiresan, K. and Manivannan, S. Cellulase production by Penicillium fellutanum isolated from coastal mangrove rhizosphere soil. Research Journal of Microbiology. 2010;5(11):1160-1164.
- Kaur, H.P. and Kaur, G. OPTIMIZATION OF CULTURAL CONDITIONS FOR PECTINASE PRODUCED BY FRUIT SPOILAGE FUNGI. International journal of advances in research. 2014;3(4):851-859.
- Koser, S., Anwar, Z., Iqbal, Z., Anjum, A., Aqil, T., Mehmood, S. and Irshad, M. Utilization of Aspergillus oryzae to produce pectin lyase from various agro-industrial residues. Journal of Radiation Research and Applied Sciences. 2014;7(3):327-332.
CrossRef - Kumar, C.G., Kamle, A., Mongolla, P. and Joseph, J. Parametric optimization of feruloyl esterase production from Aspergillus terreus strain GA2 isolated from tropical agro-ecosystems cultivating sweet sorghum. Journal of microbiology and biotechnology. 2011;21(9):947-953.
CrossRef - Ma, Y., Sun, S., Hao, H. and Xu, C. Production, purification and characterization of an exo-polygalacturonase from Penicillium janthinellum sw09. Anais da Academia Brasileira de Ciências. 2016;88:479-487.
CrossRef - Miller GL Anal.Chem. 1959;31:426-428.
CrossRef - Padmavathi, A. and Ram, M.R., STUDIES ON PECTINOLYTIC BACTERIA USEFUL IN FRUIT JUICE INDUSTRY. 2016;4(6):75-82.
- Patil, S.R. and Dayanand, A., 2006. Exploration of Regional Agrowastes for the Production of Pectinase by Aspergillus niger. Food Technology & Biotechnology. 44(2).
- Pedrolli, D.B. and Carmona, E.C. Pectin lyase from Aspergillus giganteus: comparative study of productivity of submerged fermentation on citrus pectin and orange waste. Applied biochemistry and microbiology. 2009;45(6):610.
CrossRef - Phutela, U., Dhuna, V., Sandhu, S. and Chadha, B.S. Pectinase and polygalacturonase production by a thermophilic Aspergillus fumigatus isolated from decomposting orange peels. Brazilian Journal of Microbiology. 2005;36(1):63-69.
CrossRef - Pinheiro, V.E., Desagiacomo, C.C.V., Michelin, M., Maller, A., Monteiro, L.M.O., Jorge, J.A. and Polizeli, M.D.L.T.D. Neosartorya glabra polygalacturonase produced from fruit peels as inducers has the potential for application in passion fruit and apple juices. Brazilian Journal of Food Technology. 2017;20:1-11.
CrossRef - Ruiz, H.A., Rodríguez-Jasso, R.M., Rodríguez, R., Contreras-Esquivel, J.C. and Aguilar, C.N. Pectinase production from lemon peel pomace as support and carbon source in solid-state fermentation column-tray bioreactor. Biochemical engineering journal. 2012;65:90-95.
CrossRef - Semenova, M.V., Sinitsyna, O.A., Morozova, V.V., Fedorova, E.A., Gusakov, A.V., Okunev, O.N., Sokolova, L.M., Koshelev, A.V., Bubnova, T.V., Vinetskii, Y.P. and Sinitsyn, A.P. Use of a preparation from fungal pectin lyase in the food industry. Applied Biochemistry and Microbiology. 2006;42(6):598-602.
CrossRef - Sethi, B.K., Nanda, P.K. and Sahoo, S. Enhanced production of pectinase by Aspergillusterreus NCFT 4269.10 using banana peels as substrate. 3 Biotech. 2016;6(1):36.
- Siddiqui, M.A., Pande, V. and Arif, M. Production, purification, and characterization of polygalacturonase from Rhizomucor pusillus isolated from decomposting orange peels. Enzyme research. 2012.
CrossRef - Siddiqui, M.A., Veena, P. and Arif, M. Polygalacturonase production from Rhizomucor pusillus isolated from fruit markets of Uttar Pradesh. African Journal of Microbiology Research. 2013;7(3):252-259.
- Soares, M.M.C.N., Da Silva, R., Carmona, E.C. and Gomes, E. Pectinolytic enzyme production by Bacillus species and their potential application on juice extraction. World Journal of Microbiology and Biotechnology. 2001;17(1):79-82.
CrossRef - Taragano, V., Sanchez, V.E. and Pilosof, A.M.R. Combined effect of water activity depression and glucose addition on pectinases and protease production by Aspergillus niger. Biotechnology letters. 1997;19(3):233-236.
CrossRef - Velho, S. and DeSouza, J. Studies on pectinolytic fungi from the mangrove sediments. Mahasagar. 1982;15(3):167-173.


