Introduction
Agricultural practices depend on the various factors like rain fall, weather conditions, soil fertility and the presence of pests such as weeds. Weeds are unwanted plants that compete with crop plants and affect their quality and quantity.1,2 Moreover, weeds are also known to deteriorate soil, pollute water and cause disease in the nearby plants due to the release of poisonous chemicals. Modern agriculture practices for the control of weeds are largely on the use of chemical herbicides. However, synthetic herbicides are associated with toxic effects on plants, animals and human beings.3 Limitations of synthetic herbicides can be overcome by the development of bioherbicides. Therefore, efforts are being made to develop herbicides from natural products such as Essential Oils (EOs) from aromatic plants.4,5 Callistemon viminalis plant is a good option for EO extraction since it can survive in dry season and poor soil, and provides large biomas.6 Amaranthus viridis, Echinochloa crus-galli L. and Phalaris minor weeds are responsible for huge economic losses.7 Keeping the above facts in the mind, the experimental work was designed to assess the phytotoxic potential of C. viminalis EO against E. crus-galli L., P. minor and A. viridis for the exploration of its bio herbicidal nature.
Material and Methods
Study Material
About 700 g leaves of test plant i.e., C. viminalis were collected from the campus of Central University of Punjab, Bathinda (30.1700° N, 76.4500° E). Seeds of test weeds (E. crus-galli L., P. minor and A. viridis) were procured from Punjab Agriculture University Ludhiana, Punjab, India.
Extraction of EO
EO from leaves of C. viminalis was extracted for 3 hrs by steam distillation method using Clevenger’s apparatus and stored at 4°C until evaluation for chemical composition and biochemical studies.
Composition of EO
Chemical composition along with the identification of the major compounds present in the EOs was studied using Gas Chromatograph coupled with Mass Spectrophotometer (GC-MS) (Shimadzu QP 2010 Mass Spectrophotometer). The following conditions were applied during the GC-MS.
Detector: Flame Ionization Detector (FDI)
Column: Stabil Wax with 30×0.25 mm length and 0.25 µm film thickness
Carrier Gas: Helium
Oven temperature: 40°C (held isothermally for 4 min) and increased at a rate of 4°C per min up to 220°C (isothermally for 5 min).
Further analysis was done on mass spectrometer and linear velocity of 38.5 cm sec-1. The mass spectra were screened in the range of m/z 40-600 amu. The compounds identification was carried out by comparing Kovats / retention indices (RI) with reference to C7-C30 series of n-alkanes (Supelco, Bellefonte, PA, USA). Some compounds were searched using reference book.8
Phytotoxicity of EOs
Seeds of test weeds were imbibed in distilled water for 12 hrs and grown in Petri dishes (Ø=15 cm) lined with filter paper. EO (0.5, 1, 2, and 4 µl) was spread on the inner side of lid of Petri dish and sealed with Parafilm to avoid volatilization. A similar set up with distilled water served as control. The whole set up was kept for seven days in growth chamber under controlled conditions. After seven days, the germinated seedlings of control as well as treatments were assessed for several parameters such as percent germination, seedling length and dry weight.9 Chlorophyll content10,11 and cellular respiration were also determined.12
Assays for Anti-Oxidant Enzymes
Enzyme extraction
Tissue homogenized in phosphate buffer (0. 1 M) along with centrifugation for 15 minutes at 10,000 rpm was stored at 4°C for biochemical studies.
Antioxidant Enzyme Activities
Ascorbate peroxidase (APX; EC 1.11.1.11)
The reaction mixture required for the assessment of APX consisted of PO43- buffer (25 Mm, pH 7.0), EDTA (0.1 mM), ascorbate (0.25 mM), H2O2 (1 mM) and enzyme extract (0.2 ml). Decline in absorbance of reaction mixture was measured at 290 nm.13 Extinction coefficient of 2.8 mM-1cm-1 was used for measuring the enzyme activity. Amount of enzyme that was required to oxidize 1 mM ascorbate min-1 was defined as one enzyme unit.
Guaiacol peroxidase (GPX; EC 1.11.1.7)
Oxidation of guaiacol was used for measuring the activity of GPX.14 The reaction mixture required for the determination of GPX consisted of PO43- buffer (25Mm, pH 7.4), guaiacol (0.05%), H2O2 (1 mM), EDTA (0.1 mM) and enzyme extract (0.2 ml). Extinction coefficient of 26.6 mM-1cm was used for calculating the enzyme activity. Increase in absorbance of the reaction mixture upon the addition of enzyme extract was measured at 470nm and expressed as EU mg–1 protein. Amount of enzyme required to catalyze the oxidation of 1mM guaiacol min−1 was defined as one enzyme unit.
Calculation and statistics
Experiments were conducted in a completely randomized block design. Every experiment was repeated two times. For each treatment three replicates were maintained. Experimental data were subjected to one way ANOVA with Tukey’s test.
Results and Discussion
GCMS analysis showed that EO was composed of 22 compounds constituting nearly 99.90% of volatile oil. The average yield of EO was calculated 0.853±0.009% (w/w). Major compounds identified were 1, 8-cineole (64.5%), α-terpineol (19.7%), + (-)-limonene (4.7%), trans-geraniol (2.0%) and linalool (1.43%). The oil was comprised of monoterpene hydrocarbons (50%), oxygenated monoterpenes (33.33%), sesquiterpene hydrocarbons (11.55%) and oxygenated sesquiterpenes (5.5%). The similar composition and EOs yield was also reported by Srivastava et al.,6 plant sample collected from Indian sub-continent. Further Oyedeji et al.,15 and de Oliveira et al.,21 also reported 0.9% and 1.42 % of yields of EOs respectively from the plant collected from African subcontinent. The variations in the yield are mainly due to the changes in the geographical and climatic conditions.
C. viminalis EO was observed to be phytotoxic against test weeds as it was affecting physiological parameters like inhibition of germination, decrease in seedling growth and dry weight as shown in Table 1. Previously, 1, 8-cineol, α-terpineol, limonene, linalool and α- and β- pinene were also reported as major constituents of EOs.15 These constituents were also reported as potent phytotoxic compounds by previous researchers. Our finding on seed germination and other physiological parameters were having homology with the previous studies.16 Maximum effect on germination of A. viridis as compared to P.minor and E.crus galli L. was probably due to small sized seeds and smooth seed coat of A.viridis.17 Chlorophyll content and cellular respiration in seedlings of test weeds was reduced due to exposure of C. viminalis EO, showing that EO was affecting the energy metabolism of weed plants (Table 2). Reduction in chlorophyll content and cellular respiration in the treated seedlings was due to the alteration of leaf diffusibility, transpiration rate and stomatal aperture that leads to a change in the rate of photosynthesis.18 Decrease in cellular respiration of test plants was due to the oxidative damage of mitochondria that is responsible for ATP production. As a result energy supply gets hindered and plant growth gets retarted19 EO induced stress environment for the test plants that led to the enhancement in the activity of antioxidant enzymes so as to combat the stress. With an increase in the concentration of EO, there was a progressive elevation in the activities of APX and GPX. In case of E. cruss-galli L., APX activity elevated from 5-33 µmol min–1 g –1 f. wt and 4-28 µmol min–1 g –1 f. wt for roots and shoots, respectively and the GPX activity was found in the range of 2-6 µmol min–1 g –1 f. wt both in roots and shoots (Fig 1 and Fig 2). In case of A. viridis the activities of both the test enzymes were at their highest for 1-4 µl of EO treatment (Fig 1 and Fig 2). For P. minor, APX activity was reported to be 2-22 mol min–1 g –1 f. wt and 3-18 µmol min–1 g –1 f. wt. in roots and shoots, respectively, for 0.5-4 µl EO treatment. GPX activity was observed to be 2-12 µmol min–1 g –1 f. wt and 2-6 µmol min–1 g –1 f. wt. for 0.5-4 µl, both in roots and shoots, respectively (Fig 1 and 2). Up regulation of antioxidant enzymes activities was reported due to the generation of reactive oxygen species (ROS) in response to the EOs dose. Elevation in antioxidant enzyme activities suggested the induction of oxidative stress in tissues.20
Table 1: Effect of Essential oil from Callistemon viminalis on percent germination and Dry weight of E. crus-galli, P. minor and A. viridis.
| Volume of oil (µl) |
Effect of EOs on Percent Dry weight , Germination and Seedling length |
|||||||||||
|
E. crus-galli |
P. minor |
A. viridis |
||||||||||
| %Germination | % Dry weight | Seedling length (cm) | % Germination | % Dry weight | Seedling length in cm | % Germination | % Dry weight | SeedlingLength (cm) | ||||
| 0 | 100±0.024a | 100±0.056a | 20±0.018a | 100±0.001a | 100±0.021a | 19±0.012a | 100±0.001a | 100±0.021a | 12±0.066a | |||
| 0.5 | 81.37±0.054b | 74.44±0.019b | 15±0.053b | 77.77±0.023b | 72±0.034b | 12±0.024b | 69±0.054b | 72±0.034b | 8.6±0.039b | |||
| 1.5 | 79.41±0.045b | 62.22±0.046c | 8.6±0.042c | 69.44±0.023c | 65.55±0.023c | 8.2±0.03c | 54.55±0.021b | 65.55±0.023c | 6.3±0.013c | |||
| 2.5 | 68.74±0.020c | 56.22±0.067d | 3.6±0.028d | 61.11±0.033d | 51.11±0.022d | 5.1.±0.042d | 44.31±0.089b | 51.11±0.022d | 2.3.±0.030d | |||
| 5 | 44.98±0.063d | 37.77±0.034e | 2.1.±0.052e | 46.66±0.067e | 38.88±0.076e | 1.8.±0.052e | 17.24±0.027c | 38.88±0.017e | 1.2.±0.023e | |||
Data is represented as mean ± Standard Error, a-e: In a column, means followed by same alphabet represents significant difference according to one-way ANOVA followed by post hoc Tukey’s test at P>0.05.
Table 2: Effect of EOs on Chlorophyll and Percent Cellular Respiration
|
Table 2: Effect of EOs on Chlorophyll and Percent Cellular Respiration |
||||||
| Volume of EOs (µl) | Effect of EOs on Percent Chlorophyll Content and Cellular Respiration | |||||
|
E. crus-galli L. |
P. minor |
A. viridis |
||||
|
% Chlorophyll |
% Respiration |
% Chlorophyll |
% Respiration |
% Chlorophyll |
% Respiration |
|
|
0 |
100.00±0.014a |
100±0 a |
100 ±0.051e |
100±0.004a |
100 |
100±0.004a |
|
0.5 |
90.78±0.061b |
88.4±0.038 b |
64.34 ±0.034b |
79.41±0.045b |
68.97±0.019b |
47.51±0.047b |
|
1.5 |
55.94±0.021c |
46.8±0.049 c |
54.39 ±0.052c |
51.53±0.013b |
48.76±0.045c |
19.53±0.073b |
|
2.5 |
43.53±0.18d |
26.6±0.090 d |
41.23 ±0.057d |
37.77±0.034e |
36.15±0.017d |
7.77±0.034e |
|
5 |
34.33±0.062e |
14±0.021e |
39.45.±0.02e |
16.94±0.021c |
24.24±0.021e |
——- |
Data is represented as mean ± Standard Error, a-e: In a column, means followed by same alphabet represents significant difference according to one-way ANOVA followed by post hoc Tukey’s Test at P>0.05.
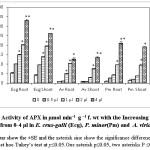 |
Figure 1: Activity of APX in µmol min–1 g –1 f. wt with the Increasing the amount EOs from 0-4 µl in E. crus-galli (Ecg), P. minor(Pm) and A. viridis(Av). The error bar show the ±SE and the asterisk sine show the significance difference on applying post hoc Tukey’s test at p≤0.05.One asterisk p≤0.05, two asterisks P ≤0.01. Click here to View figure |
 |
Figure 2: Activity of GPX in µmol min–1 g –1 f. wt with the Increasing the amount EOs from 0-4 µl in E. crus-galli( Ecg), P.minor(Pm) and A.viridis(Av). The error bar show the ±SE and the asterisk sine show the significance difference on applying post hoc Tukey’s test at p≤0.05.One asterisk p≤0.05, two asterisks P ≤0.01. Click here to View figure |
Conclusion
Experimental study led to the conclusion that C. viminalis EO could be employed as potential herbicide for weed management in agro ecosystems.
Acknowledgements
The authors are thankful to SERB (Grant no SR/FT/LS-66/2011), Department of Science and Technology, Government of India, for the financial assistance.
Conflicts of Interests
Authors declare no conflicts of interests.
Source of Funding
SERB (Grant no SR/FT/LS-66/2011), Department of Science and Technology, Government of India.
References
- Chauhan, B.S., Singh, R.G., and Mahajan, G. Ecology and management of weeds under conservation agriculture: A review. Crop Protection;38(SupplementC). 2012;57-65..
- Zimdahl, R.L. Fundamentals of weed science. Academic Press, 4th Edition. 2013;17.
- Zhao, S., Liu, J., Zhao, F., Liu, W., Li, N., Suo, Q., Zhao, J., and Zhao, L. Sub-acute exposure to the herbicide atrazine suppresses cell immune functions in adolescent mice. Bioscience trends. 2013;7(4):193-201.
CrossRef - Abad, M.J., Bedoya, L.M., Apaza, L., and Bermejo, P. The Artemisia L. genus: a review of bioactive essential oils. Molecules. 2012;17(3):2542-2566.
CrossRef - Jawahar, S., Deepika, A.L., Kalaiyarasan, C., and Suseendran, K. Herbicidal efficacy of eucalyptus oil on parthenium (Parthenium hysterophorus L.) control. Lifesciences Leaflets. 2013;3(3):79-88.
- Srivastava, S., Ahmad, A., Syamsunder, K., Aggarwal, K., and Khanuja, S. Essential oil composition of Callistemon viminalis leaves from India. Flavour and fragrance Journal. 2003;18(5):361-363.
CrossRef - Johnson, W.G., Bradley, P.R., Hart, S.E., Buesinger, M.L., and Massey, R.E. Efficacy and Economics of Weed Management in Glyphosate-Resistant Corn (Zea mays) L. Weed Technology. 2000;14(1):57-65..
CrossRef - Adams, R.P. Identification of essential oil components by gas chromatography/mass spectrometry. Allured publishing corporation. 2007.
- Azirak, S., and Karaman, S. Allelopathic effect of some essential oils and components on germination of weed species. Acta Agriculturae Scandinavica Section B-Soil and Plant Science. 2008;58(1):88-92.
CrossRef - Arnon, D.I. Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant physiology. 1949;24(1):1:.
CrossRef - Hiscox, J. T, and Israelstam, G. A method for the extraction of chlorophyll from leaf tissue without maceration. Canadian Journal of Botany. 1979;7(12):1332-1334.
CrossRef - Steponkus, P.L., and Lanphear, F. Refinement of the triphenyl tetrazolium chloride method of determining cold injury. Plant Physiology. 1967;2(10):1423-1426.
CrossRef - Nakano, Y., and Asada, K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant and cell physiology. 1981;22(5):867-880.
- Egley, G., Paul Jr, R., Vaughn, K., and Duke, S. Role of peroxidase in the development of water-impermeable seed coats in Sida spinosa L. Planta. 1983;157(3):224-232.
CrossRef - Oyedeji, O.O., Lawal, O.A., Shode, F.O., and Oyedeji, A.O.. Chemical composition and antibacterial activity of the essential oils of Callistemon citrinus and Callistemon viminalis from South Africa. Molecules. 2009;4(6):1990-1998.
CrossRef - Kaur, S., Singh, H.P., Mittal, S., Batish, D.R., and Kohli, R.K. Phytotoxic effects of volatile oil from Artemisia scoparia against weeds and its possible use as a bioherbicide. Industrial Crops and Products. 2010;2(1):54-61.
CrossRef - Kordali, S., Cakir, A., Ozer, H., Cakmakci, R., Kesdek, M., and Mete, E. Antifungal, phytotoxic and insecticidal properties of essential oil isolated from Turkish Origanum acutidens and its three components, carvacrol, thymol and p-cymene. Bioresource Technology. 2008;99(18):8788-8795.
CrossRef - Kaur, S., Singh, H. P., Batish, D. R., and Kohli, R. K.. Chemical characterization and allelopathic potential of volatile oil of Eucalyptus tereticornis against Amaranthus viridis. Journal of plant interactions. 2011;6(4):297-302.
CrossRef - Vaid, S., Batish, D. R., Singh, H. P., and Kohli, R. K.. Phytotoxic effect of eugenol towards two weedy species. The Bioscan. 2010;5(3):339-341.
- Ahuja, N., Singh, H.P., Batish, D.R., and Kohli, R.K. Eugenol-inhibited root growth in Avena fatua involves ROS-mediated oxidative damage. Pesticide Biochemistry and Physiology. 2015;118:64-70.
CrossRef - de Oliveira, C. M., das Graças Cardoso, M., da Silva Figueiredo, A. C., de Carvalho, M. L. M., de Miranda, C. A. S. F., Albuquerque, L. R. M., . . . de Andrade Santiago, J.. Chemical Composition and Allelopathic Activity of the Essential Oil from Callistemon viminalis (Myrtaceae) Blossoms on Lettuce (Lactuca sativa L.) Seedlings. American Journal of Plant Sciences. 2014;5:3551-3557..
CrossRef

