Introduction
Anthurium, a genus of the family Araceae, is indigenous to tropical America. It is popular cut flower in tropical and subtropical countries. Numerous Anthurium species are produced and traded internationally as cut-flowers, flowering potted plants and landscape plants. Obviously there is huge demand for quality planting material. The three basic propagation methods for Anthurium, propagation by seed, traditional vegetative and tissue culture are used for conventional production. However, as cross pollinating species, the off springs show poor uniformity. Besides poor germination and short viability span make seed propagation difficult. Vegetative propagation is slow and time consuming to achieve large scale production of propagules. Further, Anthurium plants grow slowly and would have little commercial potential without multiplication of elite cultivars through plant tissue culture. Micropropagation of Anthurium has been achieved through adventitious shoots formation from callus and direct shoot regeneration1,2,3,4,5 with various tissues including leaf, petiole, spadix, spathe, seed, lateral bud and shoot tips. However, it appears early callusing response, fast and high multiplication rate, fast shoot elongation and high survival rate of plantlets in hardening are factors to be improved through further research. In this view, in the present study, in vitro morphogenetic responses such as culture establishment, callusing response, callus proliferation and shoot multiplication rate been researched employing different explant type and stage/age of explants in elite Anthurium cv. Tropical Red, Acropolis, Sun Glow, Esmeralda, Chaco, Pistachio, Neveda and Safari.
Materials and Methods
Media preparation and sterilization
Media compositions for callus induction, proliferation, shoot bud induction, shoot elongation and rooting were standardized in preliminary studies conducted in our laboratory.
Callusing, proliferation and shoot initiation medium (A1)
MS salts [6], 100 mg/ L inositol, 0.4mg/ L Thymine Hcl, 30gm/ L sucrose, 0.2gm/L 2,4-D, 1mg/l BAP and pH adjusted to 5.8±0.05
Shoot elongation and rooting medium (A2)
MS salts, 100 mg/l inositol, 0.4mg/ L thymine HCL, 1mg/ L pyridoxine HCL, 1mg/ L calcium pentothenate, 1mg/ L Nicotinic acid, 1.5 mg/ L BAP, 0.3mg/l IAA and pH adjusted to 5.8±0.05.Medium was boiled with 6.5gm/ L agar, dispensed 30-35ml into pre sterilized 250ml glass culture bottles, autoclaved in steam sterilizer (Nat steel Pvt. Ltd., India) at 121oC and 18lbs for 18 minutes and stored in media storage room at ambient temperature for 7-10 days before usage.
Table 1: Effect of explants on culture establishment and callusing in Anthurium cultivars
|
Cultivar |
Leaf lamina with mid rib (LLMR) |
Leaf tip (LT) |
petiole with Leaf (PL) |
Candle |
petiole |
| Tropical Red |
Callusing |
NR/BC |
Callusing |
NR/BC |
NR/BC |
| Acropolis |
Callusing |
NR/BC |
Callusing |
NR/BC |
NR/BC |
| Sun Glow |
Callusing |
NR/BC |
Callusing |
NR/BC |
NR/BC |
| Esmeralda |
Callusing |
NR/BC |
Callusing |
NR/BC |
NR/BC |
| Chaco |
Callusing |
NR/BC |
Callusing |
NR/BC |
NR/BC |
| Pistachio |
Callusing |
NR/BC |
Callusing |
NR/BC |
NR/BC |
| Nevada |
Callusing |
NR/BC |
Callusing |
NR/BC |
NR/BC |
| Safari |
Callusing |
NR/BC |
Callusing |
NR/BC |
NR/BC |
| Callusing response (days) |
60-75 |
— |
90-100 |
— |
— |
|
Callusing response (%) |
40-50% |
— |
60-85% |
— |
|
|
Appearance of bacteria (days) |
35-40 (IVth) |
25-30 (IIIrd) |
35-40 (Vth) |
8-10 (Ist) |
15-20 (IInd) |
|
Explants shows bacteria (%) |
Upto 25%
|
Upto 100%
|
Upto 25%
|
Upto 100%
|
Upto 100%
|
Note: NR-no response; BC-Bacteria
Explants collection, sterilization and inoculation
Healthy mother plant of various cultivars free from pest and disease were selected, leaf, petiole and candles were excised, immediately stalks were dipped into sterile water and brought to laboratory. They were washed twice in sterile, soaked in solution containing Bavistin (200 mg/100 ml sterile water) and citrimide (50mg/100ml sterile water) with 2 drop soap oil, kept in shaker for one hour, water washes 3-4 times. Then were immersed in 2% sodium hypochlorite for four minutes, washed twice in sterile water at two minutes interval, taken to Laminar Air Flow chamber, changed into fresh sterile flask, treated with 0.01% mercuric chloride for five minutes, washed 4 times in sterile water at three minutes interval, dipped for 30 seconds in 70% ethanol and washed twice in sterile water at 2 minutes interval.
Experiments and incubation
Experiments on type of explants
Sterilized explants were excised into shoot tip (ST), petiole, petiole with leaf (PL) leaf lamina with mid rib (LLMR-0.5 cm both side) and candles (0.5 cm), inoculated into A1 medium and incubated in dark conditions at 25±1oC.
Experiments on stage of leaf
Leaves of various growth stage viz., stage 1 (Just opened leaf having brown color and very soft texture), stage 2 (Young leaf having light/pale green color with brown tinge and smooth texture) and stage 3 (Fully matured leaf having dark green color with rough texture) were collected from different cultivars, sterilized, excised, inoculated in A1 medium and incubated in dark at 25±1oC.
Table 2: Effect of growth stage of leaf on in vitro morphogenetic response in Anthurium cultivars
|
Cultivar |
Stage 1 leaf (Days) |
Stage 2 leaf (Days) |
Stage 3 leaf (Days) |
Stage1 leaf: Response (%) |
Stage2 leaf: Response (%) |
Callus Multiplication rate |
Shoot multipli- cation rate |
| Tropical Red |
90-100 |
30-35 |
60-75 |
40.29±12.32 |
87.50±8.65 |
1:5.0 |
1:4.2 |
| Acropolis |
90-100 |
40-45 |
60-65 |
24.28±8.34 |
53.33±5.43 |
1:2.9 |
1:2.5 |
| Sun Glow |
100-120 |
50-60 |
75-80 |
35.23±7.97 |
80.20±6.47 |
1:5.0 |
1:4.0 |
| Esmeralda |
90-100 |
40-45 |
60-65 |
53.34±10.36 |
66.66±6.72 |
1:4.0 |
1:3.0 |
| Chaco |
100-120 |
45-50 |
75-80 |
33.30±9.64 |
60.20±5.75 |
1:3.5 |
1:2.7 |
| Pistachio |
100-120 |
50-60 |
75-80 |
40.20±8.93 |
72.38±5.32 |
1:4.0 |
1:3.8 |
| Nevada |
100-120 |
40-45 |
75-90 |
35.26±10.43 |
59.63±6.31 |
1:4.5 |
1:3.6 |
| Safari |
100-120 |
40-45 |
75-90 |
30.30±9.12 |
55.97±6.12 |
1:4.9 |
1:3.3 |
| General Response |
Very late |
Early |
Late response |
Low |
High |
2.9-5.0 |
2.5-4.2 |
| SEM |
1.11 |
0.93 |
|||||
| CD |
3.27 |
2.87 |
Experiments on callus proliferation and shoot multiplication rate
Callus was separated from initiated cultures, five callus clumps of ~0.5 cm2 size has been inoculated per culture bottle containing A1 medium and incubated at 25±1oC in dark conditions for proliferation and induction of shoots. Shoots of >1.5 cm with a 2-3mm callus at base were separated, eight shoots were inoculated per culture bottle containing A2 medium and incubated in 16/8 hour light by incandescent tubes maintained at 25±1oC for elongation and rooting.
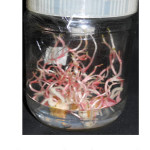 |
Figure 1: Morphogenetic responses in Anthurium cv. Tropical Red. |
Observations, data collection and analysis
Observations on callus induction and bacterial appearance were recorded after four weeks of inoculation till 10th week. Observations on shoot bud induction and elongation were recorded after 6 weeks of inoculation till 12th week. All experiments were repeated for four times with 5 replications. Percent callusing response and bacterial appearance were calculated. Callus proliferation and shoot multiplication rates were calculated (no. of bottles having 5 clumps of ~0.5 cm obtained from a bottle of culture). The results are presented as mean± standard errors.
Results and Discussion
Effect of explants, growth stage of explants and genotype on in vitro morphogenetic response has been studied in Anthurium cv. Tropical Red, Acropolis, Sun Glow, Esmeralda, Chaco, Pistachio, Neveda and Safari. Shoot tip (ST), petiole, petiole with leaf (PL) leaf lamina with mid rib (LLMR-0.5 cm both side) and candles were experimented for in vitro responses. 40-50% and 60-85% of LLMR and PL explants showed callusing response, respectively in 60-100 days in various cultivars (Table 1). Callusing was first observed along the cut ends of leaf lamina especially at the mid rib and vein region of explants. LT, petiole and candle explants did not show any kind of response. Foliar explants exhibit more potential for callus induction when they contained midrib or vein which was consistent with results reported earlier.4 Visual bacteria started appearing in explants and subsequently explants started turning into brown color. Petiole and candle explants were first to show bacteria, browning. All of them died in 15-20 days. Leaf tip explants initially turned yellow, were second to show bacteria and browning. All of them died in 25-30 days (Table 1). Unresponsive LLMR (up to 25%) and PL (up to 25%) explants also showed bacteria, browning and died, but less compared to other explants.
Callusing response was tested in explants of three growth stages of leaves. In vitro morphogenetic response in explants is expressed in accordance with favorable characteristics, achieved by selection of explants right growing stage characterized by color, texture, maturity etc. In general, among explants of three growth stages of leaves employed explants of stage- 2 leaves showed early (30-50 days) callusing response compared to stage -1 (90-120 days) and stage-3 (60-90 days) leaves in various cultivars (Table 2). In particular, cv. Tropical Red showed early callusing response (30-35 days) and Pistachio and Sun Glow (50-60 days) showed late response and callusing response in other varieties ranged between times taken by these two varieties using explants of growth stage-2 leaves. An earlier study reported that superior in vitro morphogenetic response from relatively older explants to younger one.4 Contrary to this, explants from folded brown leaves2 and newly expanded brown leaves exhibited better callusing response in Anthurium cultivars whereas explants from green leaves showed oxidation and turned necrotic and dead later.3,5,7 Further, time taken for callus induction was above 45 days in those studies [2][3][4][5][7]. However, in this study both younger (growth stage-1) and older (growth stage-3) explants showed delayed in vitro response compared to young (growth stage-2) explants.
Rate of callus response was tested in explants of growth stage-2 and stage-3. In general, the rate of callusing response from explants of stage-2 leaves was better (53-87%) which also showed good callus development compared to explants of growth stage-3 leaves (24-53%) in various cultivars. In particular, callusing rate was highest and lowest in cv. Tropical red (87.50±8.65) and cv. Acropolis (53.33±5.43) respectively and the same in other cultivars ranged between these two cultivars employing explants of growth stage-2 leaves. In earlier studies, callus induction rate was 52.9% using pale green leaves,4 81% newly expended brown leaves [5][3][7] and 67% folded brown leaves.2 In this study, rate of callusing response was in accordance with those studies in explants from growth stage-1, whereas the same was fast and high in explants of growth stage-2 leaves (young, pale green and smooth texture) in all cultivars. Hence, employing appropriate explants of right growth stage found to be of prime importance to obtain fast as well as high rate of callusing response.
Incubation of callus cultures for 15-20 days after 35 days (callus proliferation period), 8-10 shoots of 1.5-2.5 cm in height per clump has been recorded. Shoots were slender and etiolated and upon shifted into light, they started turning green. Similarly, the etiolated shoots produced in dark were transformed into green shoots when incubated under light.4 Eight shoots of >1.5 cm were inoculated per bottle containing A2 medium and incubated in light for further shoot elongation and rooting. Shoot multiplication rate was high in Anthurium cv. Tropical Red, and Sun Glow (>4 folds), medium in cv. Esmeralda, Pistachio, Chaco, Safari and Nevada (3-4 folds) and low in Acropolis (<3folds). Earlier, 8 shoots sprouts per explants4,7 and 12-15 shoots per explants5 has been reported. In this study, shoots grown up to 4-5.3 cm in height in 45-55 days (Figure1), simultaneously rooting appeared in third week of inoculation and rooted plantlets were ready in 65-80 days after inoculating into A2 medium in various cultivars. Incubation period for elongation and rooting appears as 2-3 weeks more compared to earlier studies.4,5,7 However, though plantlets were ready by 7-8 weeks after inoculation into A2 medium, extending incubation in same medium for another 3-4 weeks found to help in higher plantlet survival in hardening. Overall results shows genotype has also got strong impact on in vitro morphogenetic responses. Rooted plantlets were washed thoroughly in running tap water, hardened in 1:1 ratio soil rite and coco-peat mix in portrays, transferred to beds prepared with coconut coir-pith, 95% plants survived, appeared normal and flowered.
In conclusion, LLMR and PL explants and explants of young pale green leaves with smooth texture showed fast and high in vitro morphogenetic responses in all eight elite cultivars of Anthurium. These results would help in standardizing high throughput protocols for commercial production of quality planting material.
Acknowledgement
Author is grateful to Shri. H. S. Shivakumar, Additional Director of Horticulture, Shri. Krishnamoorthy, Joint Director of Horticulture (Biotechnology) and Chandrashekar, AHO, Hulimavu, PB 7648, BG Road, Bangalore-560 076, Department of Horticulture, Karnataka, where most of the work has been carried out. Thanks are also due to Mr. Y. C. and Shri. Umapathi and Shri. Jayanna, Managing Director of Meghana tissue culture Nursery, Davanagere for encouraging to carry out a part of work.
Callus obtained was creamy, compact, and slow growing and five callus clumps of ~0.5 cm2 size was subcultured per bottle containing A1 medium and incubated in dark for proliferation. About 3-5 fold increase in volume of calli clumps was achieved after 30-35 days of incubation in various cultivars. On proliferated calli, shoot buds originated as protuberances, which ranged from 30-60nos in various cultivars. Anthurium cv. Tropical Red, Sun Glow, Safari and Nevada showed fast and high callus proliferation rate (45-50 days and >4.5 folds; Table 2), cv. Esmeralda, Chaco, Pistachio showed fast and medium callus proliferation rate (45-50 days and <4.0 folds) and cv. Acropolis, showed slow and less callus proliferation rate (60 days and <3 folds). A previous study reported that 2-3 fold increase in volume of callus.4
References
- Hamidah M., Karim A.G.A. and Debergh P., Plant Cell Tis. Org. Cult. 1997;48:189.
CrossRef - Joseph M., Martin K. P., Mundasssery J. and Philip V.J., Ind. J. Exptl. Bio. 2003;41:154.
- Martin K. P., Joseph D., Madassery J., Philip V.J., In vitro Cell Devtl. Biol.- Plant. 2003;39:500.
CrossRef - Bejoy M., Sumitha V. R. and Anish N. P., Biotech. 2008;7:134.
CrossRef - Atak C. and Celik O., Pak J Bot. 2009;41:1155.
- Murashige T. and Skoog F., Physiol. Plantarum. 1962;15:473–497.
CrossRef - Viegas J., Rocha D.R.T.M., Moura F., De Rosa L.D., De Souza L.D., De Souza A.J.,
- Correa S.G.M. and De Silva T.A.J., Ornamental Biotech. 2007;1:61.
