Introduction
A goal of aquaculture is to produce a healthy fish to assure the maximum profit. Diseases and unregulated water quality management in the culture systems interferes this.17,92,103 Application of antibiotics for the treatment of bacterial diseases was encouraged in the past.118 However, aquaculture depends on antibiotics is now criticized due to the following reasons. An antibiotic kills both the good and bad microbes in the gut of the animals. Also, continual usages of antibiotics lead animals to become resistant to pathogenic bacteria which lower the treatment effect. As using antibiotics in the aquaculture is severely condemned farmers are now looking for alternative methods to replace the use of antibiotics in disease control.35,69
Basic resource needed for aquaculture is the water and land. In most of the places water and land available for fish culture is very less, thus farmers intend to go for intensive culture,29 but intensification results in deterioration of water quality which causes stress to the fish followed by disease outbreak.107 Some intensive production techniques such as re-circulatory aquaculture systems (RAS) are generated to solve these concerns; however, they are not economically beneficial to the farmers as using RAS is quite expensive. The generation of a cost efficient technology would overcome these problems.29
Biofloc is a technology followed in the fish and shrimp farming; its outstanding feature is that they contain the mixture of bacteria, algae and other detritus which would be available feed for the fishes of omnivorous feeding habits.11,29,39 A growing body of literature has recognized the positive influence of the biofloc technology on growth, non-specific immunity and disease prevention in fish.37,40,61,134,138 It also helps in the improvement of water quality in fish farming.29 On the other hand, probiotics are specific microbial strains; its beneficial roles are known as they favourably contain all the necessary functions as biofloc does.52 Despite biofloc and/or probiotics are adopted by farmers for the practical reasons discussed, an occurrence of certain diseases are still common in fish as they reflect in the form of lower survival rates at the farms.126 As diseases acquired by animals are often linked to specific bacteria, the action of specific antagonistic/beneficial bacteria would favorably minimize these problems.139 Therefore, it was hypothesized that addition of specific, known probiotic bacteria to the biofloc proliferate the bacterial population either in the water or animals gut in order to suppress the potentially harmful pathogenic strains.1,31,62,77,146 Based on the hypothesis, recent studies have been attempted in these area, and reports seems to suggest that addition of probiotics to the biofloc further improve the water quality, animal growth, immunity, and survival of the animals than those of biofoc alone does.1, 31,62,77,146 A study on the influences of exogenous probiotics on biofloc based aquaculture system is a novel and integrative approach; but very less explored. Considering the practical merits of the technology mentioned above, significant studies are required to be conducted in the area on commercially cultivable fish species for the economic and environmental friendly sustainable aquaculture.
Biofloc
What is Biofloc Technology?
In general, biofloc is the macro-aggregation of bacteria, algae, detritus and other decomposed components.11 According to Decamp and co-authors, it is the combination of bacteria, diatoms, zooplankton, protozoa, macro-algae, feces, uneaten feed, and exoskeleton from dead organisms.39 As said by Hargreaves and co-authors, it is a group of biotic and abiotic particulate components suspended in the water which includes bacteria, planktons, and other organic materials.63
Principle and Concept
The main principle of this technique is the practice of nutrient recycling.109 It is originated depends on the maintenance of carbon/ nitrogen supplementation to pond water.11 Initially researchers acquired the knowledge of carbon/ nitrogen for the production of heterotrophic bacteria, which in reverse they feed are for the fish and shrimp.8 A ratio of the carbon/ nitrogen (C/N) is managed to stimulate the growth of heterotrophic bacteria to produce microbial biomass.9 Supplemented carbon will help to hold the excreted ammonia from the animals,11 and by the proper inclusion of carbon and nitrogen to the system ammonia in the water will be altered into bacterial biomass.119
Reasons to Maintain C/N Ratio
The maintenance of C/N ratio is quite prerequisite for controlling of accumulating organic nitrogen and for the production of microbial communities in the water.45 The inorganic nitrogen is converted into organic nitrogen when C: N ratio is sufficient to produce bacterial cells; preferably 5:1.88 As carbohydrate is involved in the part of respiration process, during aerobic situations the condition of C: N ratio must be more than bacterial body compositions.46 It was found that around 10 mg NH4+-N/L can be completely absorbed when glucose was added as a substrate and when the maintenance of C/N ratio was 10:110. To minimize the artificial feed requirement, the practice of increasing C: N of higher than 10:1 by utilizing different low-cost carbon sources which are locally obtainable is common in biofloc waters.28 Apart from reducing the feed cost, utilization of biofloc components will also decrease the amount of protein in the feed.9,63 It was established that the accumulation of toxic inorganic components including, NH4+ and NO2– will be stopped in the water when the maintenance of C/N ratio is high in the biofloc system as the ammonium consumption by the microbial community will increase.11,84 Earlier findings in the literature attempted to see the production of microbial protein by varying levels of C: N ratio in the feed. The reports show that floc generation was high in the tanks received with low protein in the feed than that of high protein.13,14 Therefore, biofloc system may not need much protein supplementation and as such this technique can be used without increasing the protein content in the feed as demonstrated by earlier workers.9,63 Fontenot and co-authors studied the manipulating four levels of C: N ratios (5:1, 10:1, 20:1 and 30:1) for the removal of inorganic nitrogen from waste water from the shrimp pond. It was noticed that the maximum removal of inorganic nitrogen is contributed by C: N ratio of 10:1.50 Previous workers have also assessed the impact of C/N ratio for giant fresh water prawn in the periphyton-based aquaculture system; the ponds received with periphyton as a substrate had higher production of heterotrophic bacteria and prawn production.7
Different Sources of Carbon
It is suggested that economic use of carbon for biofloc technology depends on locally available industrial by-products. Reports say that cheapest carbon sources such as plant meals (tapioca, wheat, corn, rice, etc.), molasses and glycerol can be applied in the pond water to develop bioflocs.28 In addition to this, the mixture of different pelletized plant meals131 or low protein ingredients contains high C: N ratio is also be used to enrich the bioflocs.14,19 Although carbon sources act as a substrate for the microbial protein cell production, the mode of action differs between different carbon sources.9 Various authors were used different carbon source for the production of heterotrophic bacteria; molasses from solid fish waste,119 acetate, glycerol and glucose,31 tapioca flour,64,65 wheat flour.14 Previous study established that every one gram of carbohydrate, the carbon yield is 0.4 gram.89 Earlier reports also saying that 20g carbohydrate will be required for the immobilization of on gram of mineral nitrogen.9
Importance of Biofloc Technology in Aquaculture
Biofloc technology is reliable for the cost effective, environmental friendly fish production.29 It is a preferable technique for facing the economic, ecological and social issues relevant to current aquaculture.110 The system has an advantage in intensive farming practices.29 The practice of rearing aquatic animals in the biofloc was established in the many countries. Today, this technology is productively successful in the commercial shrimp farming to the countries including Asia, China, America, Italy, Brazil and South Korea.46 Besides this, most of the universities, colleges and research centers are currently working on biofloc technology to explore this fundamental technique thoroughly.46 An important feature of this technology is ammonia wastes are consumed by bacteria for their growth that increases the microbial biomass yield as well as improves the water quality.9,93 Besides these, the technology carries a lot of benefits from nursery rearing to the all stages of rearing animals including the augmentation in the growth and immunity of animals, water quality, less cost for feed, reduced water consumption (zero water exchange), supplying sufficient quality nutrients and lesser environmental damage to the culture system.9,21,32,71,78,93,102,129,142 These features attract the farmer communities to implement this technique in the farm.21,26
The following subheading covers the role of biofloc on different fields of aquaculture. Overview of some of the study conducted in fish with reference to biofloc based culture systems are listed in the Table 1.
Water Quality Management
Biofloc technology offers an ample advantage ensuring zero water exchange through minimal consumption of water and less water pollution.46 The elimination of pathogen entry is guaranteed by this technique as there is no re-entry of water is needed.10 Biofloc technology is applied for decreasing the effluent discharge, preventing risks from the disease outbreak, protecting the water free from pathogen entry; thus, ultimately improve the biosecurity at the farm level.22 Regarding the presence of microorganisms, biofloc play a major role in the management of water quality.84,91 In order to attain more growth, fish fed with a lot of feed. As aqua feeds are rich in protein that contain 65% of nitrogen content, it is considered that most of the uneaten feeds that present in the water damage the pond water and threaten the animals to disease susceptibility.51 Uneaten feed present in the water columns not only deteriorate the culture water, but it also unnecessarily involves with the wastage of money. Overfeeding is often common practice in the farming which increases the nitrogen content in the feed and when fish utilize this nitrogen through the feed it is excreted as ammonia which is toxic to the fish.51 It was demonstrated in the earlier findings that adopting biofloc technology would solve the problems concerned with ammonia toxicity as increasing consumption of nitrogen by heterotrophic bacteria rapidly increases the nitrification process, which ensures the reduction in the concentration of ammonium in the culture systems.63 The study also demonstrated that the production rate for heterotrophic bacteria for the utilization of ammonium is 10 times greater by heterotrophic bacteria as compared to that of nitrifying bacteria.63
Feeding, Growth and Metabolism
Generally it is considered that biofloc contributes significant amounts of nutrition to the farm animals.84,91 Previous authors indicating that the augmentation of animal growth is due to utilization of algae, bacteria which contains adequate nutrients that support the growth.46 It is known that aquaculture can’t be sustainable without supplementary feed as it relies on 50 to 60 % of artificial feed which is about 60% of the total operating cost. In order to reduce the feed costs, methods including the addition of live feeds are followed as an alternate to supplementary feeds.81 Nevertheless, none of the methods can replace the supplementary feed. It is supported by previous authors that implementing biofloc technique farmers can able to minimize the dependence of supplementary feed to the greater as microbial protein from biofloc origin has higher bioavailability than feed protein.8 Prior studies reported that animal reared in the biofloc water reduces the FCR and feed costs.46 The results from the earlier studies also indicated that L. vannamei can replace the supplementary feed of up to 29 % if biofloc method opts for the culture.21 In addition to these, the available reports also show that there was 20% improvement in feed utilization found in tilapia reared in Biofloc.11
Earlier study reported that the bacterial biomass yield per every gram of carbon used as a substrate is 0.5 g.29 It was reported in the early study that production of bioflocs takes place when the microbial concentration reaches 107 CFU/ml.21 The group matters (algae, detritus and other decomposed components) in the water including bacteria are attached with one another and forming a floc, which is called as biofloc.8 In biofloc technology, the obtainability of biofloc as feed to the animals is available whole the day; therefore, the dependence of artificial feed and the expenditures for feed and feeding will be dramatically reduced for following this method.10 Biofloc will be a main feed for filter feeders such as tilapia,10,13,30 shrimp,21,64,131 sturgeon and snook.123 However, utilization capacity of this fluctuates from animals of other feeding habits (omnivorous and carnivorous); thus, the concept of biofloc as a feed ingredient has been proposed. From this idea, biofloc from the pond water can also be collected and processed for feed supplementation.9,10,27,78,79
Immune Response and Disease Resistance
Literature appears to suggest that biofloc contains the abundant amounts of beneficial bacteria which help in the improvement of immunity to the animals.37,40,61,95 Further evidence supporting that there was a significant improvement in the non-specific immunity obtained by the animals when animals cultured in the biofloc water.39,134,138 Asaduzzaman and co-authors found that survival rate was higher with increasing the abundance of the heterotrophic bacteria in periphyton based prawn culture system.7 Biofloc bacteria have poly –β- hydroxybutyrate (PHB), which terminate the pathogenic bacterial attack to the farming animals.40,61 It is speculated that the presence of heterotrophic microbial biomass in the biofloc tends to mitigate the invasion of pathogenic bacteria.46 Kim and co-authors claims that decreasing in the mortality rate can be seen when the biofloc treated animals were injected with the potentially harmful bacteria.55
Advantage of Biofloc Technology in Fish Culture Systems
- This technology is basically of zero water exchange oriented i.e. water exchange is not required in the culture ponds; therefore it required less water input which is not only economical to the farmers, but these will also minimize the pathogenic entry of animals through water and certify for more biosecurity in the fish culture. It also promises the less environmental impacts and footprints.142
- This technology allows the animals to rear under the higher stocking density with effective feed management.28,29
- The requirement for the feed is considerably less as biofloc itself will be a feed for the cultivable animals, which results in the lower FCR.2,43,89 Therefore, application of the technology will reduce the feed cost to the farmers.
- Biofloc increase the survival of fish since the beneficial microorganisms dominate in the biofloc act as an antagonism to the pathogenic bacteria which prevent the disease outbreak and expand the percentage of survival during the harvest. This way (beneficial) bacteria present in the biofloc prevent the colonization of any harmful bacteria that ensure the highest survival rate of the fish in the farms.76,117,102 In nutshell, biofloc act as an immunostimulants to the aquatic animals.30
- Biofloc enhance the gene expression of immune related genes such as ProPO1, ProPO2, PPAE, ran, mas and SP1 in shrimps to protect them from the harmful diseases.18 Therefore, biofloc technology would be a preventive solution to many of the emerging diseases in shrimp farming.
- Unlike artificial feeds, biofloc is available whole the day which facilitates animals to eat whenever they feel eating. This certifies the improvement in the body weight of the aquatic animals when reared under this system.8,10
- Biofloc bacteria produce the polyhydroxybutyrate (PHB) which are beneficial in the digestion and metabolism of fatty acid and growth increment to the fish.36
- Biofloc waters rich in the heterotrophic bacteria which utilize the toxic nitrogenous matters as a substrate for their growth that helps maintaining the water quality through reducing the organic loads as well as biochemical oxygen demand of the system.11,21,64,142
Biofloc technology in aquaculture with special attention to Indian authors
Despite the vast growth of biofloc research in outside the country, only little research has been carried out in India. The list of Indian authors who worked in the area is discussed as follows: In the year 2012, Prajith and co-authors reported that giant freshwater prawn reared under the biofloc had better growth, nutrient utilization and further suggested that it can be used in prawn farming to improve the ecological and economical sustainability of prawn farmers.106 In the year 2015, Mahanand and co-authorsworked in the biofloc reported that when rohu fed fish artificial feed and biofloc in wet form at the ratio of 1:1, the growth parameters were significantly higher.85 They concluded that microorganism community such as protozoa grazers, rotifers, bacteria and diatoms were represented in the biofloc would be the reason for the improved growth. Again, the similar results were demonstrated by the same authors in rohu when reared under the biofloc technology.86 During the year 2015, Faizullah and co-authorsassessed the impact of biofloc technology on the growth of goldfish young ones and reports suggested that growth and survival was significantly higher when reared under biofloc as compared to the normal water culture system.47 The similar results were once again evidenced by same authors in the goldfish fry reared under biofloc.48 One more study in the same year (2015) was conducted by Sharma and co-authors in rohu. The results suggested that biofloc can be a feed for this species without inferring the optimal growth as that of normal water systems.124 The authors were also noticed that feed cost was reduced up to 50% using this technique. Recent studies on biofloc attempted by Harini and co-authors in Blue morph suggesting that fish reared under the biofloc significantly improved the growth and survival.66 Biofloc technology is popularized and practiced in the certain foreign countries. But the technology have not yet popularised in India for fish farming. This is probably due to the deficiency of disseminating this technology and lack of awareness to farmers regarding the merits of the technology over other culture systems. It is value to note that many earlier works have been done in the field, but most of them are outside the country (India) and only few Indian workers were worked in this area as mentioned. But according to the primary information gained by the authors, it come to know that some fish and shrimp farmers of Tamil Nadu, Andhra Pradesh and Haryana state of India were recently established applying the biofloc technology at their farms.
Table 1: Some of the study conducted in fish with reference to biofloc based culture systems.
| S. No | Species studied | Duration of study | Results acquired in the study with biofloc |
| 1. | Litopenaeus vannamei | 35 days | Significant growth increment and reduced feed cost78. |
| 2. | Oreochromis sps. | 14 weeks | Improvement in the water quality, fish survival and minimization in the external feed requirement42. |
| 3. | Litopenaeus vannamei | 30-day | Promoted the animal growth, health, digestion and feed utilization performances144. |
| 4. | Farfantepenaeus paulensis | 15 days | Increased survival and growth rates of shrimp44. |
| 5. | Rhamdia quelen | 21-day | Increased the larval survival and stress mitigation104. |
| 6. | Marsupenaeus japonicus | 106-day | Comparing with the control group, the ammonium and nitrite concentration was significantly reduced in the bioflocs treatment groups147. |
| 7. | Labeo rohita | 90 days | Reduced the artificial feed reliance and improved the utilisation of bioflocs as feed to 50%124. |
| 8. | Oreochromis niloticus | N/A | Fish survival was 100% and results in the utilization of biofloc as food13. |
| 9. | Litopenaeus vannamei | 2 weeks | Biofloc improved the growth and immune-related gene expression75. |
| 10. | Litopenaeus vannamei | 34 days | There was a significant increase in the survival rate, in addition to increases in growth120. |
| 11. | Litopenaeus vannamei | Effectively improved the water quality, bacterial activities and zooplankton growth; consequently resulted in the better growth performances53. | |
| 12. | Litopenaeus vannamei | 13 weeks | Affected the nitrogen cycling pathways and de-nitrification process109. |
| 13. | Penaeus monodon | 60-day | Gave the beneficial effects on growth performances and digestive enzyme activities6. |
Probiotics
Definition and its Debate
Probiotic is a Greek word derivative of pro and bios; “pro means promoting and bio means life”.58 The first probiotics discovered is the fermented milk, which contains lactic acid bacteria (LAB). According to Metchnikoff, probiotics are live microbes consumed with the aim to improve the health of the host organisms.90 In later year, parker, defined they are bacteria and its constituent which plays a role in the maintenance of healthy intestine.101 Later, Fuller, redefined probiotics are feed supplement which contains live microbes that positively disturb the host intestine results in the improvement of intestinal microbial balance.52 As the probiotic effect in the intestinal microbial balance has not been observed in some fish, Tannock redefined that they are live microbial cells utilized as a dietary supplements to improve the host health.130 As stated by Gatesoupe, probiotics are single or the combination of viable microorganisms which improves the indigenous micro floral property to the host.54
The definitions above mentioned are widely accepted for the terrestrial animals; however, still there is a debate for the aquatic animals. As the aquatic animals have very close relationship with water and environment, the definition was reformed that they are live, viable microbial addition, results in the beneficial modification of host by having an association with microbial community or along with feed administration it improves the nutritional value of feed and/ or improve the immunity of host.138 At present, the scientific understanding on the probiotics study implies that even non-viable microbial constituents too can be administered for beneficially disturb the host intestine. Hence, Salminen and co-authors redefined that they are either microbial cell preparations or microbial cell components that exerts beneficial impact to the host health.116 Based on above concept, eventually Irianto and Austin redefined that they are entire microbial cell components that exerts beneficial effect to the host health.70
Table 2: Some of the study conducted in fish with reference to probiotics supplementation.
| S. No | Species studied | Strains used for study | Days of study | Results acquired in the study |
| 1. | Litopenaeus vannamei | B. subtilis | 60 days | No improvement on survival, final weight, FCR and water quality49. |
| 2. | Oreochromis niloticus | B. subtilis and L. acidophilus | 60 days | Improved the disease resistance and growth performance5. |
| 3. | Litopenaeus vannamei | B. subtilis | 14 days | Improved the larval survival rate, development, stress resistance and immune status82. |
| 4. | Litopenaeus vannamei | Bacillus species | N/A | Improved the growth, survival and some water quality parameters such as pH, ammonia and nitrite as compared to controls97. |
| 5. | Clarias gariepinus | L. acidophilus | 21 days | Significantly improved the haematology parameters and histopathology4. |
| 6. | Penaeus vannamei | B. coagulans | N/A | Significantly increase survival rate and digestive enzyme activities149. |
| 7. | Penaeus vannamei | Bacillus sp | 28 days | Positive effects on enzyme activity and resulted in an increase in the growth performances140. |
| 8. | Clarias gariepinus | Lactobacillus and Bifidobacterium | 90 days | Improved the growth performance and blood parameters12. |
| 9. | Oncorhynchus mykiss | Enterobacter amnigenus | N/A | Improved the health status20. |
| 10. | Litopenaeus vannamei | B. licheniformis, B. megaterium | 60 day | Effectively enhanced both digestive enzyme activity and non-specific immunity simultaneously80. |
| 11. | Sparus aurata | Lactobacillus spp. | 31 days | No effect on growth parameters and digestive enzyme activities128. |
| 12. | Paralichthys olivaceus | L. lactis | 5 weeks | Enhanced the immune response and effectively controlled bacterial infection67. |
| 13. | Penaeus monodon | Bacillus S11 (probiont) | 90-days | Enhanced both cellular and humoral immune defense93. |
| 14. | Penaeus monodon | B. subtilis | N/A | The growth of pathogenic V. harveyi was effectively controlled137. |
The Need of Probiotics?
A significant demand for fishery commodities urges many farmers to go for intensive farming.15 But, disease outbreaks are very common in the intensive practices.103 To prevent the disease, use of antimicrobial drugs are common in the aquaculture.118 However, using these drugs is criticized as they kill both adverse and beneficial microbes.69 Also, they establish the resistance to those bacteria during continuous application.3 Also, they transfer some antibiotic resistant genes to the consumers as well.59,121 The application of chemotherapeutics to the system degrades the aquatic ecosystem as residues stay in the system.113 At present, governments and many social welfare organizations restricted the usage of antimicrobial drugs.83 Due to these concerns, it is the need for farmers to adopt techniques that eliminate the use of antimicrobial drugs usage.69 Defoirdt and co-authors recommend the use of probiotics is potent alternatives to the use of antibiotics in culture systems.40 An alternative to the use of anti-microbial drugs are immunostimulants such as vaccines and probiotics.99 Both methods use microbes for the action. But vaccines are better than probiotics; however, they can be applied for only single disease and commonly cultured shrimps lack with the adaptive immune system. Thus, the applications of probiotics are important for controlling the disease.125
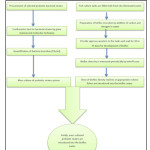 |
Figure 1: Schematic representation of biofloc technology integrated with exogenous probiotics. Click here to View figure |
 |
Figure 2: Experimental set up for rearing animals on biofloc water with supplemented probiotics. Click here to View figure |
Characteristics of an Ideal Probiotic
Many authors have been defined the characteristics of an ideal Probiotic.73,111,138,141 According to the available works of literature, an ideal probiotic strain must have following characteristics.
- It should be non-pathogenic to host.
- It taxonomy must have confirmed.
- It should have a potential to grow and survive in the host.
- It must survive even during unfavorable conditions generated in the digestive tract due to the production of gastric acid and bile juices.
- It should have capable enough to produce antimicrobial constituents to kill the invading pathogenic bacteria.
- It should modulate the host immune response and offer a health benefit.
- It must be genetically stable.
- It must survive during processing and storage conditions.
- It should be viable even at high centration.
- It should have desired organoleptic and technological characteristics to be included for fermentation methods.
Types of Probiotics
Depending on the way in which the probiotic bacteria are introduced to the animal, it differs. There are three types of probiotics such as soil, feed and water probiotics. But, only two types of probiotics (feed and water probiotics) are majority used in aquaculture.114 In feed probiotics, the probiotic strain is introduced to animals via feed.54 In water probiotics, the bacterial spores are directly added to the culture water.148 Timmermans and co-authors reported that water probiotics are especially important to the early larval stages and small fishes as they are very less exposure to artificial feed.133 Various authors mentioned that water probiotics have a significant role in the water quality management.23,91,138 Cabak and co-authors reported gram-positive bacteria are more efficient for the conversion of organic carbon to the bacterial biomass.23 Verschuere and co-authors reported that gram-positive Bacillus sp. improved the water quality parameters when they added into the rearing water.138 Moriarty, indicated that seawater receives with an inoculum containing frozen cells reduced the time taken for nitrification process of about 30 %.91
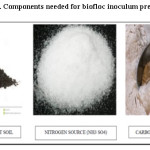 |
Figure 3: Components needed for biofloc inoculum preparation. Click here to View figure |
 |
Figure 4: Observation of biofloc volume in imhoff cone. Click here to View figure |
Applications in Aquaculture
Various studies have analyzed the use of probiotic bacteria to promote the health of the organisms.4,5,15,38,56,60,91,138 Conducted research on probiotics has shown many beneficial impacts to the health of cultured animals, including growth and immunity.39,134,138 Probiotics have many mechanisms of action: the competitive exclusion of pathogenic bacteria, serving as a nutrient source and contributing to enzymatic digestion of animals, beneficial effects on water quality, and improvement of the animal’s immune response.15,34,98,138 Many bacteria are being explored to be used as a probiotic strain as they contain the growth, immune stimulatory effects and resistance against pathogenic microbes.73 Previous studies indicated that the addition of probiotics in the water or feed increases growth, immunity, reduces animals to expose pathogenic bacteria and stops the growth of harmful pathogens.94,108,112,140 There is rapidly growing literature on the application of probiotics which indicates that it is one of the important methods developed to control disease at the farm; therefore, the addition of probiotics is common practice in fish farming.18 Aquatic farming is surrounded by environment that facilitates the natural uptake of potentially harmful opportunistic pathogens by animals through the water.24 Surrounding bacteria are continuously ingested when the fish is drinking; thus, harmful pathogenic microorganisms will reach high densities in the animal tissues. Especially this is the case with filter feeders which ingest bacteria at a high rate from the culture water, causing a bacterial infection to the animals.135 Previous reports in fish suggested that probiotics reduce the loads of harmful bacterial in the fish tissues.15,34,73,74,94,140 It has been reported that lactic acid bacteria such as lactobacilli and bifidobacteria are helping to reduce the gastrointestinal tract (GIT) pH by converting lactose into lactic acid.122 In this manner, colonization of many bacteria in the GIT is barred. Previous studies have also proven that spore-forming Bacillus sp generates antimicrobial peptides which offer immuno-stimulatory effect to the animals.16 Probiotic strains are either used as a single bacterial strain or multi strains which contain more than one strain. The available evidence seems to suggest that multi strain probiotics deliver synergistic effect which leads to an extra protection to the animal health.132 Dalmin and co-authors indicated that Bacillus sp. enhanced the growth, immunity and water quality in Penaeus monodon culture system.33 The findings from previous results confirmed that rainbow trout improved the activity of leukocytes, phagocytes and the resistance against Vibrio sp. when Clostridium butyricum bacteria were orally given.115 Besides, it was also reported that lactic acid bacterium Lactobacillus rhamnosus in the feed administration motivated the respiratory burst activity in rainbow trout.96 Some available reports in the literature appears to support that some probiotic bacteria are effective against some viruses such as Infectious hematopoietic necrosis virus (IHNV), Oncorhynchus masou virus (OMV) and Poliovirus.41,57,72
Overview of some of the study conducted in fish with reference to probiotics supplementation is listed in the table 2.
Advantages of Probiotics Application in Fish Culture Systems
- Probiotics are single bacteria act as a growth promoter via improving the digestibility of nutrients through the colonization of beneficial bacteria in the gastrointestinal tract of the animals.15
- Probiotics improve the water quality.34,98 Most of the gram-positive probiotic bacteria are are very efficient in converting the organic matter to CO2.138 Also, most of the probiotic bacteria which are heterotrophic in nature are efficiently utilize the toxic nitrogenous matters available in the pond water for their growth.105
- Probiotic bacteria also have synergetic effect.127 It communicates each other’s and does not allow harmful pathogens to attach for the binding sites for nutrients.143
- Probiotic bacteria have capacity to increase the rate of expression of immune related genes that improve the immune status of the animals.99,100 Probiotics also have phagocytic, antibacterial and antiviral activity against pathogenic bacteria.96,115
- Probiotic microorganisms show bactericidal and bacteriostatic effect against pathogenic bacteria due to the inhibitive influence of probiotic bacteria against harmful bacteria, production of enzymes that kills the harmful bacteria and creating the acid pH in the intestine of animals to kill the harmful bacteria lives in the low pH.87
- Probiotic bacteria produce the digestive enzymes such as proteases, amylases and lipases to improve the nutrient digestion.87 It also promote the growth factors such as vitamins, fatty acids, and amino acids to metabolise the digested nutrients to the cells for absorption.15
- Probiotics also offers stress tolerance to the animals by reducing the metabolic136 and oxidative stress factors.25
- Certain probiotics include B. subtilis produce essential vitamins such as vitamin B1 and vitamin B12 which help in the animal’s growth, metabolism and reproduction.55,72
Present status on influence of exogenous probiotic bacteria on biofloc based fish culture systems
Exogenous supplementation of probiotics to the biofloc is very recent area, still in the experimental level studies. So far, only few attempts have been successfully performed globally to identify the beneficial effects of exogenous probiotic strains on biofloc based aquaculture.1,31,62,77,146 An attempted works documented the positive results on growth and survival of the aquatic animals.1,31,62,77,146 In-vitro study by Hutabarat and co-authors (2013) reported that when biofloc inoculated with probiotic bacterium, Bacillus cereus produced higher amounts of polyhydroxybutyrate (PHB) which is one of the main component believed to have role in the energy reserve and growth acceleration in fish.68 Crab and co-authors reported that biofloc based brine shrimp tanks immunized with Bacillus sp. were reduced almost five times of pathogenic vibrio load than the control tanks.31 Krummenauer and co-authors analyzed the effect of commercial bacterial probiotics on a Litopenaeus vannamei based biofloc culture system.77 In this study, Vibrio parahaemolyticus infected to animals, and results show that experimental fishes received probiotic strains on biofloc system had significantly higher growth rate and survival than that of control. Aguilera-Rivera and co-authors observed the probiotic effect of biofloc in Pacific white shrimp, Litopenaeus Vannamei. This study reported that Vibrio load was reduced in the biofloc tanks treated with the probiotics.1 The study conducted by Yusuf and co-authors (2015) reported that biofloc added with Bacillus sp., showed highest growth and feed conversion ratios in African catfish.146 The findings of Hapsari, 2016 also supported that biofloc inoculated with Bacillus cereus improved the growth performances and reduced the FCR in African catfish.62 It can also be noted that authors of the paper are presently working in the area of current topic, results yet to be published. Nevertheless, schematic representation of the technology, experimental set up, components required for biofloc preparation, besides with the observation during the work was displayed in figure 1-4.
Pros/cons and future prospects of Biofloc technology together with supplementation of exogenous probiotic bacteria
Results of recent studies supported that supplementing the probiotics to biofloc helps in the growth, digestion, metabolism and disease resistance to the animals together with improving the water quality in the culture systems.1,31,62,77,146 This is probably a result of the ability of supplemented probiotic bacterial groups that dominate the other bacteria to minimize the pathogenic loads in the fish tissues. Despite the presence of bacteria in the biofloc or supplemented probiotics bacteria exhibits the mitigating effects on pathogenic microbes in the fish that ensures the non-specific immunity to the cultivable organisms, there is still dearth of knowledge in understanding the exact mechanisms behind how bacteria communicate each other (quorum sensing) to perform these effects. Therefore, future studies must be required in these areas to disclose the exact scientific reasons for these, so that this technology will be more scientific oriented. Bioflocs together with probiotics is reliable technique to aquaculture industry, but farmers needed to be satisfied with economic benefit; thus, require economic study. As the immunological effect differs among the strains and species; hence the specific actions of different strains must be explored among the different cultivable animals.138 The utilization of probiotic strains is species-specific,145 therefore, this technique should be scrutinized with various commercially cultivable fishes. An animal’s capacity to utilize various components varies among species10,13,21,30,64,123,131 therefore, animals preference utilize biofloc and probiotics must be well studied. The feed utilization test must be done to identify potential candidate species would be best for the culture; and also at what extend these techniques can reduce the feed cost must be available to the farmers for the implementation of this technology. Research must also explore how biofloc contributes to the improvement of growth and animal health performances. Bioflocs have an adequate amount of protein, lipid, carbohydrate and ash content for use as an aquaculture feed.29 However, the composition of amino acid and fatty acid is less studied; thus, careful investigation on nutritional composition must be completed to find out whether % of any nutrients in excess which responsible for the improvement of growth in the animal.
Conclusion
In conclusion, the paper demonstrated the new technique i.e. “Supplementation of probiotics to the biofloc”. Nevertheless, according to the best of author’s knowledge, at present no farmers in the country are following this technique. Therefore, keeping the practical advantages discussed in the technique, we hope that this technology will be shortly disseminated to be implemented by the fish and shrimp farmers of India and other countries for the sustainable production of aquatic animals in the cost effective and environmental friendly approach.
Acknowledgements
Authors are thankful to the Director of ICAR- Central Institute of Fisheries Education (CIFE), Mumbai, India for the provision given to carry out this study. Authors would like to thank scientists, staffs and students of Aquaculture Division (CIFE) for the contribution and support in the present study.
References
- Aguilera-Rivera, D., Prieto-Davó, A., Escalante, K., Chávez, C., Cuzon, G. and Gaxiola, G., Probiotic effect of FLOC on Vibrios in the pacific white shrimp Litopenaeus vannamei. Aquaculture, 424: 215-219 (2014)
CrossRef - Aiyushirota, I., Konsep Budidaya Udang Sistem Bakteri Heterotrop Dengan Bioflocs (2009)
- Akinbowale, O.L., Peng, H. and Barton, M.D., Antimicrobial resistance in bacteria isolated from aquaculture sources in Australia. Journal of Applied Microbiology, 100(5): 1103-1113 (2006)
CrossRef - Al‐Dohail, M.A., Hashim, R. and Aliyu‐Paiko, M., Evaluating the use of Lactobacillus acidophilus as a biocontrol agent against common pathogenic bacteria and the effects on the haematology parameters and histopathology in African catfish Clarias gariepinus juveniles. Aquaculture Research, 42(2): 196-209 (2011)
CrossRef - Aly, S.M., Ahmed, Y.A.G., Ghareeb, A.A.A. and Mohamed, M.F., Studies on Bacillus subtilis and Lactobacillus acidophilus, as potential probiotics, on the immune response and resistance of Tilapia nilotica (Oreochromis niloticus) to challenge infections. Fish & Shellfish Immunology, 25(1): 128-136 (2008)
CrossRef - Anand, P.S., Kohli, M.P.S., Roy, S.D., Sundaray, J.K., Kumar, S., Sinha, A., Pailan, G.H. and kumar Sukham, M., Effect of dietary supplementation of periphyton on growth performance and digestive enzyme activities in Penaeus monodon. Aquaculture, 392: 59-68 (2013)
CrossRef - Asaduzzaman, M., Wahab, M.A., Verdegem, M.C.J., Huque, S., Salam, M.A. and Azim, M.E., C/N ratio control and substrate addition for periphyton development jointly enhance freshwater prawn Macrobrachium rosenbergii production in ponds. Aquaculture, 280(1):117-123 (2008)
CrossRef - Avnimelech, Y., Biofloc technology. A practical guide book. The World Aquaculture Society, Baton Rouge (2009)
- Avnimelech, Y., Carbon/nitrogen ratio as a control element in aquaculture systems. Aquaculture, 176(3): 227-235 (1999)
CrossRef - Avnimelech, Y., Feeding with microbial flocs by tilapia in minimal discharge bio-flocs technology ponds. Aquaculture, 264(1): 140-147 (2007)
CrossRef - Avnimelech, Y., Kochva, M. and Diab, S., Development of controlled intensive aquaculture systems with a limited water exchange and adjusted carbon to nitrogen ratio. Israeli Journal of Aquaculture-Bamidgeh, 46(3): 119-131 (1994)
- Ayoola, S.O., Ajani, E.K. and Fashae, O.F., Effect of probiotics (Lactobacillus and Bifidobacterium) on growth performance and hematological profile of Clarias gariepinus juveniles. World journal of fish and Marine Sciences: 5(1): 01-08 (2013)
- Azim, M.E. and Little, D.C., The biofloc technology (BFT) in indoor tanks: water quality, biofloc composition, and growth and welfare of Nile tilapia (Oreochromis niloticus). Aquaculture, 283(1): 29-35 (2008a)
CrossRef - Azim, M.E., Little, D.C. and Bron, J.E., Microbial protein production in activated suspension tanks manipulating C: N ratio in feed and the implications for fish culture. Bioresource Technology, 99(9): 3590-3599 (2008b)
CrossRef - Balcázar, J.L., De Blas, I., Ruiz-Zarzuela, I., Cunningham, D., Vendrell, D. and Múzquiz, J.L., The role of probiotics in aquaculture. Veterinary microbiology, 114(3): 173-186 (2006)
CrossRef - Barbosa, T.M., Serra, C.R., La Ragione, R.M., Woodward, M.J. and Henriques, A.O., Screening for Bacillus isolates in the broiler gastrointestinal tract. Applied and environmental microbiology, 71(2): 968-978 (2005)
CrossRef - Bondad-Reantaso, M.G., Subasinghe, R.P., Arthur, J.R., Ogawa, K., Chinabut, S., Adlard, R., Tan, Z. and Shariff, M., Disease and health management in Asian aquaculture. Veterinary parasitology, 132(3): 249-272 (2005)
CrossRef - Browdy, C., Hargreaves, J., Hoang, T. and Avnimelech, Y., Biofloc Technology and Shrimp Disease Workshop (2013)
- Browdy, C.L., Ray, A.J., Leffler, J.W. and Avnimelech, Y., Biofloc‐based Aquaculture Systems. Aquaculture Production Systems, 278-307 (2012)
- Burbank, D.R., Shah, D.H., LaPatra, S.E., Fornshell, G. and Cain, K.D., Enhanced resistance to coldwater disease following feeding of probiotic bacterial strains to rainbow trout (Oncorhynchus mykiss). Aquaculture, 321(3): 185-190 (2011)
CrossRef - Burford, M.A., Thompson, P.J., McIntosh, R.P., Bauman, R.H. and Pearson, D.C., The contribution of flocculated material to shrimp (Litopenaeus vannamei) nutrition in a high-intensity, zero-exchange system. Aquaculture, 232(1): 525-537 (2004)
CrossRef - Burford, M.A., Thompson, P.J., McIntosh, R.P., Bauman, R.H. and Pearson, D.C., Nutrient and microbial dynamics in high-intensity, zero-exchange shrimp ponds in Belize. Aquaculture, 219(1): 393-411 (2003)
CrossRef - Čabak, V., Dickerson, J.W.T. and Stanier, M.W., Response of young rats to rehabilitation with diets containing different amounts of protein after deprivation of protein or of calories. British Journal of Nutrition, 17(01): 601-616 (1963)
CrossRef - Cahill, M.M., Bacterial flora of fishes: a review. Microbial ecology, 19(1): 21-41 (1990)
CrossRef - Castex, M., Lemaire, P., Wabete, N. and Chim, L., Effect of dietary probiotic Pediococcus acidilactici on antioxidant defences and oxidative stress status of shrimp Litopenaeus stylirostris. Aquaculture, 294(3): 306-313 (2009)
CrossRef - Cohen, J., Samocha, T.M., Fox, J.M. and Lawrence, A.L., Biosecured production of juvenile Pacific white shrimp in an intensive raceway system with limited water discharge. Aquaculture Engineering, 32: 425-442 (2005)
CrossRef - Crab, R., Avnimelech, Y., Defoirdt, T., Bossier, P. and Verstraete, W., Nitrogen removal techniques in aquaculture for a sustainable production. Aquaculture, 270(1): 1-14 (2007)
CrossRef - Crab, R., Chielens, B., Wille, M., Bossier, P. and Verstraete, W., The effect of different carbon sources on the nutritional value of bioflocs, a feed for Macrobrachium rosenbergii postlarvae. Aquaculture Research, 41(4): 559-567 (2010a)
CrossRef - Crab, R., Defoirdt, T., Bossier, P. and Verstraete, W., Biofloc technology in aquaculture: beneficial effects and future challenges. Aquaculture, 356: 351-356 (2012)
CrossRef - Crab, R., Kochva, M., Verstraete, W. and Avnimelech, Y., Bio-flocs technology application in over-wintering of tilapia. Aquacultural Engineering, 40(3): 105-112 (2009)
CrossRef - Crab, R., Lambert, A., Defoirdt, T., Bossier, P. and Verstraete, W., The application of bioflocs technology to protect brine shrimp (Artemia franciscana) from pathogenic Vibrio harveyi. Journal of applied microbiology, 109(5): 1643-1649 (2010b)
CrossRef - Cuzon, G., Lawrence, A., Gaxiola, G., Rosas, C. and Guillaume, J., Nutrition of Litopenaeus vannamei reared in tanks or in ponds. Aquaculture, 235(1): 513-551 (2004)
CrossRef - Dalmin, G., Kathiresan, K. and Purushothaman, A., Effect of probiotics on bacterial population and health status of shrimp in culture pond ecosystem. Indian journal of experimental biology, 39(9): 939-942 (2001)
- Dan, S. and Hamasaki, K., Evaluation of the effects of probiotics in controlling bacterial necrosis symptoms in larvae of the mud crab Scylla serrata during mass seed production. Aquaculture international, 23(1): 277-296 (2015)
CrossRef - Daniel, N., Amit Kumar., Mohamed Faizullah. M., and Nageswari, P., Investigation of Vitamin C [Ascorbic Acid] in Nutrition and Health of Fish. Aquaculture times, 2(1): 20-24 (2016)
- De Schryver, P., Boon, N., Verstraete, W. and Bossier, P., The biology and biotechnology behind bioflocs. In Biofloc technology: a practical guide book (pp. 199-215). World Aquaculture Society (WAS) (2012)
- De Schryver, P., Sinha, A.K., Kunwar, P.S., Baruah, K., Verstraete, W., Boon, N., De Boeck, G. and Bossier, P., Poly-β-hydroxybutyrate (PHB) increases growth performance and intestinal bacterial range-weighted richness in juvenile European sea bass, Dicentrarchus labrax. Applied microbiology and biotechnology, 86(5): 1535-1541 (2010)
CrossRef - De Souza, D.M., Suita, S.M., Leite, F.P.L., Romano, L.A., Wasielesky, W. and Ballester, E.L.C., The use of probiotics during the nursery rearing of the pink shrimp Farfantepenaeus brasiliensis (Latreille, 1817) in a zero exchange system. Aquaculture Research, 43(12): 1828-1837 (2012)
CrossRef - Decamp, O., Moriarty, D.J. and Lavens, P., Probiotics for shrimp larviculture: review of field data from Asia and Latin America. Aquaculture Research, 39(4): 334-338 (2008)
CrossRef - Defoirdt, T., Halet, D., Vervaeren, H., Boon, N., Van de Wiele, T., Sorgeloos, P., Bossier, P. and Verstraete, W., The bacterial storage compound poly‐β‐hydroxybutyrate protects Artemia franciscana from pathogenic Vibrio campbellii. Environmental microbiology, 9(2): 445-452 (2007)
CrossRef - Direkbusarakom, S., Yoshimizu, M., Ezura, Y., Ruangpan, L. and Danayadol, Y., g., the dominant flora in shrimp hatchery against some fish pathogenic viruses. Journal of Marine Biotechnology, 6: 266-267 (1998)
- Ekasari, J. and Maryam, S., Evaluation of biofloc technology application on water quality and production performance of red tilapia Oreochromis sp. cultured at different stocking densities. Hayati journal of Biosciences, 19(2): 73-80 (2012)
- Ekasari, J., crab, R. and Verstraete, W., Primary nutritional content of bio-flocs cultured with different organic carbon sources and salinity. Hayati Journal of Biosciences, 17(3): 125-130 (2010)
CrossRef - Emerenciano, M., Ballester, E.L., Cavalli, R.O. and Wasielesky, W., Effect of biofloc technology (BFT) on the early post larval stage of pink shrimp Farfantepenaeus paulensis: growth performance, floc composition and salinity stress tolerance. Aquaculture International, 19(5): 891-901 (2011)
CrossRef - Emerenciano, M., Ballester, E.L., Cavalli, R.O. and Wasielesky, W., Biofloc technology application as a food source in a limited water exchange nursery system for pink shrimp Farfantepenaeus brasiliensis (Latreille, 1817). Aquaculture Research, 43(3): 447-457 (2012)
CrossRef - Emerenciano, M., Gaxiola, G. and Cuzon, G., Biofloc technology (BFT): a review for aquaculture application and animal food industry. Biomass Now: Cultivation and Utilization. Rijeka, Croatia: InTech, Rijeka, Croatia: 301–328 (2013)
- Faizullah, M., Rajagopalsamy, C.B.T., Ahilan, B. and Francis, T., Impact of Biofloc Technology on the Growth of Goldfish Young Ones. Indian Journal of Science and Technology, 8(13): 1-8 (2015a)
CrossRef - Faizullah, M., Rajagopalsamy, C.B.T., Ahilan, B. and Francis, T., Fffect of biofloc technology in the nursery rearing of gold fish. Journal of aquaculture in the tropics, 30(3-4): 59-171 (2015b)
- Far, H.Z., Saad, C.R.B., Daud, H.M., Harmin, S.A. and Shakibazadeh, S., Effect of Bacillus subtilis on the growth and survival rate of shrimp (Litopenaeus vannamei). African Journal of Biotechnology, 8(14) (2009)
- Fontenot, Q., Bonvillain, C., Kilgen, M. and Boopathy, R., Effects of temperature, salinity, and carbon: nitrogen ratio on sequencing batch reactor treating shrimp aquaculture wastewater. Bioresource Technology, 98(9): 1700-1703 (2007)
CrossRef - Francis-Floyd, R., Watson, C., Petty, D. and Pouder, D.B., Ammonia in aquatic systems. University of Florida IFAS Extension Publication,FA-16 (2009)
- Fuller, R., History and development of probiotics. In Probiotics. Springer Netherlands, 1-8 (1992)
CrossRef - Gao, L., Shan, H.W., Zhang, T.W., Bao, W.Y. and Ma, S., Effects of carbohydrate addition on Litopenaeus vannamei intensive culture in a zero-water exchange system. Aquaculture, 342: 89-96 (2012)
CrossRef - Gatesoupe, F.J., The use of probiotics in aquaculture. Aquaculture, 180(1): 147-165 (1999)
CrossRef - Ghosh, S., Sinha, A. and Sahu, C., Effect of probiotic on reproductive performance in female livebearing ornamental fish. Aquaculture Research, 38(5): 518-526 (2007)
CrossRef - Gildberg, A., Mikkelsen, H., Sandaker, E. and Ringø, E., Probiotic effect of lactic acid bacteria in the feed on growth and survival of fry of Atlantic cod (Gadus morhua). In Asia-Pacific Conference on Science and Management of Coastal Environment, Springer Netherlands, 279-285 (1997)
CrossRef - Girones, R., Jofre, J.T. and Bosch, A., Isolation of marine bacteria with antiviral properties. Canadian journal of microbiology, 35(11): 1015-1021 (1989)
CrossRef - Gismondo, M.R., Drago, L. and Lombardi, A., Review of probiotics available to modify gastrointestinal flora. International journal of antimicrobial agents, 12(4): 287-292 (1999)
CrossRef - Goossens, D., Jonkers, D., Stobberingh, E., Bogaard, A.V.D., Russel, M. and Stockbrugger, R., Probiotics in gastroenterology: indications and future perspectives. Scandinavian Journal of Gastroenterology-Supplements, 38(239): 15-16 (2003)
- Gram, L., Melchiorsen, J., Spanggaard, B., Huber, I. and Nielsen, T.F., Inhibition of Vibrio anguillarum by Pseudomonas fluorescens AH2, a Possible Probiotic Treatment of Fish. Applied and Environmental Microbiology, 65(3): 969-973 (1999)
- Halet, D., Defoirdt, T., Van Damme, P., Vervaeren, H., Forrez, I., Van de Wiele, T., Boon, N., Sorgeloos, P., Bossier, P. and Verstraete, W., Poly-β-hydroxybutyrate-accumulating bacteria protect genotobiotic Artemia franciscana from pathogenic Vibrio campbellii. FEMS microbiology ecology, 60(3): 363-369 (2007)
CrossRef - Hapsari, F., The effect of fermented and non fermented biofloc inoculated with bacterium Bacillus cereus for catfish (Clarias gariepinus) juveniles. AACL Bioflux, 9(2): 334-339 (2016)
- Hargreaves, J.A., Photosynthetic suspended-growth systems in aquaculture. Aquacultural engineering, 34(3): 344-363 (2006)
CrossRef - Hari, B., Kurup, B.M., Varghese, J.T., Schrama, J.W. and Verdegem, M.C.J., Effects of carbohydrate addition on production in extensive shrimp culture systems. Aquaculture, 241(1): 179-194 (2004)
CrossRef - Hari, B., Kurup, B.M., Varghese, J.T., Schrama, J.W. and Verdegem, M.C.J., The effect of carbohydrate addition on water quality and the nitrogen budget in extensive shrimp culture systems. Aquaculture, 252(2): 248-263 (2006)
CrossRef - Harini, C., Rajagopalasamy, C.B.T., Kumar, J.S.S. and Santhakumar, R., Role of Biofloc in the Growth and Survival of Blue morph, Pseudotropheus saulosi. Indian Journal of Science and Technology, 9(8): 1-7 (2016)
CrossRef - Heo, W.S., Kim, Y.R., Kim, E.Y., Bai, S.C. and Kong, I.S., Effects of dietary probiotic, Lactococcus lactis subsp. lactis I2, supplementation on the growth and immune response of olive flounder (Paralichthys olivaceus).Aquaculture, 376: 20-24 (2013)
CrossRef - Hutabarat, J., Prayitno, S.B. and Darmanto, Y.S., The effect of different C: N and C: P ratio of media on the content of polyhydroxybutyrate in biofloc inoculated with bacterium Bacillus cereus. Journal of Coastal Development, 16(2): 114-120 (2013)
- Huys, G., Bartie, K., Cnockaert, M., Oanh, D.T.H., Phuong, N.T., Somsiri, T., Chinabut, S., Yusoff, F.M., Shariff, M., Giacomini, M. and Teale, A., Biodiversity of chloramphenicol-resistant mesophilic heterotrophs from Southeast Asian aquaculture environments. Research in microbiology, 158(3): 228-235 (2007)
CrossRef - Irianto, A. and Austin, B., Probiotics in aquaculture. Journal of fish diseases, 25(11): 633-642 (2002)
CrossRef - Izquierdo, M., Forster, I., Divakaran, S., Conquest, L., Decamp, O. and Tacon, A., Effect of green and clear water and lipid source on survival, growth and biochemical composition of Pacific white shrimp Litopenaeus vannamei. Aquaculture Nutrition, 12(3): 192-202 (2006)
CrossRef - Kamei, Y., Yoshimizu, M., Ezura, Y. and Kimura, T., Screening of bacteria with antiviral activity from fresh water salmonid hatcheries. Microbiology and immunology, 32(1): 67-73 (1988)
CrossRef - Kesarcodi-Watson, A., Kaspar, H., Lategan, M.J. and Gibson, L., Probiotics in aquaculture: the need, principles and mechanisms of action and screening processes. Aquaculture, 274(1): 1-14 (2008)
CrossRef - Kesarcodi-Watson, A., Miner, P., Nicolas, J.L. and Robert, R., Protective effect of four potential probiotics against pathogen-challenge of the larvae of three bivalves: Pacific oyster (Crassostrea gigas), flat oyster (Ostrea edulis) and scallop (Pecten maximus). Aquaculture, 344: 29-34 (2012)
CrossRef - Kim, S.K., Pang, Z., Seo, H.C., Cho, Y.R., Samocha, T. and Jang, I.K., Effect of bioflocs on growth and immune activity of Pacific white shrimp, Litopenaeus vannamei postlarvae. Aquaculture Research, 45(2): 362-371 (2014)
CrossRef - Krummenauer, D., Peixoto, S., Cavalli, R.O., Poersch, L.H. and Wasielesky, W., Superintensive culture of white shrimp, Litopenaeus vannamei, in a biofloc technology system in southern Brazil at different stocking densities. Journal of the World Aquaculture Society, 42(5): 726-733 (2011)
CrossRef - Krummenauer, D., Poersch, L., Romano, L.A., Lara, G.R., Encarnação, P. and Wasielesky Jr, W., The effect of probiotics in a Litopenaeus vannamei biofloc culture system infected with Vibrio parahaemolyticus. Journal of Applied Aquaculture, 26(4): 370-379 (2014)
CrossRef - Kuhn, D.D., Boardman, G.D., Lawrence, A.L., Marsh, L. and Flick, G.J., Microbial floc meal as a replacement ingredient for fish meal and soybean protein in shrimp feed. Aquaculture, 296(1): 51-57 (2009)
CrossRef - Kuhn, D.D., Lawrence, A.L., Boardman, G.D., Patnaik, S., Marsh, L. and Flick, G.J., Evaluation of two types of bioflocs derived from biological treatment of fish effluent as feed ingredients for Pacific white shrimp, Litopenaeus vannamei. Aquaculture, 303(1): 28-33 (2010)
CrossRef - Kumar, P.N.J., Jyothsna, R.S., Reddy, M.H. and Sreevani, Sreemanthula., Effect of Bacillus subtilis and Lactobacillus rhamnosus incorporated probiotic diet on growth pattern and enzymes in Penaeus vannamei. International journal of life science and pharma research, 3(4): 6-11 (2013)
- Lim, L.C., Dhert, P. and Sorgeloos, P., Recent developments in the application of live feeds in the freshwater ornamental fish culture. Aquaculture, 227(1): 319-331 (2003)
CrossRef - Liu, K.F., Chiu, C.H., Shiu, Y.L., Cheng, W. and Liu, C.H., Effects of the probiotic, Bacillus subtilis E20, on the survival, development, stress tolerance, and immune status of white shrimp, Litopenaeus vannamei larvae. Fish & Shellfish Immunology, 28(5): 837-844 (2010)
CrossRef - Love, D.C., Rodman, S., Neff, R.A. and Nachman, K.E., Veterinary drug residues in seafood inspected by the European Union, United States, Canada, and Japan from 2000 to 2009. Environmental science & technology, 45(17): 7232-7240 (2011)
CrossRef - MacIntosh, R.P., Changing paradigms in shrimp farming: IV: low protein feeds and feeding strategies. Global Aquaculture Advocate, 2: 40-47 (2000)
- Mahanand, S.S., Moulick, S. and Rao, P.S., Optimum formulation of feed for Rohu, Labeo rohita (Hamilton), with biofloc as a component. Aquaculture International, 21(2): 347-360 (2013a)
CrossRef - Mahanand, S.S., Moulick, S. and Rao, P.S., Water quality and growth of Rohu, Labeo rohita, in a biofloc system. Journal of applied Aquaculture, 25(2): 121-131 (2013b)
CrossRef - Martínez Cruz, P., Ibáñez, A.L., Monroy Hermosillo, O.A. and Ramírez Saad, H.C., Use of probiotics in aquaculture. ISRN microbiology (2012)
CrossRef - McCarty, P.L., Environmental biotechnology: principles and applications. Tata McGraw-Hill Education (2012)
CrossReMegahed, M.E., The effect of microbial biofloc on water quality, survival and growth of the green tiger shrimp (Penaeus semisulcatus) fed with different crude protein levels. Journal of the Arabian Aquaculture Society, 5(2):119-142 (2010) - Metchnikoff, E., The Prolongation of Life. Optimistic Studies. William Heinemann, London (1907)
- Moriarty, D.J., The role of microorganisms in aquaculture ponds. Aquaculture, 151(1): 333-349 (1997)
CrossRef - Moriarty, D.J.W., Control of luminous Vibrio species in penaeid aquaculture ponds. Aquaculture, 164(1): 351-358 (1998)
CrossRef - Moss, S.M., LeaMaster, B.R. and Sweeney, J.N., Relative Abundance and Species Composition of Gram‐Negative, Aerobic Bacteria Associated with the Gut of Juvenile White Shrimp Litopenaeus vannamei Reared in Oligotrophic Well Water and Eutrophic Pond Water. Journal of the World Aquaculture Society, 31(2): 255-263 (2000)
CrossRef - Nayak, S.K., Probiotics and immunity: a fish perspective. Fish & shellfish immunology, 29(1): 2-14 (2010)
CrossRef - Nhan, D.T., Wille, M., De Schryver, P., Defoirdt, T., Bossier, P. and Sorgeloos, P., The effect of poly β-hydroxybutyrate on larviculture of the giant freshwater prawn Macrobrachium rosenbergii. Aquaculture, 302(1): 76-81 (2010)
CrossRef - Nikoskelainen, S., Ouwehand, A.C., Bylund, G., Salminen, S. and Lilius, E.M., Immune enhancement in rainbow trout (Oncorhynchus mykiss) by potential probiotic bacteria (Lactobacillus rhamnosus). Fish & shellfish immunology, 15(5): 443-452 (2003)
CrossRef - Nimrat, S., Suksawat, S., Boonthai, T. and Vuthiphandchai, V., Potential Bacillus probiotics enhance bacterial numbers, water quality and growth during early development of white shrimp (Litopenaeus vannamei). Veterinary microbiology, 159(3): 443-450 (2012)
CrossRef - Olafsen, J.A., Interactions between fish larvae and bacteria in marine aquaculture. Aquaculture, 200(1): 223-247 (2001)
CrossRef - Panigrahi, A. and Azad, I.S., Microbial intervention for better fish health in aquaculture: the Indian scenario. Fish physiology and biochemistry, 33(4): 429-440 (2007)
CrossRef - Panigrahi, A., Kiron, V., Satoh, S., Hirono, I., Kobayashi, T., Sugita, H., Puangkaew, J. and Aoki, T., Immune modulation and expression of cytokine genes in rainbow trout Oncorhynchus mykiss upon probiotic feeding. Developmental & Comparative Immunology, 31(4): 372-382 (2007)
CrossRef - Parker, R.B., Probiotics, the other half of the antibiotic story. Animal Nutrition and Health, 29(29): 4-8 (1974)
- Pérez-Fuentes, J.A., Pérez-Rostro, C.I. and Hernández-Vergara, M.P., Pond-reared Malaysian prawn Macrobrachium rosenbergii with the biofloc system. Aquaculture, 400: 105-110 (2013)
CrossRef - Piccolo, G., Bovera, F., Lombardi, P., Mastellone, V., Nizza, S., Di Meo, C., Marono, S. and Nizza, A., Effect of Lactobacillus plantarum on growth performance and hematological traits of European sea bass (Dicentrarchus labrax). Aquaculture international, 23(4): 1025-1032 (2015)
CrossRef - Poli, M.A., Schveitzer, R. and de Oliveira Nuñer, A.P., The use of biofloc technology in a South American catfish (Rhamdia quelen) hatchery: Effect of suspended solids in the performance of larvae. Aquacultural Engineering, 66: 17-21 (2015)
CrossRef - Porubcan, R.S., Reduction in chemical oxygen demand and improvement in Penaeus monodon yield in ponds inoculated with aerobic Bacillus bacteria. In Proceedings of the Program and Abastracts of the 22nd Annual Conference and Exposition. World Aquaculture Society (1991)
- Prajith, K.K. and Madhusoodana, K.B., Application of Biofloc Technology (BFT) in the nursery rearing and farming of giant freshwater prawn, Macrobrachium rosenbergii (deMan) (Doctoral dissertation, Cochin University of Science and Technology). PP.226 (2011)
- Pulkkinen, K., Suomalainen, L.R., Read, A.F., Ebert, D., Rintamäki, P. and Valtonen, E.T., 2010. Intensive fish farming and the evolution of pathogen virulence: the case of columnaris disease in Finland. Proceedings of the Royal Society of London B: Biological Sciences, 277(1681), pp.593-600.
CrossRef - Pybus, V., Loutit, M.W., Lamont, I.L. and Tagg, J.R., Growth inhibition of the salmon pathogen Vibrio ordalii by a siderophore produced by Vibrio anguillarum strain VL4355. Journal of Fish Diseases, 17(4): 311-324 (1994)
CrossRef - Ray, A.J., Dillon, K.S. and Lotz, J.M., Water quality dynamics and shrimp (Litopenaeus vannamei) production in intensive, mesohaline culture systems with two levels of biofloc management. Aquacultural Engineering, 45(3): 127-136 (2011)
CrossRef - Ray, A.J., Shuler, A.J., Leffler, J.W. and Browdy, C.L., Microbial ecology and management of biofloc systems. The Rising Tide, Proceedings of the Special Session on Sustainable Shrimp Farming. Baton Rouge, LA, pp.255-266 (2009)
- Reid, G., Sanders, M.E., Gaskins, H.R., Gibson, G.R., Mercenier, A., Rastall, R., Roberfroid, M., Rowland, I., Cherbut, C. and Klaenhammer, T.R., New scientific paradigms for probiotics and prebiotics. Journal of clinical gastroenterology, 37(2): 105-118 (2003)
CrossRef - Rengpipat, S., Rukpratanporn, S., Piyatiratitivorakul, S. and Menasaveta, P., Immunity enhancement in black tiger shrimp (Penaeus monodon) by a probiont bacterium (Bacillus S11). Aquaculture, 191(4): 271-288 (2000)
CrossRef - Rico, A., Phu, T.M., Satapornvanit, K., Min, J., Shahabuddin, A.M., Henriksson, P.J., Murray, F.J., Little, D.C., Dalsgaard, A. and Van den Brink, P.J., Use of veterinary medicines, feed additives and probiotics in four major internationally traded aquaculture species farmed in Asia. Aquaculture, 412: 231-243 (2013)
CrossRef - Ringø, E. and Birkbeck, T.H., Intestinal microflora of fish larvae and fry. Aquaculture research, 30(2): 73-93 (1999)
CrossRef - Sakai, M., Yoshida, T., Atsuta, S. and Kobayashi, M., Enhancement of resistance to vibriosis in rainbow trout, Oncorhynchus mykiss (Walbaum), by oral administration of Clostridium butyricum bacterin. Journal of Fish Diseases, 18(2): 187-190 (1995)
CrossRef - Salminen, S., Ouwehand, A., Benno, Y. and Lee, Y.K., Probiotics: how should they be defined?. Trends in food science & technology, 10(3): 107-110 (1999)
CrossRef - Samocha, T.M., Patnaik, S., Speed, M., Ali, A.M., Burger, J.M., Almeida, R.V., Ayub, Z., Harisanto, M., Horowitz, A. and Brock, D.L., Use of molasses as carbon source in limited discharge nursery and grow-out systems for Litopenaeus vannamei. Aquacultural Engineering, 36(2): 184-191 (2007)
CrossRef - Schmidt, A.S., Bruun, M.S., Dalsgaard, I., Pedersen, K. and Larsen, J.L., Occurrence of antimicrobial resistance in fish-pathogenic and environmental bacteria associated with four Danish rainbow trout farms. Applied and environmental microbiology, 66(11): 4908-4915 (2000)
CrossRef - Schneider, O., Sereti, V., Eding, E.H. and Verreth, J.A., Molasses as C source for heterotrophic bacteria production on solid fish waste. Aquaculture, 261(4): 1239-1248 (2006)
CrossRef - Schveitzer, R., Arantes, R., Baloi, M.F., Costódio, P.F.S., Arana, L.V., Seiffert, W.Q. and Andreatta, E.R., Use of artificial substrates in the culture of Litopenaeus vannamei (Biofloc System) at different stocking densities: effects on microbial activity, water quality and production rates. Aquacultural engineering, 54: .93-103 (2013)
- Schwarz, S., Kehrenberg, C. and Walsh, T.R., Use of antimicrobial agents in veterinary medicine and food animal production. International journal of antimicrobial agents, 17(6): 431-437 (2001)
CrossRef - Senok, A.C., Ismaeel, A.Y. and Botta, G.A., Probiotics: facts and myths. Clinical Microbiology and Infection, 11(12): 958-966 (2005)
CrossRef - Serfling, S.A., Microbial flocs: Natural treatment method supports freshwater, marine species in recirculating systems. Global Aquaculture Advocate, 9(June): 34-36 (2006)
- Sharma, D.A., Sharma, K. and Sangotra, R., Biofloc culture and its utilisation as feed in limited water exchange system for the culture of Labeo rohita. 3(2): 185-193 (2015)
- Smith, V.J., Brown, J.H. and Hauton, C., Immunostimulation in crustaceans: does it really protect against infection?. Fish & Shellfish Immunology, 15(1): 71-90 (2003)
CrossRef - Snieszko, S.F., The effects of environmental stress on outbreaks of infectious diseases of fishes. Journal of Fish Biology, 6(2): 197-208 (1974)
CrossRef - Su, P., Henriksson, A., Nilsson, C. and Mitchell, H., Synergistic effect of green tea extract and probiotics on the pathogenic bacteria, Staphylococcus aureus and Streptococcus pyogenes. World Journal of Microbiology and Biotechnology, 24(9): 1837-1842 (2008)
CrossRef - Suzer, C., Çoban, D., Kamaci, H.O., Saka, Ş., Firat, K., Otgucuoğlu, Ö. and Küçüksari, H., Lactobacillus spp. bacteria as probiotics in gilthead sea bream (Sparus aurata, L.) larvae: effects on growth performance and digestive enzyme activities. Aquaculture, 280(1): 140-145 (2008)
CrossRef - Tacon, A.G.J., Cody, J.J., Conquest, L.D., Divakaran, S., Forster, I.P. and Decamp, O.E., Effect of culture system on the nutrition and growth performance of Pacific white shrimp Litopenaeus vannamei (Boone) fed different diets. Aquaculture nutrition, 8(2): 121-137 (2002)
CrossRef - Tannock, G.W., Modification of the normal microbiota by diet, stress, antimicrobial agents, and probiotics. Gastrointestinal microbiology, 2: 434-465(1997)
- Taw, N., Biofloc technology expanding at white shrimp farms. Global aquaculture advocate (2010)
- Timmerman, H.M., Koning, C.J.M., Mulder, L., Rombouts, F.M. and Beynen, A.C., Monostrain, multistrain and multispecies probiotics- a comparison of functionality and efficacy. International journal of food microbiology, 96(3): 219-233 (2004)
CrossRef - Timmermans, L.P., Early development and differentiation in fish. Sarsia, 72(3-4): 331-339 (1987)
CrossRef - Tseng, D.Y., Ho, P.L., Huang, S.Y., Cheng, S.C., Shiu, Y.L., Chiu, C.S. and Liu, C.H., Enhancement of immunity and disease resistance in the white shrimp, Litopenaeus vannamei, by the probiotic, Bacillus subtilis E20. Fish & Shellfish Immunology, 26(2): 339-344 (2009)
CrossRef - Van Hai, N., Research findings from the use of probiotics in tilapia aquaculture: a review. Fish & shellfish immunology, 45(2): 592-597 (2015)
CrossRef - Varela, J.L., Ruiz-Jarabo, I., Vargas-Chacoff, L., Arijo, S., León-Rubio, J.M., García-Millán, I., del Rio, M.M., Moriñigo, M.A. and Mancera, J.M., Dietary administration of probiotic Pdp11 promotes growth and improves stress tolerance to high stocking density in gilthead seabream Sparus auratus. Aquaculture, 309(1): 265-271 (2010)
CrossRef - Vaseeharan, B.A.R.P. and Ramasamy, P., Control of pathogenic Vibrio spp. by Bacillus subtilis BT23, a possible probiotic treatment for black tiger shrimp Penaeus monodon. Letters in applied microbiology, 36(2): 83-87 (2003)
CrossRef - Verschuere, L., Rombaut, G., Sorgeloos, P. and Verstraete, W., 2000. Probiotic bacteria as biological control agents in aquaculture. Microbiology and molecular biology reviews, 64(4), pp.655-671.
CrossRef - Villamil, L., Figueras, A., Planas, M. and Novoa, B., Pediococcus acidilactici in the culture of turbot (Psetta maxima) larvae: administration pathways. Aquaculture, 307(1): 83-88 (2010)
CrossRef - Wang, Y.B., Effect of probiotics on growth performance and digestive enzyme activity of the shrimp Penaeus vannamei. Aquaculture, 269(1): 259-264 (2007)
CrossRef - Wang, Y.B., Tian, Z.Q., Yao, J.T. and Li, W.F., Effect of probiotics, Enteroccus faecium, on tilapia (Oreochromis niloticus) growth performance and immune response. Aquaculture, 277(3): 203-207 (2008)
CrossRef - Wasielesky, W., Atwood, H., Stokes, A. and Browdy, C.L., Effect of natural production in a zero exchange suspended microbial floc based super-intensive culture system for white shrimp, Litopenaeus vannamei. Aquaculture, 258(1): 396-403 (2006)
CrossRef - Westerdahl, A.L.L.A.N., Olsson, J.C., Kjelleberg, S.T.A.F.F.A.N. and Conway, P.L., Isolation and characterization of turbot (Scophtalmus maximus)-associated bacteria with inhibitory effects against Vibrio anguillarum. Applied and Environmental Microbiology, 57(8): 2223-2228 (1991)
- Xu, W.J. and Pan, L.Q., Effects of bioflocs on growth performance, digestive enzyme activity and body composition of juvenile Litopenaeus vannamei in zero-water exchange tanks manipulating C/N ratio in feed. Aquaculture, 356: 147-152 (2012)
CrossRef - Yeung, P.S.M., Sanders, M.E., Kitts, C.L., Cano, R. and Tong, P.S., Species-specific identification of commercial probiotic strains. Journal of Dairy Science, 85(5): 1039-1051 (2002)
CrossRef - Yusuf, M.W., Utomo, N.B.P. and Yuhana, M., Growth Performance of Catfish (Clarias gariepinus) in Biofloc-Based Super Intensive Culture Added with Bacillus sp. Journal of Fisheries and Aquatic Science, 10(6), p523 (2015)
CrossRef - Zhao, P., Huang, J., Wang, X.H., Song, X.L., Yang, C.H., Zhang, X.G. and Wang, G.C., The application of bioflocs technology in high-intensive, zero exchange farming systems of Marsupenaeus japonicus. Aquaculture, 354: 97-106 (2012)
CrossRef - Zhou, X., Tian, Z., Wang, Y. and Li, W., Effect of treatment with probiotics as water additives on tilapia (Oreochromis niloticus) growth performance and immune response. Fish physiology and biochemistry, 36(3): 501-509 (2010)
CrossRef - Zhou, X.X., Wang, Y.B. and Li, W.F., Effect of probiotic on larvae shrimp (Penaeus vannamei) based on water quality, survival rate and digestive enzyme activities. Aquaculture, 287(3): 349-353 (2009)
CrossRef
