Introduction
Abiotic stress is the prime factor reducing the crop growth and productivity of the plant.1 One such major constraint is salinity. It was increased year by year due to limited rainfall, poor management practices, changing lifestyles and wake of industrialization. Scientist2 reported that, half of the irrigation schemes were affected by salinity in worldwide. Scientists observed the negative effects of salinity from their studies, reduction of enzyme activity, reduced soluble protein content which ultimately reduced the crop growth and final yield.3 Okra is commonly growing in tropics and subtropical areas of India and sensitive to salinity environmental conditions. Under severe saline conditions there will be total failure of the crop. Many scientific reports proposed the positive effects of PGRs and nutrients on plant growth under salinity. Salicylic acid is a simple phenolic compound involved in mitigation of saline stress.4 Egamberdieva (2009)5 reported that, to break the dormancy state of the seeds, gibberellic acid plays a important role. Brassinolide treatment enhanced the proline content in oryza sativa seedling under salinity.6 Foliar spray of ascorbic acid protected the photosynthetic apparatus,7 increased the chlorophyll a, chlorophyll b, carotenoids and total pigment of tomato plant leaves.8 The present study was designed to study the effect of different plant growth regulators and nutrients on the enzyme activity, soluble protein content and growth of the bhendi hybrid and ultimately mitigation of salinity effects.
Materials and Methods
Pot culture experiment was conducted in glasshouse at Department of Crop Physiology, TNAU, Coimbatore. The pot mixture comprises of red soil, sand and vermicompost in the proportion of 3:1:1. The recommended fertilizers like 20 Kg N, 50 Kg P and 30 Kg K as basal were also mixed with the pot mixture. Bhendi is a direct sown crop hence salinity (250 mM) was imposed from the sowing onwards and it was end with the final harvest. The PGRs and nutrient solutions were given as foliar spray at 25 and 45 days after sowing and the observations were recorded at 40, 60 and 80 DAS.
Table 1: Effect of PGRs and nutrients on plant height (cm) and soluble protein content (mg g-1) of bhendi under salinity.
|
S.No |
Treatments |
Plant height (cm) |
Soluble protein (mg g-1) |
||||||
|
40 DAS |
60 DAS |
80 DAS |
Mean |
40 DAS |
60 DAS |
80 DAS |
Mean |
||
|
1 |
T1: Absolute control (Without salinity) |
32.70 |
54.93 |
68.43 |
52.02 |
12.74 |
17.62 |
9.54 |
13.30 |
|
2 |
T2: Control (Salinity) |
24.01 |
40.91 |
49.74 |
38.22 |
8.41 |
10.26 |
5.27 |
7.98 |
|
3 |
T3: Gibberellic acid (10 ppm) |
27.83 |
52.54 |
55.80 |
45.39 |
10.56 |
15.87 |
8.09 |
11.51 |
|
4 |
T4: Brassinolide (0.5 ppm) |
29.74 |
53.83 |
63.81 |
49.13 |
11.80 |
16.18 |
9.23 |
12.40 |
|
5 |
T5: Salicylic acid (100 ppm) |
31.61 |
54.20 |
65.44 |
50.42 |
11.65 |
15.73 |
9.13 |
12.17 |
|
6 |
T6: Ascorbic acid (100 ppm) |
28.30 |
51.72 |
61.72 |
47.25 |
10.59 |
14.54 |
8.12 |
11.08 |
|
7 |
T7: Benzyl amino purine (5 ppm) |
26.52 |
46.84 |
53.50 |
42.29 |
10.87 |
15.82 |
8.60 |
11.76 |
|
8 |
T8: K2SO4 (0.5%) + FeSO4 (0.5%) + Borax (0.3%) |
26.03 |
48.60 |
60.21 |
44.95 |
9.53 |
15.23 |
8.35 |
11.04 |
|
9 |
T9: 19:19:19 (1%) |
25.51 |
47.21 |
54.84 |
42.52 |
10.35 |
15.37 |
8.11 |
11.28 |
|
SEd |
0.53 |
1.00 |
1.39 |
0.27 |
0.36 |
0.18 |
|||
|
CD (P=0.05) |
1.12 |
2.10 |
2.93 |
0.58 |
0.76 |
0.39 |
|||
DAS – Days After Sowing
Soluble protein content in the leaf was estimated by using Folin Ciocalteau reagent by following the procedure described by Lowry et al. (1950).9 250 mg of leaf sample was macerated with 10 ml of phosphate buffer and the content was centrifuged at 3000 rpm for about 10 minutes. The supernatant was collected and made up to 25 ml. one ml of the supernatant was mixed with 5 ml of alkaline copper tartarate reagent and 0.5 ml of folin ciocalteau reagent and the OD value was measured at 660 nm in the spectrophotometer. The soluble protein content was expressed as mg g-1 fresh weight by using bovine serum albumin as the standard.
The proline content was estimated by acid ninhydrin protocol given by Bates et al.,(1973)10 and expressed in mg g-1. The leaf sample (0.5g) was homogenized with 10 ml of 3 per cent sulphosalicylic acid and centrifuged at 3000 rpm for 10 minutes. Two ml of the supernatant was taken and 2 ml of glacial acetic acid, 2 ml of ortho phosphoric acid and 2 ml of acid ninhydrin were added. The contents were allowed to react at 100°C for 1 hour and incubating in ice for 10 minutes. The content was transferred into separating funnel and 4 ml of toluene and mixed vigorously for 15 to 20 seconds. The chromophore containing toluene was aspired from the aqueous phase and optical density was read at 520 nm.
Nitrate reductase activity was estimated in fully expanded functional leaves following the method of Nicholas et al.,(1976)11 and expressed as μmol NO2 g-1 h-1. Plant height was measured from the ground level to the tip of the growing point and expressed cm. The total weight of fruits harvested from each plant of all pickings was added and average yield per plant was worked out and expressed in gram per plant. The data on various parameters were analyzed statistically as per the procedure suggested by Gomez and Gomez (1984).12
Table 2: Effect of PGRs and nutrients on proline content (μg g-1) and NR activity (µmol NO2 g-1 h-1) under salinity.
|
S.No |
Treatments |
Proline content (μg g-1) Days after sowing |
Nitrate reductase activity (µmol NO2 g-1 h-1) Days after sowing |
||||||
|
40 |
60 |
80 |
Mean |
40 |
60 |
80 |
Mean |
||
|
1 |
T1: Absolute control (Without salinity) |
216.56 |
247.16 |
265.42 |
243.05 |
155.50 |
164.14 |
146.54 |
155.39 |
|
2 |
T2: Control (Salinity) |
248.34 |
282.75 |
304.60 |
278.56 |
94.26 |
98.07 |
78.47 |
90.27 |
|
3 |
T3: Gibberellic acid (10 ppm) |
256.75 |
286.36 |
307.73 |
283.61 |
119.67 |
128.58 |
105.87 |
118.04 |
|
4 |
T4: Brassinolide (0.5 ppm) |
268.16 |
299.54 |
313.61 |
293.77 |
128.71 |
132.70 |
112.00 |
124.47 |
|
5 |
T5: Salicylic acid (100 ppm) |
270.08 |
303.46 |
328.13 |
300.56 |
133.98 |
145.64 |
123.01 |
134.21 |
|
6 |
T6: Ascorbic acid (100 ppm) |
271.56 |
300.26 |
312.05 |
294.62 |
131.01 |
142.01 |
120.51 |
131.18 |
|
7 |
T7: Benzyl amino purine (5 ppm) |
259.52 |
288.16 |
311.63 |
286.44 |
127.80 |
133.13 |
112.58 |
124.50 |
|
8 |
T8: K2SO4 (0.5%) + FeSO4 (0.5%) + Borax (0.3%) |
254.02 |
285.08 |
305.54 |
281.55 |
118.85 |
122.05 |
100.97 |
113.96 |
|
9 |
T9: 19:19:19 (1%) |
256.43 |
289.37 |
309.18 |
284.99 |
114.10 |
126.22 |
109.66 |
116.66 |
|
SEd |
5.92 |
5.98 |
4.75 |
2.27 |
2.44 |
3.15 |
|||
|
CD (P=0.05) |
12.44 |
12.57 |
9.99 |
4.78 |
5.12 |
6.61 |
|||
DAS – Days After Sowing
Results
The maximum soluble protein content was observed at 60 DAS and then declined. Absolute control recorded highest soluble protein content (17.62 mg g-1) while control plants recorded the lowest amount (10.26 mg g-1). Among the treatments, brassinolide recorded 16.18 mg g-1 of soluble protein followed by salicylic acid (15.73 mg g-1).
The data on proline content showed an increased trend with respect to plant age. The proline content was showed high which respect to its stressful environment. Absolute control recorded the lowest mean proline content of 243.05 μg g-1 while the control recorded 278.56 μg g-1. The result showed that the significant difference among the treatments was observed. Higher mean proline content was observed in salicylic acid (300.56 μg g-1) followed by ascorbic acid (294.62 μg g-1).
The nitrate reductase activity was increased up to 60 DAS further it was reduced. The mean nitrate reductase activity was higher in absolute control of 155.39 μg NO2 g-1 h-1 and the lower mean value was recorded in control (90.27 μg NO2 g-1 h-1). Among the treatments, salicylic acid registered highest NR activity (145.64 μg NO2 g-1 h-1) followed by ascorbic acid (142.01 μg NO2 g-1 h-1) at 60 DAS.
The trend line of the plant height was increased with respect to its age of the plant. Among the PGRs and nutrient treatments, the maximum plant height of 65.44 cm was recorded by salicylic acid treatment followed by brassinolide (63.81 cm) at 80 DAS.
The absolute control recorded maximum fruit yield of 387.52 g plant-1 and control recorded minimum fruit yield of 238.50 g plant-1. The application of PGRs and nutrient treatments showed the significant increment of the fruit yield. In this regard, brassinolide recorded highest fruit yield (364.59 g plant-1) followed by salicylic acid (350.37 g plant-1).
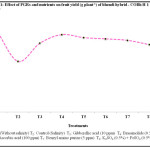 |
Figure 1: Click here to View figure |
Discussion
The plant normally contains 6 to 13 mg of soluble protein per gram of leaf sample.13 However the present study recorded the soluble protein content up to 17 mg per gram of leaf sample. In present study, it was observed that 40 per cent decrement of soluble protein content by salinity. This might be due to breakdown of proteins by proteolytic process and diversion of energy for growth and metabolism to overcome the stress situations. Brassinolide treatment increased the soluble protein content by 35.65 per cent followed by salicylic acid (34.43%) over control. Present findings coincide with Tania Das and Shukla (2011)14 who stated that, soluble protein content was decreased under the saline stress. However, rice plants treated with 8 μM brassinolide showed increased amount of soluble protein content over control.
Proline accumulation in plant cell lead to the osmotic adjustment at the cellular level acts as osmoprotectant and macromolecules stabilization.15 Proline accumulation in plants exposed salinity stress is due to low activity of oxidant enzymes.16 Increasing stress conditions lead to increase the proline content in the plant. In the present study, when the plants exposed to saline environmental condition proline content was increased up to 12.74 per cent over absolute control. Present finding was similar to earlier reports that proline content significantly increased in common bean17 and soybean18 under salt stress. The plant growth regulators and nutrients treatment increased the proline content over the control. In the present experiment concluded that, salicylic acid treatment increased the proline content by 7.32 per cent followed by ascorbic acid (2.75%) over control plants. This may be due to salicylic acid enhanced the accumulation of amino acid under stress through maintaining the proline biosynthetic pathway under stress. In chickpea plants, Asadi et al.,(2013)19 stated that, when the seeds primed with SA showed an increased proline content under salinity.
Nitrate reductase (NR) is an enzyme mediates the conversion of nitrate to nitrite, which involved in amino acid and protein synthesis.20 NR activity gives the best indication of nitrogen status of the plant and related to the normal growth and development of the plant21 and indicator of stress tolerant capacity. In the present study, NR activity was decreased up to 41.91 per cent in leaves due to salinity. This decline in NR activity may be due to the presence of high salt ions reduced the rate of enzyme synthesis and enzyme activity. In this present investigation, salicylic acid treatment increased NR activity up to 32.74 per cent of followed by ascorbic acid (31.19%) over control. This may be due to salicylic acid enhances the uptake of nitrate during stress condition. Similar results obtained by Dar et al., (2007)22 who stated that, mungbean plants treated with salicylic acid (0.1 mM) increased the NR activity 5.1 per cent and 1.5 per cent at 20 and 40 DAS respectively under salinity.
Plant height is considered as one of the important morphological characters determining growth and development of the crop. Presence of high salt concentration beyond their level of consumption leads to decrease the plant height. The present study showed the decrement of plant height by 26.52 per cent in saline environment which may be partly due to the building of salts in the apoplast of the growing tissues leading to cell and tissue dehydration. The results was supported by Iqbal et al.,(2015)23 who stated that, the plant height was decreased with increasing sodium chloride in the growth medium of citrus rootstocks. In this present investigation, salicylic acid (SA) increased plant height up to 24.19 per cent followed by brassinolide (22.20%) over control under saline condition. This might be due to SA altered the auxin, cytokinin and ABA balances in plants under stress condition which resulted in stem elongation and increased plant height. These results are in agreement with scientists24,25 who reported that exogenous foliar application of SA ameliorated the adverse effects of salt stress on growth of barley and wheat.
Salinity causes fruit yield reduction due to reduction of yield parameters like number of fruits per plant and average fruit weight of the plant. In the present study, 38.46 per cent of fruit yield was decreased in salt stressed plants over absolute control (Fig. 1). The reduction shown by the okra plants in the saline condition may be due to the high salt concentrations in the soil which caused shrinkage of cell contents, damage of membrane, disturbed avoidance mechanism, unbalanced nutrition, reductions in the development and differentiation of the tissues. These entire factors contribute towards the reduction in plant yield.26 In present investigation, brassinolide increased the fruit yield up to 34.58 per cent followed by salicylic acid (31.93%) and ascorbic acid (30.79%) over control (Fig. 1). Exogenous application of SA enhanced photosynthetic rate and chlorophyll contents ultimately yield in maize under salt stress.27 Increased seed weight by BR due to enhanced source sink relationship.28 Similar effects were obtained by Xu (2007)29 when spray application of epibrassinolide to sorghum plants at heading and grain filling stages.
Acknowledgements
The authors are thankful to the Professor and Head, Department of Crop Physiology, TNAU, Coimbatore for providing moral support, glasshouse and laboratory facilities to conduct the experiment.
References
- Boyer J. S., Plant Productivity and Environment. Sci., 218: 443-448 (1982).
- CrossRef
- Flowers T. and Yeo A., Breeding for salinity resistance in crop plants: where next. Aus. J Plant Physiol., 22: 875–884 (1995).
CrossRef - Memon S. A. Hou X. and Wang L. J., Morphological analysis of salt stress response of pak Choi. EJEAFChe, 9(1): 248–254 (2010).
- Ahmad M. Zahir A. Naeem Asghar, H. and Asghar M., Inducing salt tolerance in mung bean through coinoculation with rhizobia and plant-growth-promoting rhizobacteria containing 1-aminocyclopropane-1-carboxylate deaminase. Can. J Microbiol., 57 (7): 578–589 (2011).
CrossRef - Egamberdieva D., Alleviation of salt stress by plant growth regulators and IAA producing bacteria in wheat. Acta Physiol. Plant., 31: 861-864 (2009).
CrossRef - Sharma I. Ching I. Saini S. Bhardwaj R. and Pati, P. K., Exogenous application of brassinosteroid offers tolerance to salinity by altering stress responses in rice variety Pusa Basmati-1. Plant Physiol.Biochem., 69: 17–26 (2013).
CrossRef - Khan A. Ahmad M.S.A. Athar H.U.R. and Ashraf M., Interactive effect of foliar applied ascorbic acid and salt stress on wheat (Triticum aestivum L.) at the seedling stage. Pak. J Bot., 38: 1407-1414 (2006).
- El Sayed. Hameda El Sayed Ahmed. Salih A.M. Baziad. and Reem A.A.S. Basaba., Application of Exogenous Ascorbic Acid on Tomato (Solanum lycopersicum L.) seeds under NaCl Salinity Stress. Int. J Cur. Res. Biosci. Plant Biology, 2(5): 33-46 (2015).
- Lowry O. H. Rosebrough N. J. Farr A. L. and Randall R. J., Protein measurement with the folin phenol reagent. J Biol. Chem., 193(1): 265-275 (1950).
- Bates L. S. Waldren R. P. and Teare I. D., Rapid determination of proline for water stress studies. Plant Soil, 39: 205-207 (1973).
CrossRef - Nicholas J. C. Harper J. E. and Hageman R. H., Nitrate reductase activity in soybeans. Effect of light and temperature. Plant Physiol., 58: 731-735 (1976).
CrossRef - Gomez K. A. and Gomez A. A., Statistical procedures for agricultural research. (2nd Ed.) John Wiley and sons, New York, USA (1984).
- Metodiev M. and Demirevska-Kepova K., Rubisco quantitation in leaves of different barley varieties by enzyme-linked immunosorbent assay. J. Exp. Bot., 247: 155–158 (1992).
CrossRef - Tania Das. and Shukla Y. M., Effect of brassinolide on biochemical constituents in rice (Oryza sativa L.) under salinity stress. The Asian J Exp. Chem., 6(1): 22-25 (2011).
- Tawfik K. M., Evaluating the use of rhizobacterin on cowpea plants grown under salt stress. Res. J Agri. Biol. Sci., 4(1): 26-33 (2008).
- Sudhakar C., Change in the antioxidant enzyme efficacy in two high yielding genotypes of mulberry (Morus alba L.) under NaCl salinity. Plant Sci., 161: 613-619 (2001).
CrossRef - Khadri M. Tejera N. A. and Lluch C., Alleviation of salt stress in common bean by exogenous abscisic acid supply. J. Plant Growth Regul., 25: 110-119 (2006).
CrossRef - Yoon J. Y. Hamayun M. Lee S. K. and Lee I. J., Methyl jasmonate alleviated salinity stress in soybean. J Crop Sci. Biotech., 12: 63-68 (2009).
- Asadi M. Heidari M. A. Kazemi M. and Filinejad A. R., Salicylic acid induced changes in some physiological parameters in chickpea (Cicer arietinum L.) under salt stress. J Agri. Tech., 9(2): 311-316 (2013).
- Solmonson L. P. and Barber M. J., Assimilatory nitrate reductase functional properties and regulation. Ann. ReviewPlant Physiol., 41: 225-253 (1990).
- Srivastava H. S., Regulation of nitrate reductase activity in higher plants. Phytochem., 17: 725-733 (1980).
CrossRef - Dar Z. M. Hemantaranjan A. and Pandey S. K., Effects of salicylic acid on growth, nitrate reductase activity and mineral uptake by mungbean (Vigna radiata L.) Under induced salinity. Legume Res., 30(2): 133-136 (2007).
- Iqbal N. Umar S. and Khan N.A., Nitrogen availability regulates proline and ethylene production and alleviates salinity stress in mustard (Brassica juncea). J Plant Physiol., 178: 84-91 (2015).
CrossRef - El Tayeb M. A., Response of barley grains to the interactive effect of salinity and salicylic acid. Plant Growth Regul., 45: 215-224 (2005).
CrossRef - Arfan M. Habib A. and Ashraf M., Does exogenous application of salicylic acid through the rooting medium modulate growth and photosynthetic capacity in two differently adapted spring wheat cultivars under salt stress. J Plant Physiol., 164(6): 685-694 (2007).
CrossRef - Aslam R. Boston N. Amen N. Marie M. and Safdar W., A critical review on halophytes: salt tolerance plant. J Medicinal Plant Res., 5(33): 7108-7118 (2011).
- Tufail A. Muhammad Arfan. Ali Raza Gurmani. Abdullah Khan. and Asghari Bano., Salicylic acid induced salinity tolerance in maize (Zea Mays). Pak. J Bot., 45(1): 75-82 (2013).
- Abdel Hamid. and Ebitahal M., Physiological effect of some phytoregulators on growth productivity and yield of wheat plant cultivated in new reclaimed soil. Ph.D. degree, Botany Department Univ. college of women for Arts, Science and Education-Ain Shams Univ. Cairo, Egypt (2008).
- Xu H.L., Effect of exogenous epibrassinolide and abscisic acid on grain yield of sorghum plants growing under soil water deficit. In Dryland Crop Production: Technology Break through and study cases. H.L. Xu, Ed (Trivandrum, India: Research Signpost). 195-202 (2007).
