Introduction
Grasshoppers and locusts are major pests in most countries of the Sahel zone like Senegal. In invasion years, their control requires annually large amounts of synthetic pesticides sprayed over about 1,000,000 ha per year (Launois Luong et al., 1988). In locust invasion years, about 2,000,000 ha are treated with pesticides to achieve a control over the large migrating locusts and grasshoppers populations (Anon., 1997). Besides negative economic impact, should be added other impacts on the environment and on health of humans as well as on animals. With respect to negative impact on environment, the decontamination of 1 ton of sand contaminated with pesticides is estimated at 26,000,000 CFA. With a total of 40,000 tons of soil to be decontaminated in pesticide storages sites in 2004, a total of 1,040 billion CFA was needed to mitigate environmental pollution (Locustox, 2009).
Gradually, environment friendly alternatives pesticides are being developed to control locusts. Biopesticides like those having insect pathogenic microorganisms as active agent are increasingly developed for operational use in several countries of the Sahel zone.
In West Africa, particularly in Senegal, Metarhizium acridum, a locust pathogenic fungus gave excellent test results in controlling grasshoppers. The fungal strain was further formulated to a biopesticide, found to be very effective against locusts and grasshoppers. Those insects belong to the order Orthoptera, and consist among others, with Schistocerca gregaria, Oedaleus senegalensis, Hieroglyphus daganensis, Locusta migratoria, etc. The spores of Metarhizium acridum represent the active ingredient of the bioinsecticide, intended for locust control. However, the widespread use of this biopesticide in operational conditions in Senegal is facing a technical problem due to a lack of stability of the active ingredient during storage. In fact, the biological effectiveness of the product in operational conditions drops below the critical rate of 85% infectivity (Jaronski, 2000) in different environments. Nonetheless, the feasibility of this control method, in particular during the hot rainy season, characterized with rapid buildup of locust populations, and a worsening of roads and transportation systems, requires the pesticides to be stored in close proximity to the agrosystems at risk and applied as needed.
The present work therefore was intended to look at the influence of temperature on the survival of the active ingredient of a Metarhizium acridum based biopesticide in different formulations, with special focus on the storage facilities of the National Plant Protection Organization over the country.
Materials and Methods
The Fungal Biocontrolagent
Metarhizium acridum strain IMI 330189 is the active ingredient of the biopesticide. It is a fungus of the class ascomycete with Metacordyceps sp as teleomorph. Species of the genus Metarhizium live naturally in the soil litter almost everywhere in the world. Metarhizium acridum strain IMI 330189 was isolated from a parasitized Ornithacris cavroisi found in Niger (Kooyman, 2007). The biopesticide is formulated as a powder composed with dry spores or spores mixed with vegetable oil as oil formulation.
Study Sites
The study of the viability of the spores of Metarhizium acridum in different formulations of the biopesticide during storage was carried out in the storage facilities of“Direction de la Protection des Végétaux” (DPV), the National Plant Protection Organization. Those storage facilities are located in different cities scattered over the whole country, like Richard Toll, Louga, Gossas, Nioro duRip and Dakar.They are designed for almost permanent storage of insecticides for locust control targeting O. senegalensis and S. gregaria.The mean temperatures and relative humidity recorded in the storages facilities during the experiment are listed in Table 1.
Storage Test
The study of the viability of the spores of Metarhizium acridum in different formulations of the biopesticide during storage was carried out in the storage facilities of “Direction de la Protection des Végétaux” (DPV), the National Plant Protection Organization. Those storage facilities are located in different cities scattered over the whole country, like Richard Toll, Louga, Gossas, Nioro du Rip and Dakar. They are designed for almost permanent storage of insecticides for locust control targeting O. senegalensis and S. gregaria. The mean temperatures and relative humidity recorded in the storages facilities during the experiment are listed in Table 1.
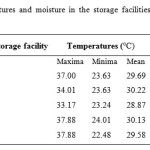 |
Table 1: Mean temperatures and moisture in the storage facilities of DPV during the test period. |
Samples the biopesticide in dry spores formulation as well as oil formulation were placed in test tubes. For dry spores formulation, 1 g of powder was sampled and 5ml of oil formulation. In each storage facility, 5 tubes for oil formulation and 5 other tubes for dry spores formulation were kept. The germination rate was recorded before storage and every 30 days, as described by Moore et al., (1996). For the germination test, 1 ml was sampled from oil formulation containing tubes and 0.1 g from dry formulation treatment. The samples were kept in small vials closed with parafilm and placed in a humid chamber for 24 hours (Moore et al., 1996). Thereafter, 10 ml sterile water and a droplet of Tween 80 were added prior to centrifugation for 5 minutes at 2500 rotations / min. The solvent was gently removed and the pellet, composed with spores is mixed with 10 ml sterile water and Tween 80. This spore suspension is used for germination test by plating a 20, 50 and 100 µL into petri dishes containing Sabouraud Dextrose Agar culture media. For the oil formulation, the sampled volume was completed to 10 ml with sterile water and Tween 80. The mixture was centrifuged and the pellet collected and suspended in 10 ml sterile water and Tween 80 before being plated onto the culture media.
Effect of Temperature on Spore Germination, Mycelial Growth and Sporulation
For the study of mycelial growth and sporulation ability, petri dishes with the culture media were inoculated with a 6 mm diameter of 4-5 days old culture of the fungus and incubated at different temperatures. For sporulation performance mature conidia were harvested after 3 weeks of incubation at constant temperatures in the dark. To collect the spores, a fine brush and 10 ml sterile water and Tween 80 were used to prepare a suspension of spores. The concentration of the suspension of spores obtained was determined using the grid cell of Neubauer (Tefera and Pringle, 2003).
Mycelial growth of the fungus at different temperatures was measured by taking the average of two perpendicular diameters of colony in the petri dish. The diameter was measured every 24 hours for 11 days (Tefera and Pringle, 2003).
The effect of a range of constant temperatures was evaluated on the germination of spores extracted from the biopesticide. The number of germinated conidia was determined by over a 20 conidia sample, repeated 10 times after 6 hours, 12 hours, 18 hours and 24 hours of incubation.
Data Analysis
An analysis of variance was performed for data with a normal distribution. Data expressed in percentages were first normalized by arcsinus transformation. The statistical analysis software Mini tab 13th edition for Windows was used. Means were separated using the Student test & Newman Keuls at 5 %.
Results
Effect of Different Temperatures on Germination of Spore of M. Acridum
The spore germination of M. acridum at different temperatures gave variable results (Fig. 1). The highest rates were obtained at 33 °C and 28 °C with 18% and 21.12% respectively after only 6 hours of incubation. The germination rate reached over 90% after 24 h. When temperatures reached 35 °C and 37 °C, the germination rate did not cross the 10% bar after 24 hours. The analysis of variance showed a highly significant difference between treatments (p ≈ 0.000).
 |
Figure 1: Influence of different temperatures on spore germination of M. acridum (n = 10; p ≈ 0.000). |
Effect of Different Temperatures on Mycelial Growth of M. Acridum
A daily average mycelia growth of 3.648 mm was observed at 28 °C. It decreased down to 1.93 mm at 33 °C, and further to 0.78 mm at 35 °C and 0,012 °C at 37 mm. The colony diameter was subsequently the highest at 28 °C and 33 °C, with respectively 40 mm and 25 mm after 12 days (Fig. 2). Colony size reached barely 10 mm and 6 mm respectively at 35 °C and 37 °C after 12 days of incubation. The analysis of variance showed highly significant differences between treatments (p <0.001).
 |
Figure 2: Influence of different temperatures on the mycelial growth of M. acridum (P <0.001). |
Effect of Temperature on the Production of Conidia
At 28 °C, about 3*108 spores per petri dish were produced (Fig. 3). Spore production reached 6.8*107 spores per petri dish at 33 °C and was suppressed at 35 °C and 37 °C. Variance analysis showed highly significant differences (p <0.001).
 |
Figure 3: Production of spores by M. Acridum under different incubation temperatures (n = 10; p < 0.001) |
Effect of Storage in Operational Conditions on Survival of M. Acridum
The storage test in DPV facilities over different sites in Senegal gave the results in figure 4. The survival rate was higher for dry spores formulation compared to oil formulation. This survival rate was over 90% for spores in dry formulation in all sites. This level of survival rate of spores was recorded for oil formulation only in Dakar. The general trend for oil formulation shows a quick decrease from the second month of storage on wards to reach values below 30% at the end of the experiment, 90 days later. Variance analysis showed highly significant differences between treatments (p <0.001).
 |
Figure 4: Rate of germination of spores of M. Acridum in operational conditions of storage in different sites in Senegal (n = 5; p < 0.001) (SH: Oily suspension, SS: dry spores). |
Discussion
Fungal growth results from spore germination, mycelial growth and sporulation to achieve a virulence and good dispersal over space and time. These parameters are often directly affected by climatic factors such as moisture level or temperature (Luz et al., 1998). In the present study, germination of spores and mycelial growth of M. Acridum are higher at 28°C and 33°C. Higher temperatures lead to a significant decrease of these biological parameters, with a survival of the biological agent compromised at 37°C.
Locusts are known to hang at the top herbs to enjoy the sun and raise their body temperature. If this behavior occurs at 37 °C, for a long period, the progression of fungal infections may slow down. In fact, Ouedraogo (1996) and Dimbi et al., (2004) showed that mortality of migratory locust goes from 78 % in the shade, down to 21% in the sun within 6 days. It should however be reminded that daily temperatures in the Sahel are very variable and furthermore, locust are more frequent in habitats with milder temperatures. These environments are more favorable for germination, mycelial growth and sporulation of the fungus.
Sporulation of M. acridum is abundant at 28 and 33 °C. It is completely inhibited at 37 °C, as already reported by Maniania and Una (2001). Fargues et al., (1997) demonstrated the existence of a positive correlation between virulence, spore germination, mycelial growth and spore production. According to these authors, spore production depends of dry matter of the mycelium produced that is affected by climatic factors but also the culture substrate as well. These observations seem to point in the direction of the use of fungal strains best adapted to the ecological conditions of the target area. This assumption, however, should be extensively studied to better understand the conditions of use of M. acridum in the areas of migration of locusts across Africa. With a very low germination rate of spores, poor mycelial growth and no sporulation at 37 °C, this strain of M. acridum may be safe to human. These observations are consistent with the findings of EPA (2002) and Mancebo et al., (2005) who reported no significant risk for humans and mammals, in relation with the use of M. acridum.
The survival rate of spores of M. acridum in the 2 formulations showed a good performance of the dry spores within 3 months, covering the whole rainy season, at which crops are at risk. In operational conditions, dry spores formulation could be stored in the target sites to benefit from local availability, facilitating the use of the biocontrol product in the field. The oil formulation in contrast, is not suited to high temperature and low moisture conditions for a long period. However, its use could be justified within 1 month as the threshold for obtaining a good efficiency is greater than 85% (Junkins and Grzywacz, 2000). The position of the central storage facility in Dakar at the seaside, with reduced variations of temperatures while benefiting from high moisture could be the reason for the prolonged good conservation of the product over the 3 months in contrast to the inland sit
References
- Anonyme, UNITAR/IOMC/FISC Dakar Sénégal pp 32 – 74 (1997).
- Bischoff, J.F., Rehner, S.A., Humber, R.A., Mycologia 101, 512-530. (2009).
CrossRef - Dimbi S, Maniania KN, Lux AS, and Mueke M., BioControl 49, 83-94. 2004.
CrossRef - EPA. Environmental Protection Agency, Office of Pesticide Programs, 2002 edition, 40p (2002).
- Fargues J, Goettel MS, Smits N, Ouedraogo A and Rougier M., Mycologia 89, 383-392. (1997).
CrossRef - Jaronski S., USDA ARS Northern Plains Agricultural research laboratory, Sidney MT. 7p (2000).
- Junkins NE and Grzywacz D., Biocontrol Sci Tech 10, 753-777 (2000).
CrossRef - Kooyman C., Atelier international sur l’avenir des biopesticides en lutte contre le criquet pèlerin Dakar Saly 49p (2007).
- Launois Luong MH, Launois M and Rachadi T., Collection Acridologie Opérationnelle no 3. PRIFAS. Acridologie Opérationnelle Ecoforce® Internationale. Département GERDAT (1988).
- Locustox, Rapport de mission CERES-Locustox, Dakar 25p (2009).
- Luz C, Tigano MS, Silva IG, Cordeiro CM and Aljanabi S., Mem Inst Oswaldo Cruz 93 (6), 839-846 (1998).
CrossRef - Mancebo A, Gonzalez F, Lugo S, Gonzalez B, Bada MA, Aldana L, Gonzalez Y, Artiga M and Dasha F., Pakistan Journal of Biological Sciences 8, 970-973 (2005).
- Maniania NK and Ouna E. Forth Annual Report Development of Biopesticides for Grasshopper and Locust Control in Sub-Saharan Africa. Blacksburg, pp 47-51 (2001).
- Moore D, Douro-Kpindou OK, Jenkins NE and Lomer CJ., Journal of Invertebrate Pathology 6, 51-61 (1996).
- Ouedraogo A., Thèse de Doctorat, Université de Montpelier 72p (1996).
- Tefera T and Pringle K., Biocontrol Science and Technology 13, 699-704 (2003).
CrossRef
