Introduction
Agricultural productivity and food security in Sub-Saharan Africa(SSA) are highly threatened by nutrient depletion in the soil (Diagana, 2003). About 60% of the total land area is marginally suitable for cultivation due to limited organic matter and water retention capacity while close to 30% is considered degraded and vulnerable to erosion (Asenso-Okyere and Jemaneh, 2012).Sub-Saharan Africa suffers from extensive soil degradation, threatening the livelihoods of the 70% of Africans who are involved in agriculture.As the region’s population continues to grow rapidly (3% per annum), the carrying capacity of its agricultural land is becoming lower (Diagana, 2003). As water and land become increasingly limited, improving agricultural productivity becomes a fundamental factor in addressing future food security problems in SSA (Asenso-Okyere and Jemaneh, 2012). Like other Sub Saharan countries, Nigerian soils appear to behighly depleted because farmers remove the soil nutrients without putting back anything to replenish the soil. In northern Nigeria and with the people predominantly farmers, farmlands are constantly under cultivation all year round in rainy and dry season with no fallow periods.Vegetable production occurs mainly during the dry season, while cereal crops are produced during the rainy season. With this trend in agricultural practice and its consequent effect on soil fertility, food security and livelihood of the rural people in the country is being threatened. Scientists are actively devising techniques for a more sustainable agriculture to meet the need of the growing population. Among several other efforts to address the problem of agricultural productivity in SSA is the innovation of the ecological and highly productive biochar-enriched Super Vegetable Garden (SVG) by Pro Natura International in association with the social business JTS seeds, Paris. The SVG is a combination of the Improved Tropical Garden (ITG) seeds with its corresponding kit and Pro Natura’s biochar and is applicable to all tropical zones.Among the items enclosed in the kit is the crop veil made up of transparent white cotton material. These veils help counter water evaporation, limit the duration of stomatal closure during hot hours of the day, thus increasing photosynthesis and consequent increase in vegetative growth, and also protect the crops against insect pests and heavy rainstorms (Pro Natura International, 2009). Biochar has the potential characteristic of increasing water retention capacity and nutrient especially in depleted tropical soils (Bakewell-Stone, 2011). Previous research has shown thatadding charcoal dust to soil can reverse soil fertility decline, improve crop yields, and improve plant response to fertilizer. Early adoption by farmers increased yields by approximately 23% in the first season of application, and 30% a year later (USAID, 2013).
The effect of biochar amendment on crops such as maize (Zea mays), soybean (Glycine max), radish (Raphanussativus), sorghum (Sorghum bicolor), potato (Solanumtuberosum), wheat (Triticumaestivum), pea (Pisumsativum), oats (Avenasp), rice (Oryza sativa), and cowpea (Vignaunguiculata) (Lehmann et al., 2003; Chan et al., 2007) and on sweet potato (Ipomoea batatas) yield and quality (Douet al., 2012) has been studied from different parts of the world but no study has been carried out on tomato.Biochar research in Africa has not been extensively carried out. The only exceptionsare the studies carried out by few scientists on the aspects of improving physical and chemical properties and management ofhighly weathered soils with charcoal in the tropics, and Mycorrhizal responses to biochar in soil, (Warnock, Lehmann, Kuyper and Rilig, 2007; Lehmann and Rondon, 2006; Glaser, Lehmann and Zech, 2002;).
Tomato, which is the major and widely cultivated vegetable crop in northern Nigeriais produced by farmers for economic as well as for consumption purposes. Application of innovative agricultural method to improve the yield of this cropagainst the continuing depletion of soil organic nutrients will help in improving the livelihood of this rural populace.It was therefore, the aim of this research to investigate the effect of biochar and crop veil on the growth and yield of tomato in Jos, north central Nigeria.
Material and Methods
Study Site
The research was carried out in Laminga village Jos, North central Nigeria (coordinates: 8º 57’E, 9º51’N.) about 8 km east of Jos metropolis. The presence of large expanse of relatively flat lands makes the Laminga village a predominantly agricultural area producing several tones of vegetable crops annually (fig.1). It has a mean annual rainfall of 1375 mm -1750 mm per annum with a mean temperature of 10 -13 ºC. Farming occurs almost all through the year during both rainy and dry seasons. Crops produced during the rains are majorly cereal crops such as maize, guinea corn and millet, while vegetable crops are produced largely during the dry season under irrigation. The vegetable crops produced include tomato, cucumber, green pepper, green beans, Irish potato, and green peas.
 |
Figure 1: Google Earth map of the survey plot showing the SVG and traditional beds side by side. Click here to View figure |
Experimental Design
The research was conducted in Laminga villageduring the dry season from March to June, 2012 which allows for manual irrigation of crops and application of adequate and equal amount of water to both treatments. The experiment consists of the preparation of the SVG and traditional beds, nursery production of seedlings, transplanting of seedlings on the prepared beds, post planting operations which include fertilizer application, watering, spraying insecticide and weeding. Data for growth and fruit yield were collected.
Preparation of SVG and Traditionalvegetable Beds
The same number of SVG and traditional beds (4 each) were laid out using the same measurement of 15 m2(12.5 m longand 1.2 m wide) and 1 m gap between beds to allow for accurate comparison. The dimensions of the beds were mapped out using a 100 m measuring tape, iron pegs were mounted at the 4 corners of each bed and tied round the bed dimensions with strings. For the SVG beds, trenches of 20cm wide and 45 cm deep were dug round the edges of the beds. The edges of the trenches were lined with black plastic sheets with long pieces of wood placed inside them to act as floaters and to keep the edge above the level of the growing bed (Bakewell-Stone, 2011). The earth in the middle of the growing bed was broken to the sides of the trenches to hold the lining in place. A draining trench of 50cm deeper was dug in the centre of the growing bed and filled with dried grass after which the growing beds were filled back with the earth and leveled (fig. 2). For the traditional vegetable beds, the soil was dug and raised round the bed dimensions into rectangular heaps of about 50 cm high.
The soil on both SVG and traditional beds were mixed with2 wheel barrows each of cow and poultry dungon each bed.Biochar was prepared by grinding wood charcoal into powder and sieved using a 2 mm mesh. After preparing the subsoil, 30 kg of biochar was spread on the SVGbeds as evenly as possiblebut not on the traditional beds.
 |
Figure 2:The SVG bed preparation showing the blackpolythene lining and the draining trench filled with grass. Click here to View figure |
Nursery Preparation, Transplanting and Post Planting Operations of Seedlings
A piece of land with loamy, fertile and well drained soil was selected for the nursery preparation. The soil was deeply cultivated (turned and leveled) using the pick axe, gardening fork and shovels. After the soil had been leveled, a flat bed of 4 m2 area was laid, manure obtained from cow and poultry dung was applied to enhance the soil fertility. Tomato seeds (UTC variety widely used in north central Nigeria) were broadcasted on the bed and watered frequently. Grass mulch was used to cover the bed to retain moisture in order to ensure high quality germination.The seedlings were raised in the nursery for the period of 4 weeks after which they were removed with the use of a garden fork and transplanted to the SVG plot at 30 cmspacing. The transplanted seedlings were covered with the veils a week after transplanting when the seedlings had established on the ground. The veils were maintained for 4 weeks during the vegetative growth period and withdrawn when flowering had fully set in.Two hundred and forty litres of water was applied daily to the SVG and traditional beds using a 10 litres watering can. Nitrogenous Fertilizer (NPK,20:10:10) was applied twice at 3 weeks interval at an application rate of 1 kg per 15 m2 bed per application. First fertilizer application was carried out a week after transplanting while second application was done 4 weeks after transplanting.In order to control insect pests, the plots were sprayed with insecticide after removal of the veil.
Data Collection
Data was collected using systematic random sampling. Each bed which was 12.5 metres long and 1.2 metres wide was divided into 5 plots of 2.5 by 1.2 metres each. Three plots were systematically chosen at the first, third (middle) and fifth (last) positions fromwhich the height of all the plants within the plots were measured with the use of 100 cm measuring rule.Data was collected twice; 4 and 6 weeks after planting. Data for fruit yield was collected once by counting the number of fruits on each plant within sample plots when maximum fruiting had been achieved.
Data Analysis
Data collected was analysed using Microsoft excel and R-statistical package (version 3.0.1). Normal Q-Q plot was used to test for normality of the response variable which was non-normally distributed. Mann Whitney u test was used to test for relationship in growth and yield of tomato between SVG and traditional beds.
Results and Discussion
Stem growth was significantly higher in tomatoes grown on SVG beds (with charcoal and covered with veil)than traditional beds without charcoal and without covering (Mann Whitney u test: W = 91446.5, P< 0.001; fig. 3). Fruit yield in tomato was also higher on SVG beds than traditional beds (Mann Whitney u test: W = 12434, p <0.001; fig. 4).
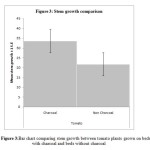 |
Figure 3: Bar chart comparing stem growth between tomato plants grown on beds with charcoal and beds without charcoal. Click here to View figure |
 |
Figure 4: Bar chart comparing fruit yield between tomato plants grown on beds with charcoal and beds without charcoal. Click here to View figure |
Plant survival and growth is critically influenced by abiotic factors including water, wind velocity, and light(Raven and Johnson, 2002).The effect of biochar and veil covering in terms of water retention may be responsible for the high growth and yield obtained in our plant. Biochar usually has the potential of activating soil micro organisms and increasing the water retention capacity of the soil thereby increasing photosynthetic rate and consequent increase in growth of plants. Due to their chemical and genetic makeup, crop plants vary in their requirement for light intensity and water availability conducive for their growth(Hogan, 2011). These basic requirements determine the locations and seasons in which they can thrive and produce. C3 plants thrive well in moderate sunlight, moderate temperature and high ground water availability(Hogan, 2011), but in dry areas especially during dry seasons, nearly all days plants encounter light intensities that exceed their photosynthetic capacity (Ort, 2001).
In order to reduce water loss in dry areas, C3 plants shut their stomata, which stops CO2 from entering the leaves, reduces the concentration of CO2 in the leaves and lowering CO2:O2 ratio therefore, increasing photorespiration which consequently limits growth (Raven and Edwards, 2001). Tomato like other C3 crop plants (Sanchez-Rocha et al., 2005), exhibits maximum photosynthetic capacity during mid-morning with photosynthesis declining throughout the afternoon as stomatal conductance declines in response to declining leaf water potentials (Wise et al., 1990). This probably explains why stem growth in tomato was about 35% higher on beds treated with charcoal and covered with veil than beds without charcoal and covering. High increase in fruit yield seem to be a consequence of the high vegetative growth of the crop since longer stems tend to provide more positions for flowers and subsequent fruits. Tomato yield from beds treated with charcoal and covering was 76% higher than the yield from beds without charcoal and covering. Application of biochar together with nitrogen fertilizer probably enhanced biochar effect on crop growth and yield. This may be because biochar serves as a carrier substrate for nitrogen (N) which increases the effectiveness of biochar by retaining and preventing the leaching of N beyond the reach of plants. Chan et al., (2007)found that plant yield decreased when biochar was applied at 10 t/ha but increased when the biochar was applied with Nfertilizer. Chan et al., (2008) reported96 % increase in radish yields from application of biochar in a greenhouse experiment and suggested that this increased yield was largely dueto the ability of biochar to increase N availability. Another study on maize reported by Major et al., (2010a) showed that maize increased to about 140% during the fourth year of biochar application and this was attributed to increased pH and nutrient retention in soil.
Though several studies have reported significant increases in crop yield with biochar, a few studies reported negative effect of biochar on crop yield. Results of studies from Schultz et al., (2014) showeda negative effect on growth and yield of oat plant with application of biochar on soil.
Conclusion
Charcoal application has been used by indigenous people from time immemorial to improve the fertility of their soil. Now scientists are finding out the importance of applying charred biomass to soils. Charcoal which is referred to as biochar when it is intended for use on the soil has been discovered by scientists as a means of replenishing soil fertility in degraded soils, and also as carbon sink in mitigating climate change. Especially in Sub Saharan Africa, biochar has been used to increase food security among rural people through its application in depleted soils to increase crop production. The result of this research like similar findings from other scientists has revealed the value of biochar in recovering soil fertility and increasing crop production in depleted Nigerian lands. With the current efforts of international and national governments and Non Governmental Organizations (NGOs) to fight poverty and to ensure food security among the largest of the world’s population, more research into soil enrichment and innovation of sustainable agricultural methods need to be given priority by researchers, government and policy makers.
Acknowledgement
We are grateful to Pro Natura International (PNI) Paris, Pro Natura International Nigeria (PNIN), the Social Development Fund of the Embassy of France in Nigeria and the A. P. Leventis Ornithological Research Institute Jos for funding the biochar project in Jos, Nigeria.
Refrences
- Diagana B., Land degradation in Sub Saharan Africa: what explains the widespread adoption of unsustainable farming practices?,Department of Agricultural Economics and Economics, Montana State University, Bozeman, MT, USA, 17 (2003).
- Asenyo-Okyere K. and Jemaneh S. Increasing agricultural productivity and enhancing food security in Africa, International Food Policy Research Institute, Washington, DC, 34 (2012).
- Pro Natura International, Super Vegetable Gardens & New Oasis, 4 (2009).
- Bakewell-Stone P., Introduction to Biochar in Tropical Agriculture, Pro Natura International, Paris, France, 51 (2011).
- U.S. Agency for International Development.Scaling biochar: Investing in soils, improving livelihoods and sequestering carbon(2013).Retrieved from http://www.usaid.gov/div/portfolio/scaling-biochar-investing on 16th April, 2014.
- Warnock D.D., Lehmann J., Kuyper T.W. &RiligM.C..Plant Soil, 300, 9–20(2007).
CrossRef - Lehmann J. and Rondon M. Bio-char soil management on highly weathered soils in the humid tropics. In Uphoff, N. (ed.) Biological Approaches to Sustainable Soil Systems, CRC Press, Boca Raton, FL, USA., 517-530 (2006).
CrossRef - Glaser B., Lehmann J. and Zech W., Biology and Fertility of Soils, 35, 219-23 (2002).
CrossRef - Lehmann J., da Silva Jr. J. P., Steiner C., Nehls T., Zech W. and Glaser B., Plant Soil, 249, 343-357 (2003).
CrossRef - Chan K. Y., Van Zwieten L., Meszaros I., Downie A. and Joseph S., Australian Journal of Soil Research, 45, 629-634 (2007).
CrossRef - Dou L., Komatsuzaki M. and Nakagawa M. Int. Res. J. Agric. Sci. Soil Sci., 2, 318-327 (2012).
- Raven P. H. and Johnson G. B., Part XI Plant Growth and Reproduction, In: Biology (6ed), Boston, McGraw-Hill, 836, (2002).
- Hogan M. C., “Respiration”. Encyclopedia of Earth. Eds. Mark McGinley and C. J. Cleveland. National Council for Science and the Environment. Washington, D.C (2011).
- Ort R. D., Plant Physiol, 125, 29-32 (2001).
CrossRef - Raven J. A. and Edwards D., J Exp Bot,52, 381–401 (2001).
CrossRef - Sanchez-Rocha S.,Vargas-Luna M., Gutiérrez-Juárez G., Franco R. H., Madueño L. and Olalde-Portugal V., J. Phys. IV France 125, 803-806, (2005).
CrossRef - Wise W.W., Frederick J. R., Alm D. M., Kramer D. M., Hesketh J. D., Crofts A. R. and Ort D. R., Plant Cell Environ,13: 923–931 (1990).
CrossRef - Chan K. Y., Van Zwieten L., Meszaros I., Downie A. and Joseph S., Australian Journal of Soil Research, 46, 437-444, (2008).
CrossRef - Major J., Lehmann J., Rondon M. and Goodale C., Glob Change Biol, 16,1366-1379 (2010a).
CrossRef - Schulz H., Dunst G. and Glaser, B.,Agronomy, 4, 34-51 (2014).
CrossRef
