Introduction
Casuarina equisetifolia L., a member of the Casuarinaceae family, native to Australia, New Guinea, Southeast Asia and India. It is a remarkable evergreen tree that exhibits both dioecious and monoecious characteristics. Casuarina equisetifolia exhibits rapid growth during its initial seven years, achieving an annual increase of 1.5 to 2.5 meters. The maximum volume yield is generally observed between the ages of 15 and 20 years, reaching approximately 7 to 10 cubic meters per hectare annually. One of the notable features of this tree is its ability to produce high-quality fuel wood and excellent charcoal. Additionally, it possesses termite resistance and exceptional durability, making it a valuable material for construction purposes. The rustling of the wind passing through its canopy creates a distinctive whistling sound, earning it the name “Gaalimara” or Wind tree. Moreover, this tree plays a crucial role in safeguarding coastal environments from storms and tsunamis, and it has become a key component in various coastal afforestation programs aimed at creating bioshields.1 Furthermore, C equisetifolia is utilized in agroforestry systems alongside vegetable and pulse crops, further highlighting its versatility.2 It is also employed in the production of paper and serves as scaffolding in building construction.3
C. equestifolia utilizes various Actinobacteria such as Frankia sp., Micromonospora sp., and others to fix nitrogen. Casuarina plants, known as good pioneer plants, can be employed in degraded lands to enhance fertility. This aids in the pedogenetic process and enables nitrogen fixation with the assistance of Frankia.4 Actinobacteria possess the capability to boost plant growth by producing growth-promoting substances like auxins, gibberellins, and more.5 Micromonospora is recognized as a root nodule endophytic bacteria that supports the growth of Casuarinas (C. equestifolia and C. junghuhiniana) and enhances biocontrol potential against Ralstonia solanacerum.6
Leifsonia sp., an endophytic bacterium found in sugarcane and grasses, has been identified by Young.7 While some species within this genus are known to be pathogenic, others have been shown to be beneficial for plants. According to Kang, the culture filtrate of L. soli has been found to significantly enhance the biomass, hypocotyl, and root lengths of cucumber seeds when compared to controls that were not inoculated or treated with distilled water.8 Lin observed that Casuarina has a rich microbiome in its litter, roots, seeds, and branchlets.9 The current study focuses on isolating Leifsonia sp. from the root nodules of C. equisetifolia and investigating its potential as a plant growth promoter in Zea mays.
Materials and Methods
Sample Processing
Root nodules obtained from the rhizospheric area of Casuarina equisetifolia were gathered. The nodules underwent three washes with tap water to eliminate any debris. Subsequently, they were surface sterilized twice with 1% Mercuric chloride (HgCl3) and rinsed five times with distilled water. The root nodules were then crushed in 1 ml of distilled water using a mortar and pestle.
Isolation of endophytic bacteria
The root nodules that had been crushed were subjected to the serial dilution technique and subsequent plating. The Defined Propionate Media (DPM) plates were then placed in an incubator at a temperature of 28°C for a duration of 21 days. Following the incubation period, the plates were carefully examined using a stereo microscope. The morphology of the colonies obtained from the isolates was duly recorded.
In Vitro Screening of Isolates for PGPR activity
Antagonistic activity
The strains were centrally inoculated on Starch Casein agar plates, followed by an incubation period at 30°C for 24 hours. The pathogens for the test was subcultured from the Forest Protection division, ICFRE-IFGTB Coimbatore stock cultures. Subsequently, different test pathogens such as Sclerotium, Alternaria, Phytophthora, Diploidia, Fusarium species were streaked along the periphery of the plates after the initial 24-hour incubation. The plates were then incubated once more at 30 °C for an additional 24 hours.10 The presence and characteristics of the inhibition zone were observed and determined to be either beneficial or not. Percent inhibition was calculated as follows,

Organic acid production
The test organism was introduced into the Minimal Salt Medium (MM9) broth and allowed to incubate for a period of 2-3 days at a temperature of 30°C. Following the incubation period, methyl red indicator was added. The alteration in color observed in the media serves as an indication of a positive result.11
Ammonia Production
After being freshly grown, the cultures were introduced to 10 ml of peptone water and left to incubate for 48 hours at a temperature of 30 °C. Subsequently, 0.5ml of Nessler’s reagent was introduced to each tube. The presence of a brownish-yellow color signifies a positive result.12
Phosphate solubilization activity
The selected isolates were introduced as spots in the middle of the Pikovaskayas medium and kept in an incubator at a temperature of 30°C for a duration of 7 days. The presence of a distinct transparent area signifies a positive outcome for phosphate solubilization, as stated by Nautiyal.13 Phosphate solubilization efficiency was calculated by,
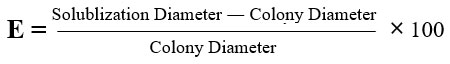
Hydrogen Cyanide Production
The chosen isolate L1 was introduced onto the nutrient media plates which were enriched with 4.4 gm of glycine per liter. Subsequently, a Whatman No.1 Filter paper that had been pre-soaked in a solution of 2% NaCO3 in 0.5% Picric acid was placed on the surface of the agar plate. The plate was then sealed using parafilm and left to incubate for a period of 4 days at a temperature of 30 °C. A change in color of the filter paper from a deep yellow hue to reddish brown was considered as a positive result.14 The quantitative analysis was carried out by employing UV-Visible Spectroscopy at wavelengths of 480, 520, and 640 nm.
IAA Production
The Actinobacterium was introduced into Luria Bertani broth supplemented with 100mg/l of tryptophan. The culture was then placed in an incubator set at 250 rpm and 30 °C for a period of 3-5 days to allow for growth. Once fully grown, the cultures were harvested and subjected to centrifugation at 10000 rpm for 10 minutes. Subsequently, 2ml of the supernatant was combined with 4 ml of Salkowski’s reagent (composed of 50 ml of 35% HClO4 in 1 ml of 0.5 M FeCl3Solution) and left to incubate in darkness for 30 minutes. The development of a pink coloration indicated a positive result.15 The Optical Density was determined through UV-Visible Spectroscopy at a wavelength of 540 nm.
Molecular Characterization
The culture underwent 16SrRNA sequencing to conduct phylogenetic and genomic analysis. The culture was identified through the utilization of BLAST software. The MEGA software was employed for neighbour joining and phylogenetic analysis.
Pot trail Analysis: (Optimization)
Different concentrations of the selected inoculant (Leifsonia sp.) were prepared. The chosen strain was administered at varying concentrations to the experimental plant Zea mays. Specifically, the concentrations used were as follows:
Treatment 1 – 25% of the strain, Treatment 2 – 50% of the strain, Treatment 3 – 75% of the strain, Treatment 4 – 100% of the strain, Treatment 5 – Control (no inoculation).
Five replicates (n=5) were maintained for each treatment. The biomass data obtained was then subjected to statistical analysis utilizing SPSS Software. To determine significant differences among the groups, Duncan’s test was conducted with a significance level set at P < 0.5%.
Results
Isolation of Endophytic bacteria
Six isolates were chosen from the spread plates, and the colony morphology of these isolates from the medium was examined. (Table 1):
Table 1: Colony morphology of the isolates, isolated from the root nodules of Casuarina equisetifolia.
| Isolates | Media | Shape | Size (cm) | Elevation | Margin | Color | Opacity |
| L1 | DPM | Circular | 1.67 | In agar | Entire | White | Transparent |
| L2 | DPM | Circular | 0.73 | In agar | Entire | White | Transparent |
| L3 | DPM | Circular | 1.43 | In agar | Entire | White | Transparent |
| L4 | DPM | Circular | 1.56 | In agar | Entire | White | Transparent |
| L5 | DPM | Circular | 0.45 | In agar | Entire | White | Transparent |
| L6 | DPM | Circular | 0.56 | In agar | Entire | White | Transparent |
Antagonistic Activity
The growth of the pathogens in the plate inhibited the isolate L4. The metabolism and physiology of L4 were significantly affected by the presence of the pathogens, leading to an inability to uptake nutrients from the media. As a result, the growth of the organism was not visible on the plate. In contrast, isolate L1 exhibited strong inhibitory effects on all pathogens, with more than a 50% reduction in growth. Diploidia was the most inhibited pathogen, followed by Phytophtora, Alternaria, Fusarium, and Sclerotium. The diameter of growth for L1 was measured at 2.5 cm. Isolate L2, on the other hand, inhibited the growth of pathogens in the order of Phytopthora, Diploidia, Alternaria, Fusarium, and Sclerotium. The growth of L2 was minimal, measuring only 0.23±0.5 cm in diameter. Similarly, L3 showed similar inhibitory effects to L2, with very limited growth observed at 0.22±0.1 cm in diameter. Isolates L5 and L6 specifically inhibited the growth of Diploidia, Phytophthora, and Alternaria.(Table 2) Among the isolates, L1, L2, L3, L5, and L6 were specifically picked for further examination, with L4 being omitted from consideration due to its reduced antagonistic activity.
Table 2: Percent Inhibition of radial growth of Pathogen was calculated using Percent inhibition formula.
| Isolates | Sclerotium | Alternaria | Phytopthora | Diploidia | Fusarium | Result |
| L1 | 73.16% | 75.00% | 89.00% | 93.00% | 68.45% | +++ |
| L2 | 62.47% | 89.34% | 75.67% | 90.12% | 73.65% | +++ |
| L3 | 42.32% | 72.45% | 66.08% | 86.71% | 67.89% | ++ |
| L4 | 0.00 | 11.92% | 23.07% | 24.10% | 13.27% | — |
| L5 | 36.28% | 84.90% | 90.62% | 75.16% | 47.83% | ++ |
| L6 | 45.23% | 76.23% | 82.33% | 76.10% | 32.17% | ++ |
(— indicates no antagonistic activity, ++ indicates antagonistic activity, +++ high antagonistic activity)
In Vitro PGPR Analysis
The L4 isolate was not accepted during this stage. The chosen isolates underwent processing for the analysis of plant growth-promoting rhizobacteria (PGPR). The presence of a green color in the organic acid production test signifies the production of specific organic acids for nutrient uptake. On the other hand, the brown color observed in isolates L5 and L6 indicates a lower amount of ammonia production. In contrast, isolates L1 and L2 exhibited a yellow color, indicating a significant amount of ammonia production. Notably, L3 produced a golden brown color, suggesting a substantial production of ammonia. Based on the color observations, the order of ammonia production is L3>L1=L2>L5=L6. IAA production was observed only in L1 isolate, which it produced pink to reddish color after 30 minutes of incubation. The isolates L2, L3, L5 and L6 was colorless to light yellow. From the primary screening the L1 isolate was screened positive as effective PGPR. The L1 isolate showed increase in the amount of IAA proportionally in increase in the concentration of tryptophan (Table 3).
Table 3: Quantitative measurement of IAA (L1) in UV-Visible spectroscopy at 540 nm in 24 and 48 hours.
| Isolate | 24hrs | 42hrs |
| L1 | µg/ml | µg/ml |
| 0.1 | 1.233 | 1.703 |
| 0.5 | 1.787 | 1.937 |
| 1 | 1.904 | 2.369 |
| 5 | 2.033 | 2.408 |
| 10 | 2.078 | 2.796 |
| 15 | 2.448 | 2.902 |
Table 4: Quantitative analysis of HCN production by L1 isolate by using UV-Visible spectroscopy (µg/ml).
| Isolate | 480 nm | 540nm | 620nm |
| L1 | 0.015 | 0.006 | 0.002 |
The L1 isolate’s HCN production caused the filter paper to exhibit an orange shade, indicating the bio control capability of the L1 isolate. The plate’s ACC deaminase activity led to the growth of approximately 30×10-1 colonies of the organism. This activity also resulted in a reduction of ethylene. Additionally, the organism L1 demonstrated phosphate solubilizing activity, with an efficiency of 50% solubilization.
Molecular Characterization of the isolate
The isolated specimen exhibited a 99.68% match with Leifsonia sp. Actinobacterium in the BLAST software analysisGene Bank Accession ID = PP542598.1 Leifsonia sp. strain Sathiqanum 16S ribosomal RNA gene, partial sequen – Nucleotide – NCBI
The FASTA sequence of the organism is presented below,
TTACTAACCGACTCCCGACTTCATGAGGTCGAGTTGCAGGACCTCAATCCGAACTGAGAGCGGCTTTTTGGGATTCGCTC
CACCTTACGGTATTGCAGCCCTTTGTACCGGCCATTGTAGCATGCGTGAAGCCCAAGACATAAGGGGCATGATGATTTGA
CGTCATCCCCACCTTCCTCCGAGTTGACCCCGGCAGTCTCCTATGAGTTCCCGCCATTACGCGCTGGCAACATAGAACGA
GGGTTGCGCTCGTTGCGGGACTTAACCCAACATCTCACGACACGAGCTGACGACAACCATGCACCACCTGTTCACGAGTG
TCCAAAGAGTTCCCTATTTCTAGGGCGTTCTCGTGTATGTCAAGCCTTGGTAAGGTTCTTCGCGTTGCATCGAATTAATC
CGCATGCTCCGCCGCTTGTGCGGGCCCCCGTCAATTCCTTTGAGTTTTAGCCTTGCGGCCGTACTCCCCAGGCGGGGCGC
TTAATGCGTTAGCTGCGACACGGAAACCGTGGAATGGTCCCCACATCTAGCGCCCAACGTTTACGGCGTGGACTACCAGG
GTATCTAATCCTGTTCGCTCCCCACGCTTTCGCTCCTCAGCGTCAGTTACGGCCCAAAGAACTGCCTTCGCCATCGGTGT
TCCTCCTGATATCTGCGCATTCCACCGCTACACCAGGAATTCCATTCTCCCCTACCGCACTCTAGTCTGCCCGTACCCAC
TGCAGGCCCGAGGTTGAGCCTCGGGTTTTCACAGCAGACGCGACAGACCGCCTACGAGCTCTTTACGCCCAATAATTCCG
GACAACGCTAGCACCCTACGTATTACCGCGGCTGCTGGCACGTAATTAGCCGGTGCTTTTTCTGCAGGTACCGTCACTTT.
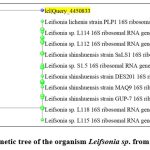 |
Figure 1: Phylogenetic tree of the organism Leifsonia sp. from BLAST software. |
Pot trial Analysis: (for optimization)
The analysis conducted on the pot trial has determined that the presence of a specified amount of Leifsonia sp. will result in an enhanced growth of Zea mays. Notably, When 50% of Leifsonia sp. was introduced, a significant improvement in plant growth was observed, followed by a 25% culture. This information is presented in table 5, sections a and b.
Table 5: (a) The table represents the germination percentage of different treatments in 5th and 10th day of inoculation & number of leaves present in the 7th and 15th day of inoculation. (a, b, c) represents the treatments are significantly different from each other in Zea mays for 5 replicates in each treatment to standardization the quantity of the culture for applying in agricultural fields.
| Treatment | Germination percentage (5th day) | Germination percentage (10th day) | Number of leaves (7th day) | Number of leaves(15th day) |
| T1 | 3.66±0.57bc | 4.66±0.577b | 2.00±0.00b | 4.33±0.57b |
| T2 | 4.00±1.00c | 5.00±1.00b | 3.00±0.00c | 6.00±0.00c |
| T3 | 2.66±0.57b | 4.00±0.577b | 2.00±0.00b | 3.00±1.00a |
| T4 | 3.66±0.57bc | 4.33±0.577b | 2.00±0.00b | 2.33±0.57a |
| T5 | 0.00±0.00a | 0.66±0.00a | 0.667±0.577a | 2.33±1.54a |
[Values within a column followed by single letters (a,b,c) show significant varietal difference by Duncan’s test]
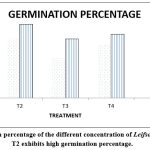 |
Figure 2: Germination percentage of the different concentration of Leifsonia sp. on Zea mays. T2 exhibits high germination percentage. |
Table 5: (b) The table represents the shoot length, girth and root length of different treatments, 15th day of inoculation. a, b, c represents the treatments are significantly different from each other in Zea mays for 5 replicates in each treatment.(n=5)
| Treatments | Shoot length (cm) | Girth (cm) | Root length(cm) |
| T1 | 10.33±1.60c | 0.436±0.046c | 9.100±0.041c |
| T2 | 8.50±0.00bc | 0.436±0.02c | 7.23±0.020bc |
| T3 | 8.16±0.28bc | 0.297±0.32b | 7.144±0.032bc |
| T4 | 6.83±1.89b | 0.440±0.36c | 5.30±0.036b |
| T5 | 1.00±1.00a | 0.103±0.105a | 1.23±0.105a |
[Values within a column followed by single letters (a, b, c) show significant varietal difference by Duncan’s test]
Discussion
Leifsonia strains have different roles, including being pathogenic, commensal, or beneficial. We successfully isolated a beneficial Actinobacterium from the root nodules of Casuarina equisetifolia. According to Karthikeyan2, Casuarina equisetifolia can fix atmospheric nitrogen through a symbiotic relationship with Frankia. Our current research has identified that the endophytic Actinobacteria Leifsonia plays a crucial role in nitrogen fixation and ammonia oxidation. Previous studies by Karthikeyan have shown that the growth and biomass of Casuarina’s are enhanced when inoculated with Frankia, are strongly increased nitrogen fixation by Frankia.16 Additionally, Leifsonia has been found to promote plant growth and biomass at specific culture concentrations. In a recent study, Karthikeyan reported that Micromonospora maritima exhibits significant antagonistic activity against Ralstonia solanacerum.6 Our observations indicate that Leifsonia sp. itself demonstrates antagonistic effects against plant pathogens such as Alternaria, Sclerotium, Phytophthora, Diploidia, and Fusarium species. The antimicrobial activity against Phytophthora and Diploidia represents a noteworthy development.
Nordsettt17 conducted research on the advantageous effects of Leifsonia sp. in enhancing water stress tolerance in plants, potentially through its involvement in bacterial osmotic stress, production of osmoprotectants, and synthesis of vitamin B9. Their in vitro studies revealed that Leifsonia acts as a Plant Growth Promoting bacterium, with L1 exhibiting superior abilities in indole-3-acetic acid (IAA) production, hydrogen cyanide (HCN) production, and phosphate solubilization compared to other isolates. Kang, observed that treatment with L. soli led to a significant increase in the growth of Waito rice seedlings compared to the control group.8 Furthermore, it was found that 50% and 25% concentrations of Leifsonia culture resulted in enhanced shoot length, girth, and root length of Zea Mays. Kang, also demonstrated that Leifsonia sp. is a highly effective plant growth promoting bacterium.18 Nordsett suggested that Leifsonia sp. holds promise as a plant growth-promoting bacterium for future applications.17. Rastogi documented the synthesis of bacterial cellulose derived from Leifsonia species, highlighting its potential applications in food packaging and medical implants.19 The current investigation emphasizes the significant role of these species in agriculture, particularly in promoting the growth of Zea mays. The current study highlights that a 50% concentration of Leifsonia culture can serve as an improved bio inoculant for plants.
Conclusion
According to the research, Leifsonia sp. has the potential to serve as a superior bioinoculant, capable of colonizing the inner tissues of the plant and functioning as an endophytic bacterium. When applied at a concentration of 50%, Leifsonia has been shown to enhance both the growth and physiological processes of the plant. This research indicates encouraging outcomes with Leifsonia sp. under controlled settings; however, subsequent investigations should prioritize field trials to evaluate the long-term effectiveness and ecological adaptability of this bacterial inoculant on C. equisetifolia. Such studies are essential for understanding the practical applicability and consistency of plant growth-promoting benefits across various soil types and climatic environments. Future investigations should seek to clarify the precise mechanisms by which Leifsonia sp. enhances growth in C. equisetifolia. This may encompass the synthesis of phytohormones (such as auxins and gibberellins), nitrogen fixation, nutrient solubilization, and the inhibition of plant pathogens. Gaining insights into these mechanisms will facilitate the advancement of efficient bio fertilizers. Evaluating the effectiveness of Leifsonia sp. as a bioinoculant for additional tree species would be beneficial, particularly within afforestation and reforestation initiatives. The wider applicability of this endophytic bacterium could yield substantial ecological and economic advantages, especially for rapidly growing species like C. equisetifolia. A thorough genomic examination of Leifsonia sp. will shed light on its genetic capabilities related to plant growth promotion, stress resilience, and compatibility with host plants. Additionally, metabolic profiling may uncover critical enzymes or metabolites produced by the bacterium that contribute to its advantageous effects. Researching the effects of Leifsonia sp. inoculation on soil health and microbial diversity is crucial for understanding its broader ecological implications and potential to enhance soil quality and ecosystem functioning.
Acknowledgement
Authors gratefully acknowledge the facilities provided by the Department of Forest Protection, Institute of Forest Genetics and Tree Breeding.
Funding Sources
The authors received no support for the research ,authorship, and /or publication of the article.
Conflict of Interest
The authors do not have any conflict of interest.
Data Availability Statement
This statement does not apply to this article.
Ethics Statement
The research didi not involve human participants, animal subjects, or any material that requires ethical approval.
Author Contributions
Jini Viju Pamboor Chack- Data collection, Analysis , Methodology and results writing- Orginal draft
Arumugam Karthikeyan- work plan, Guidance, Review and Editing of article.
References
- Karthikeyan, A., Deeparaj, B., Nepolean, P. Reforestation in bauxite mine spoils with Casuarina equisetifolia frost. and beneficial microbes. Forests, trees and livelihoods.2009. 19(2): 153-165.
CrossRef - Karthikeyan, A. Frankia strains for improving growth, biomass and nitrogen fixation in Casuarina equisetifolia seedlings. Journal of Tropical Forest Science. 2016:235-242.
- Pinyopusarerk, K.,Williams, E. R. .Range-wide provenance variation in growth and morphological characteristics of Casuarina equisetifolia grown in Northern Australia. Forest Ecology and Management. 2000. 134(1-3): 219-232.
CrossRef - Ngom, M., Gray, K., Diagne, N., Oshone, R., Fardoux, J., Gherbi, H.,Champion, A. Symbiotic performance of diverse Frankia strains on salt-stressed Casuarina glauca and Casuarina equisetifolia plants. Frontiers in Plant Science. 2016. 7:1331.
CrossRef - Marappa, N., Ramachandran, L., Dharumadurai, D., & Nooruddin, T. Plant growth-promoting active metabolites from Frankia spp. of Actinorhizal Casuarina spp. Applied biochemistry and biotechnology. 2020. 191(1):74-91.
CrossRef - Karthikeyan, Arumugam & Kanchanadevi, Kumar, Nicodemus, Abel. Effect of Frankia and Micromonospora on growth and health improvement in Casuarina clones. Journal of Forest Research. 2022.27(2).
CrossRef - Young, A. J., Nock. C. J. Molecular detection of diverse Leifsonia strains associated with sugarcane. Plant disease. 2017. 101(8):1422-1431.
CrossRef - Kang, S. M., Khan, A. L., You, Y. H., Kim, J. G., Kamran, M., Lee, I. J. Gibberellin production by newly isolated strain Leifsonia soli SE134 and its potential to promote plant growth. Journal of microbiology and biotechnology,. 2014. 24(1):106-112.
CrossRef - Lin, Q., Xu, Z., Li, M., Wang, Y., Li, L. Spatial differences in Casuarina equisetifolia L. endophyte community structure. Annals of Microbiology. 2022. 72(1): 28.
CrossRef - Rajan S &Selvi Christy. Experiments in life sciences. A laboratory Manual. India. 2010: 260-261.
- Bouizgarne, B. Bacteria for Plant Growth Promotion and Disease Management. In: Maheshwari, D. (eds) Bacteria in Agrobiology: Disease Management. Springer, Berlin, Heidelberg. 2013.
CrossRef - Cappuccino JC, Sherman N. In: Microbiology: A laboratory manual, New York. 1992: 125-79
- Nautiyal, C. S., Bhadauria, S., Kumar, P., Lal, H., Mondal, R., Verma, D. Stress induced phosphate solubilization in bacteria isolated from alkaline soils. FEMS Microbiology letters. 2000. 182(2): 291-296.
CrossRef - Lorck, H. Production of Hydrocyanic Acid by Bacteria. Physiologia Plantarum. 1948. 1:142-146.
CrossRef - Ehmann, A. The Van Urk-Salkowski reagent—a sensitive and specific chromogenic reagent for silica gel thin-layer chromatographic detection and identification of indole derivatives. Journal of Chromatography A. 1977. 132(2):267-276.
CrossRef - Karthikeyan A, Savio MDM , Nepolean P. Growth response of Casuarina junghuhniana to indigenous Frankia, arbuscular mycorhizal fungi and phosphobacterium under nursery conditions. in Zhong C (eds) Improving Smallholder Livelihood Through Improved Casuarina Productivity: Proceedings of the 4th International Casuarina Workshop. 2011. Pp131–136.
- Nordstet, N. P., Roman-Reyna, V., Jacobs, J. M., Jones, M. L. Comparative genomic understanding of gram-positive plant growth-promoting Leifsonia. Phytobiomes Journal. 2021. 5(3): 263-274.
CrossRef - Kang, S. M., Asaf, S., Kim, S. J., Yun, B. W., & Lee, I. J.. Complete genome sequence of plant growth-promoting bacterium Leifsonia xyli SE134, a possible gibberellin and auxin producer. Journal of Biotechnology. 2016.239: 34-38
CrossRef - Rastogi, Akanksha Banerjee, Rintu.Production and Characterization of cellulose from Leifsonia sp. Process Biochemistry. 2019.85:10.
CrossRef

