Introduction
Garlic (Allium sativum L) belongs to the genus Allium of the family Alliaceae. It is cultivated globally as a vegetable condiment for culinary and medicinal use. The increasing popularity of garlic is mainly attributed to its beneficial effects on human health due to the presence of allicin and other organo-sulphur compounds1. Garlic imports to Mauritius amounted to USD 3M in year 20212 and is expected to increase continuously in the coming years. The COVID-19 pandemic and the conflict between Russia and Ukraine have exacerbated the existing food insecurity issues in the country. With elevated food inflation, the purchasing power of Mauritian citizens has significantly declined. Consequently, an increase in domestic production of garlic would not only reduce the country’s reliance on imported garlic but also yield positive economic impacts for the nation.
The local production of garlic is irregular, low and restricted to the eastern part of the island. Over the last three decades, the average annual production has decreased from around 200 t during the 1980s’ to mere 31 t in 2021 This is principally attributed to the lack of improved garlic varieties and limited supply of clean planting materials. Small farmers are the main growers of garlic in Mauritius. They produce their own planting material. However, these cloves are likely the carriers of viral contamination in field and main cause of reduction in yield.
Since most garlic varieties have low flowering ability and are sterile, it is vegetatively propagated through cloves or aerial bulbils3, 4. Consequently, it is easily infected by different types of viruses such as aphid transmitted Potyviruses and Carlaviruses, mites transmitted Allexiviruses5. Also, viruses are accumulated after successive planting cycles in garlic bulbs and increased spread of viral diseases to different regions happens through infected planting material. Considerable yield losses and decrease in quality of bulbs are caused by different viral diseases, which have severe impact on the production of garlic6.
Previous studies revealed that cultivation of virus-free planting material considerably increased the productivity7. Other studies reported that expected decrease in yield of virus-free bulbs in subsequent years due to continual viral exposure were negligible. In fact, yields obtained from cultivated virus-free bulbs after five consecutive planting cycles in the field were higher than the yields of contaminated bulbs cultivated successively for the same period. Therefore, renewal of planting material periodically is required to reduce productivity loss in garlic7.
The production of virus-free planting material through traditional methods is difficult. Furthermore, garlic improvement through breeding program is challenging due to low flowering ability of garlic plants8. Therefore, other propagation methods such as tissue culture techniques can be used to produce virus-free garlic bulbs. Previous studies reported that virus-free propagules with higher yield and improved quality could be produced by meristem culture9. These techniques consist of cleaning infected bulbs and mass propagation of virus-free planting material in a short period of time8. Indeed, application of tissue culture techniques for the mass propagation of shoots and subsequent formation of multiple bulblets has high potential in garlic improvement. Other methods used for virus elimination in garlic are meristem-tip culture10, meristem culture in combination with heat treatment and meristem culture combined with chemotherapy using ribavirin11.
To date, no research work on regeneration of Mauritian virus-free garlic clones have been conducted. Therefore, the main objective of this study was to establish and optimize a reliable protocol for the production of virus-free garlic propagules that have high yield with improved quality for cultivation by Mauritian farmers.
Materials and Methods
The research was carried out between the year 2019 and 2022 at the Tissue Culture Laboratory of Food and Agricultural Research and Extension Institute (FAREI), Mauritius.
Plant Material
One imported garlic accession namely VFG158 introduced from the World Vegetable Centre (WVC) and eleven local garlic accessions viz. Beeharry, Boodnah, Bondah, Gooniah, Haulkhory, Ramdhuny, Ramjee, Rampall, Sujeebun, Sujeebun 2 and Unuth received from Agronomy Division of FAREI. The garlic accession VFG158 was selected for this study as it is adapted to the local climatic conditions. All these accessions are grown by local growers but are not registered as improved varieties. The selected garlic accessions for this study have high demand in the local market due to their high pungency.
Disinfection and Dissection of Garlic Cloves
Healthy garlic bulbs were separated into cloves and the outer bulb scales were removed. The cloves were washed with running tap water and domestic dish detergent (5ml/L). The cloves were then soaked in Benomyl 500WP solution (1%) containing two drops of surfactant (Tween 20) per 100 ml for 45 min with frequent agitation and washed under running tap water for 10 min. Thereafter, the cloves were subjected to two types of treatments; either no hot water treatment (P1) or hot water treatment, 37 °C for 10 min (P2). Thereafter cloves were surface-sterilized with 70% ethanol for 1 min and were shaken for 15 min in a 1% solution of sodium hypochlorite containing two drops of surfactant (Tween 20) per 100 ml. The cloves were then washed three times for 15 min with sterile distilled water. Garlic cloves were dissected and meristem-tips, about 1 mm in size, were exercised under laminar air-flow, using a microscope. The dome-shaped structure consisting of the shoot meristem and one or two leaf primordia were placed individually in an upright position in the appropriate initiation media for culture establishment. Inoculated explants were incubated under controlled temperature (25±2 ºC) and a photoperiod of 16 h with a light intensity of 2000 – 5000 lux from white inflorescent light.
Culture Conditions
The media were supplemented with 3% (w/v) sucrose and 0.24% (w/v) Sigma phytagel. The pH of the medium was adjusted to 5.86 before autoclaving for 15 min at 121 °C. Cultures were placed in growth room at 25 ±1.0 °C with a 16 h photoperiod and light intensity of 2000 – 5000 lux, which was illuminated by 24 W LED T8 fluorescent glass tubes. The cultures were transferred to fresh medium every 5-6 weeks.
Plantlet Establishment and Multiplication
After 15 days, the explants developed into rooted plantlets which were cultured on MS basal medium with different combinations of plant growth regulators, including 6-benzyladenine (BAP), gibberellic acid (GA3), Indole-3-butyric acid (IBA), isopentenyl adenine (2ip), kinetin and naphthaleneacetic acid (NAA), LS basal medium supplemented with plant growth regulators (BAP and NAA) and Gamborg B5 medium supplemented with plant growth regulators (BAP, NAA and Indole-3-acetic acid {IAA}). Fourteen media compositions for shoot multiplication were tested in three replicates for this study (Table 1). Each replicate consisted of five explants cultured in 5 × 11 cm bottle jar.
Bulblet Formation
Shoot cultures were sub cultured on three media compositions for bulblets formation (Table 1) and were placed in the growth room at 25 ± 1.0°C with a photoperiod of 16 h with a light intensity of 2000 – 5000 lux. Five shoots’ clumps were placed in each culture jar, and five culture jars (replicates) were used for each treatment.
Data Analysis
Data were analyzed using ANOVA and the Tukey’s multiple range tests. Statistical analysis of the data was carried out using the JASP programme package.
Table 1: Different media compositions used for plantlet establishment, multiplication and bulbification
|
Medium code |
Salts |
Kinetin (mg/L) |
BAP (mg/L) |
NAA (mg/L) |
GA3 (mg/L) |
2iP (mg/L) |
IAA (mg/L) |
IBA (mg/L) |
Sucrose (g/L) |
|
|
|
Establishment |
|||||||
|
MS hormone-free |
MS |
– |
– |
– |
– |
– |
30 |
||
|
|
|
Multiplication |
|||||||
|
G0 |
MS |
– |
– |
0.25 |
– |
0.5 |
30 |
||
|
G1 |
MS |
– |
1.5 |
0.5 |
– |
– |
30 |
||
|
G2 |
MS |
– |
– |
0.3 |
– |
3 |
30 |
||
|
G3 ( G3A+ G3B) |
The cultures were placed in G3A media then transferred to G3B after 4 weeks |
||||||||
|
G3A |
MS |
0.5 |
0.5 |
30 |
|||||
|
G3B |
MS |
2 |
2 |
30 |
|||||
|
G4 |
MS |
2 |
0.5 |
30 |
|||||
|
G5 ( G5A+ G5B) |
The cultures were placed in G5A media then transferred to G5B after 4 weeks |
||||||||
|
G5A |
LS |
0.25 |
0.23 |
30 |
|||||
|
G5B |
LS |
2.3 |
0.93 |
30 |
|||||
|
G6 |
MS |
0.5 |
2 |
30 |
|||||
|
G7 |
MS |
2 |
0.1 |
30 |
|||||
|
G8 |
MS |
30 |
|||||||
|
G9 |
Gamborg B5 |
0.5 |
0.1 |
30 |
|||||
|
G10 (G10A+G10B) |
The cultures were placed in G10A media then transferred to G10B after 4 weeks |
||||||||
|
G10A |
MS |
0.25 |
0.5 |
30 |
|||||
|
G10B |
MS |
1.5 |
0.5 |
30 |
|||||
|
G11 |
MS |
0.5 |
1 |
30 |
|||||
|
G14 |
MS |
– |
2 |
2 |
– |
– |
30 |
||
|
G15 |
MS |
– |
1 |
0.5 |
– |
– |
30 |
||
|
|
|
Bulbification |
|||||||
|
B2 |
MS |
– |
– |
– |
– |
– |
120 |
||
|
B4 |
MS |
2 |
– |
– |
1 |
– |
30 |
||
|
B5 |
MS |
– |
2 |
– |
1 |
– |
90 |
||
Gamborg B5: Gamborg B5 medium (Gamborg et al., 1968)12
LS: Linsmaier and Skoog medium (Linsmaier & Skoog, 1965)13
MS: Murashige Skoog medium (Murashige & Skoog, 1962)14
BAP: 6-Benzylaminopurine, 2iP: 6-(γ,γ-Dimethylallylamino) purine, GA3: Gibberellic acid, IAA: Indole-3-acetic acid, IBA: Indole-3-butyric acid, Kin: Kinetin, NAA: α-Naphthalene acetic acid
Results and Discussion
In tissue culture technique, contaminated explants do not develop and are thus discarded. Therefore, an effective surface sterilisation protocol should be established to obtain contamination-free explants. Two washing protocols, P1 (control) and P2 (an additional step with hot water treatment 37 °C for 10 min included) were tested for surface-sterilization of healthy garlic cloves. Protocol P2 was found to be more effective as the rate of contamination among the explants was lower than protocol P1. The contamination rate for protocol P2 varied from 5% to 10% compared to 20% to 35% for protocol P1.
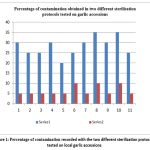 |
Figure 1: Percentage of contamination recorded with the two different sterilization protocols tested on local garlic accessions |
Effect of Different Media Formulation on Shoot Rregeneration
A total of fourteen media was tested and positive results were noted on only six shoot multiplication media: MS basal media with various growth regulators {G0 (0.25 mg/L NAA + 0.5 mg/L 2iP), G1 (1.5 mg/L BAP + 0.5 mg/L NAA), G2 (0.3 mg/L NAA + 3 mg/L 2iP), G6 (0.5 mg/L NAA + 2 mg/L 2iP), G14 (2 mg/L BAP + 2 mg/L NAA) and G15 (1 mg/L BAP + 0.5 mg/L NAA) (Table 1). Shoot proliferation was significantly better in shoot multiplication media G0, G1, G2, G6, G14, G15 compared to G3, G4, G5, G7, G8, G9, G10 and G11 (Table 1).
Highly significant difference (P< 0.05) was noted among the garlic accessions regarding the number of multiple shoot formation. Multiple shoots formation was recorded in different shoot multiplication media after 6 weeks of culture as from fourth subculture for nine garlic accessions namely, Beeharry, Boodnah. Bondah, Haulkhory, Gooniah, Ramdhuny, Rampall, Sujeebun 2 and Unuth (Fig. 3A). The other three garlic accessions: Ramjee, Sujeebun and VFG158 did not show any proliferation even after being maintained for more than 8 weeks (Fig. 3A). The highest number of shoot formation was observed in G2 (0.3 mg/L NAA+ 3 mg/L 2iP) and the lowest number of shoot formation was observed in G14 (2 mg/L BAP + 2 mg/L NAA) among the selected six shoot multiplication media Fig. 4 (B and C). It was noted that 20 to 25 % increase in shoot number was recorded in first generation followed by a multiplicative factor of 2 over generations as shown in Fig. 4 (C).
The garlic accessions could be categorised into four groups on the basic of the visual observations made during the trials and garlic accessions in the same group showed similar responses to culture media (Fig. 3 B). Furthermore, other studies suggested that shoot proliferation was induced on MS medium containing high concentrations of BA and low concentrations of NAA, IBA and IAA17. Actually, explants cultured on the six media (G0, G1, G2, G6, G14, G15 (Table 1) containing comparatively high concentrations of cytokinins (BA and 2ip) in combination with lower concentrations of auxins (NAA, IBA and IAA) resulted in good proliferation rate.
Researchers have also reported that explant with 1-2 primodial leaves in meristematic tissue have the ability to intake auxin and cytokinins that promote growth and development of the explant18. Previous studies suggested that the cytokinins 2iP and BA in the culture media have interchangeable responses in the induction of shoots17. According to researchers it is more advantageous to use 2iP in media as the shoot produced are healthier and appeared to inhibit callus formation on the basal plate of the explants. The present results are in agreement with previous studies as callus formation was observed at the basal plate of the explant in media supplemented with NAA and BA (G1, G14 and G15), while no callus was recorded with media supplemented with 2ip (G0, G2 and G6). Furthermore, researchers suggested that the absence of callus at the base of the explant is beneficial for shoot regeneration and genetic stability as it is the preferred pathway to produce true-to-type clones. In contrast to previous reports17, 19, shoot regeneration was not achieved with LS and Gamborg (B5) medium. Moreover, the present result was not in agreement with other studies which reported better shoot regeneration in media supplemented with IBA or IAA in combination with BA. Actually, root regeneration was observed instead shoot of regeneration, when the combination IBA or IAA and BA were used.
 |
Figure 2: Frequencies of multiple shoot formation recorded in garlic cultures on different shoot multiplication media. |
 |
Figure 3: Frequencies of multiple shoot formation recorded in garlic cultures. (A) Frequencies of multiple shoot formation on different shoot multiplication media during three generations. |
 |
Figure 4: Frequencies of multiple shoot formation recorded in garlic cultures on six selected media. |
Table 2: Effect of shoot multiplication media on shoot proliferation of different garlic accessions
|
Media |
Sum |
Average |
Variance |
|
G0 |
1130 |
376.67 |
73232.33 |
|
G1 |
1246 |
415.33 |
73046.33 |
|
G2 |
1238 |
412.67 |
80594.33 |
|
G6 |
1142 |
380.67 |
66012.33 |
|
G14 |
1023 |
341 |
57319 |
|
G15 |
1033 |
344.33 |
53636.33 |
Values represent sum, mean and variance of 15 explants per treatment in three repeated experiments
Table 3: Effect of shoot multiplication media on shoot proliferation during three generations
|
Generation |
Sum |
Average |
Variance |
|
Gen 1 |
888 |
148 |
389.6 |
|
Gen 2 |
1975 |
329.17 |
740.17 |
|
Gen 3 |
3949 |
658.17 |
2936.97 |
Values represent sum, mean and variance of 15 explants per treatment in three repeated experiments
Effect of Genotypes
Several studies have reported genotypic variation in shoot multiplication in garlic20, 21. In general, each genotype has different response to different media formulation. The results showed that there is high significant difference (P<0.05) in multiplication rate among garlic accessions or groups of similar accessions. The highest shoot proliferation was observed in the garlic accession Ramdhuny, while the lowest shoot proliferation was recorded in groups of similar accessions namely, Ramjee, Sujeebun and VFG 158 on different media formulations tested in the present study. The result also indicated high influence of genotype regarding multiplication of each garlic accession or groups of similar accessions on different media formulations (Fig. 3B and Fig. 4A). The result is in accordance with previous studies, which reported that garlic accessions or groups of similar accessions have different source of genetic material20, 22. It is obvious that genotypes played an important role in shoot multiplication of different garlic accessions (Fig. 3B and 4A). Furthermore, the obtained result indicated that some garlic accessions have high regeneration capacity, while others are less responsive to the effect of hormones or combinations of hormones in different media. Therefore, these findings confirmed the hypothesis of previous reports that the efficiency of tissue culture techniques in garlic is strongly genotype-dependent23.
Hyperhydricity
Hyperhydricity formerly termed as vitrification is a morphological abnormality that occurs during in vitro culture24, 25. Vitrified cultures have a glassy appearance with enlarged, thick and water-soaked translucent stems and leaves26. Several stress factors including cytokinins27, 28 and excess level of mineral elements in culture media cause this phenomenon during micropropagation. However, the factors that induce hyperhydricity in tissue culture are not fully understood. According to previous study, regeneration of normal and mature clonal shoots through micropropagation is inhibited by this physiological disorder29. Consequently, the multiplication factor and vigour of in vitro culture are affected, which a serious problem30. Furthermore, it also reduces the survival rate of plantlets in free living conditions. (Table 7). While there was significant difference (P<0.05) regarding hyperhydricity over generation. Data in Table 7 and Fig. 5 C indicated that hyperhydricity was suppressed on six media namely G0, G1, G2, G6, G14 and G15. Besides, the effect of different shoot multiplication media on hyperhydricity is shown in Fig. 5A-D. A decrease of 50% in the frequency of hyperhydricity from first to second generation and second to third generation was observed in the tested garlic accessions as shown in Fig. 2 (A). It was observed that frequency of vitrified shoots decreased and shoots proliferation increased with repeated subculture. This result is not in accordance with previous study, which reported reduction in proliferative shoots due to increase in frequency of hyperhydricity with repeated subculture31. Several researchers mentioned that BAP is a very effective cytokinins compared to kinetin, 2ip and zeatin (Z) in micropropagation of different plants32. However, previous studies reported that BAP also induced hyperhydricity and is amplified with increasing concentration of BAP33, 34. Therefore, lower concentration of BAP or 2ip was used to avoid vitrified shoots. Data in Table 2 and Fig. 5C indicated that low concentrations of BAP ranging from 1 to 2 mg/l or 2ip with concentrations ranging from 0.5 to 3 mg/l used in shoot multiplication media (G0, G1, G2, G6, G14 and G15) promoted shoot proliferation, which is similar to findings of previous studies33, 34. Other researchers also reported the influence of genotypes in micropropagation of garlic cultures, which is confirmed by the results obtained in this study (Fig. 5 B and D)20, 22. The difference in frequency of hyperhydricity in the different genotypes of the garlic accessions is evident in Table 2. It is noted that frequency of hyperhydricity was very low or nil, when garlic accessions Bondah and Boodnah were treated with the six shoot multiplication media (G0, G1, G2, G6, G14 and G15). This result confirmed the effect of genotypes in garlic accessions20, 22
 |
Figure 5: Percentage of vitrification recorded in garlic cultures. (A) Percentage of vitrification for each garlic accession on different shoot multiplication media during three generation. |
Table 4: Effect of shoot multiplication media on shoot vitrification of different garlic accessions
|
Garlic accessions |
Sum |
Average |
Variance |
|
Boodnah |
126.83 |
42.27 |
11.21 |
|
Bondah |
128.28 |
42.76 |
24.53 |
|
Ramdhuny |
158 |
52.66 |
126.49 |
|
Unuth |
194.9 |
64.96 |
118.91 |
|
Sujeebun2 |
192.21 |
64.07 |
159.57 |
|
Gooniah |
221.07 |
73.69 |
53.94 |
|
Haulkhory |
223.21 |
74.4 |
51.68 |
|
Beeharry |
221.79 |
73.93 |
78.83 |
|
Rampall |
211.52 |
70.51 |
191.82 |
|
Sujeebun |
222.23 |
74.079 |
160.61 |
|
VFG158 |
257.11 |
85.71 |
32.02 |
|
Ramjee |
259.76 |
86.58 |
27.14 |
Values represent sum, mean and variance of 15 explants per treatment in three repeated experiments
Table 5: Effect of shoot multiplication media on shoot vitrification during three generations
|
Generation |
Sum |
Average |
Variance |
|
Gen 1 |
912.76 |
76.06 |
242.44 |
|
Gen2 |
796.28 |
66.35 |
214.97 |
|
Gen3 |
707.91 |
58.99 |
214.82 |
Values represent sum, mean and variance of 15 explants per treatment in three repeated experiments
Table 6: Anova table
|
Source of Variation |
SS |
df |
MS |
F |
P<0.05 |
|
Accession |
7080.72 |
11 |
643.7 |
45.11 |
* |
|
Generation |
1759.67 |
2 |
879.83 |
61.65 |
* |
|
Error |
313.945 |
22 |
14.27 |
||
|
Total |
9154.34 |
35 |
High significance difference (P<0.05) using Tukey’s multiple range tests is indicated by the symbol*
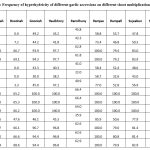 |
Table 7: Frequency of vitrification of different garlic accessions on different shoot multiplication media. |
The gradual increase in hyperhydricity among different garlic accessions with different shoot multiplication media used.
Bulblet Formation
Many advantages are associated with in vitro bulblets in garlic. These structures are good propagules, easy to maintain and manage, can be stored before hardening and do not require immediate hardening. Swelling of the bases of the shoots is an indication of bulblets formation.
In the present study, bulblets induction was achieved by transferring shoots clumps on different bulbification media: B2 (MS enriched with 12% sucrose), B4 (MS supplemented with 2mg/L Kin+1mg/L GA3 and enriched with 3%) and B5 MS supplemented with 2mg/L BAP+1mg/L GA3 and enriched with 9% sucrose). Significant differences (P<0.05) in bulblet formation were noted when the garlic cultures were incubated on different bulbification media under 25 ± 2°C and 16L/8D (Fig. 6). It was observed that shoots clumps transferred on bulbification media supplemented with BA or kinetin and gibberellic acid (B5 and B4) did not show swelling during the first six weeks of incubation in contrast to cultures transferred to media B2. Bulblet formation of shoots was observed only after eight weeks of culture for bulbification media B5 and 12 weeks for media B4. This result of early bulblet formation is comparable to previous studies, wherein researchers reported that bulblet formation is induced earlier in hormones- free media, which reduced the multiplication factor of the plantlets35, 36. Furthermore, most of the swollen shoots formed on culture media B4 did not develop into bulblets compared to the culture media B2 and B5. The main reason for the failure of the swollen portion of the shoots to form bulblets in culture media B4, could be related to the low amount of carbon source in the B4 medium as it was enriched with only 3% sucrose. According to researchers, media enriched with 12% sucrose enhanced bulblet weight, while larger bulblets are obtained in hormone-free MS medium37. However, largest and heavier bulblets were obtained in B5 medium compared to B2 medium which is in contrast to the findings of previous study37.
 |
Figure 6: Bulblet formation in different bubification media when cultured at 25±2°C and 16L/8D. |
Fig.7: Initiated meristem-tip culture and shoot development of garlic accession. (A1) Initiated meristem-tip culture {Bar=0.02 mm}. (A2) Shoots development on hormone-free medium (MS) after two weeks of culture {Bar= 1 cm}. (A3) Multiple shoots of garlic accession on G0 media (0.25mg/L NAA+0.5mg/L 2iP) after 5 weeks of culture (MS) {Bar= 1 cm}.
 |
Figure 7: Initiated meristem-tip culture and shoot development of garlic accession. (A1) Initiated meristem-tip culture {Bar=0.02 mm}. (A2) Shoots development on hormone-free medium (MS) after two weeks of culture {Bar= 1 cm}. |
 |
Figure 8: Effect of different media formulations [G0 (0.25mg/L NAA+0.5mg/L 2iP), G1 (1.5mg/L BAP+0.5mg/L NAA), G2 (0.3mg/L NAA+3mg/L 2iP), G6 (0.5mg/L NAA+2mg/L 2iP), G14(2mg/L BAP+2mg/L NAA) and G15(1mg/L BAP+0.5mg/L NAA)] on multiplication rate of garlic accession (Gooniah){Bar= 1 cm} |
 |
Figure 9: In vitro bulblets of garlic accession (Gooniah) on different bulbification media after 12 weeks of culture. (C1) Medium B2 (MS enriched with 12% sucrose) {Bar= 1 cm}. (C2) Medium B4 (MS supplemented with 2mg/L Kin+1mg/L GA3 and enriched with 3% sucrose) {Bar= 0.5 cm} (C3) Medium B5 (MS supplemented with 2mg/L BAP+1mg/L GA3 and enriched with 9% sucrose) {Bar=1 cm}. |
 |
Figure10: Hardening of garlic accessions. (D) Harvested bulblets of garlic accession (Gooniah) {Bar= 1 cm}. (E) Hardened garlic plants {Bar=1 cm}. |
Conclusion
A reliable protocol for rapid shoot regeneration and multiplication from meristem-tip culture was optimised in the present study. The different important steps of shoot regeneration, plantlets proliferation and bulblet formation were studied, which could be successfully used for mass propagation of garlic to produce virus-free planting material and increase the local garlic production. The use of in vitro techniques for garlic mass propagation will alleviate the problem of unavailability of clean planting material and continuous accumulation of viruses in the local garlic accessions.
Acknowledgement
The authors are thankful to Mr S. Sunassee for providing garlic bulbs, Mr R. Ramnauth for data analysis, Dr (Mrs) L. Unmole, Mrs S. Lutchoomun, Dr S. Ganeshan and Mr P. Erigadoo for their support and encouragement, the staff of Tissue Culture Laboratory especially Ms M. Gooransing (Trainee under Youth Employment Programme) for technical support and Directorate of Food and Agricultural Research and Extension Institution.
Funding Sources
We acknowledge funding from Food and Agricultural Research and Extension Institute and DeSIRA project
Conflict of Interest
There is no conflict of interest among the authors.
Data Availability Statement
This statement does not apply to this article.
Ethics Statement
This research did not involve human participants, animal, subjects, or any material that requires The ethical approval.
Informed Consent Statement
This study did not involve human participants, and therefore, informed consent was not required.
Authors’ Contribution
Boodhram Indira Kumari Devi: proof reading, manuscript proposal and manuscript revisions.
Greedharry Pratima: Undertaking research activities, data collection, analysis, manuscript writing and revisions.
Koyelas Chandasa: assistance provided during the initial stage of the project.
All authors have read and agreed to the published version of the manuscript.
References
- González R., Soto V., Sance M., Camargo A., Galmarini C. Variability of Solids, Organosulfur Compounds, Pungency and Health-Enhancing Traits in Garlic (Allium sativumL.) Cultivars Belonging to Different Ecophysiological Groups. Journal of Agricultural and Food Chemistry. 2009;57(21):10282-10288.
CrossRef - Mauritius Fresh Garlic market overview 2023 – Tridge. Tridge. Published 2023. Accessed May 23, 2023. https://www.tridge.com/intelligences/garlic/MU
- Kamenetsky R., Rabinowitch H.D. Floral development in bolting garlic. Sex Plant Reprod. 2001;13(4):235-241. doi:10.1007/s004970000061
CrossRef - Etoh T., Simon P. Fertility and Seed production of Garlic: Allium Crop Science. Recent Advances. 2002.
CrossRef - Cafrune E.E., Perotto M.C., Conci V.C. Effect of two Allexivirus isolates on garlic yield. Plant Dis. 2006;90(7):898-904. doi:10.1094/PD-90-0898
CrossRef - Lunello P., Di Rienzo J., Conci V.C. Yield Loss in Garlic Caused by Leek yellow stripe virus Argentinean Isolate. Plant Dis. 2007;91(2):153-158. doi:10.1094/PDIS-91-2-0153
CrossRef - Conci V.C., Canavelli A., Lunello P., et al. Yield losses associated with virus-infected garlic plants during five successive years. Plant Dis. 2003;87(12):1411-1415. doi:10.1094/PDIS.2003.87.12.1411
CrossRef - Haider S., Hossain M., Rahman S., et al. In vitro Plantlet Regeneration of Four Local Garlic (Allium sativum) Accessions of Bangladesh. Br Biotechnol J. 2015;8(3):1-12. doi:10.9734/bbj/2015/18619
CrossRef - Taşkın H., Baktemur G., Kurul M., Büyükalaca S. Use of tissue culture techniques for producing virus-free plant in garlic and their identification through real-time PCR. ScientificWorldJournal. 2013;2013:781282. doi:10.1155/2013/781282
CrossRef - Verbeek M., van Dijk P., van Well P.M.A. Efficiency of eradication of four viruses from garlic (Allium sativum) by meristem-tip culture. Eur J Plant Pathol. 1995;101(3):231-239. doi:10.1007/bf01874779
CrossRef - Kudělková M., Ondrušiková E., Sasková H. Elimination of Garlic common latent virusby meristem culture and chemotherapy. Acta Hortic. 2016;(1113):233-238. doi:10.17660/actahortic.2016.1113.35
CrossRef - Gamborg O.L., Miller R.A., Ojima K. Nutrient requirements of suspension cultures of soybean root cells. Exp Cell Res. 1968;50(1):151-158. doi:10.1016/0014-4827(68)90403-5
CrossRef - Linsmaier E.M., Skoog F. Organic growth factor requirements of tobacco tissue cultures. Physiol Plant. 1965;18(1):100-127. doi:10.1111/j.1399-3054.1965.tb06874.x
CrossRef - Murashige T., Skoog F. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol Plant. 1962;15(3):473-497. doi:10.1111/j.1399-3054.1962.tb08052.x
CrossRef - Luciani G.F., Mary A.K., Pellegrini C., Curvetto N.R. Effects of explants and growth regulators in garlic callus formation and plant regeneration. Plant Cell Tissue Organ Cult. 2006;87(2):139-143. doi:10.1007/s11240-006-9148-5
CrossRef - Mehta J., Sharma A., Sharma N., et al. An improved method for callus culture and in vitro propagation of garlic (Allium sativum L.). Int J App Biosci. 2013;1(1):1-6.
- Bhojwani S.S. In vitro propagation of garlic by shoot proliferation. Sci Hortic (Amsterdam). 1980;13(1):47-52. doi:10.1016/0304-4238(80)90021-7
CrossRef - Bhojwani S.S., Datu P.K. Production of Virus Free Plants by Tissue Culture Plant Tissue Culture. (Datu P.K., ed.). Springer; 2013.
CrossRef - Nagakubo T., Nagasawa A., Ohkawa H. Micropropagation of garlic through in vitro bulblet formation. Plant Cell Tissue Organ Cult. 1993;32(2):175-183. doi:10.1007/bf00029840
CrossRef - Moriconi D., Conci V., Nome S. Rapid multiplication of garlic (Allium sativum L.) in vitro. Phyton. 1990;51:145-151.
- Barandiaran X., Martín N., Alba C., Rodríguez-Conde M.F., Di Pietro A., Martín J. An efficient method for the in vitro management of multiple garlic accessions. In Vitro Cell Dev Biol Plant. 1999;35(6):466-469. doi:10.1007/s11627-999-0069-y
CrossRef - Seabrook J.E.A. In vitro propagation and bulb formation of garlic. Can J Plant Sci. 1994;74(1):155-158. doi:10.4141/cjps94-033
CrossRef - Barandiaran X., Martín N., Rodríguez-Conde M.F., Di Pietro A., Martín J. Genetic variability in callus formation and regeneration of garlic ( Allium sativum L.). Plant Cell Rep. 1999;18(5):434-437. doi:10.1007/s002990050599
CrossRef - van den Dries N., Giannì S., Czerednik A., Krens F.A., de Klerk G.J.M. Flooding of the apoplast is a key factor in the development of hyperhydricity. J Exp Bot. 2013;64(16):5221-5230. doi:10.1093/jxb/ert315
CrossRef - Debergh P., Harbaoui Y., Lemeur R. Mass propagation of globe artichoke (Cynara scolymus): Evaluation of different hypotheses to overcome vitrification with special reference to water potential. Physiol Plant. 1981;53(2):181-187. doi:10.1111/j.1399-3054.1981.tb04130.x
CrossRef - Debergh PC. Effects of agar brand and concentration on the tissue culture medium. Physiol Plant. 1983;59(2):270-276. doi:10.1111/j.1399-3054.1983.tb00770.x
CrossRef - Ivanova M., van Staden J. Effect of ammonium ions and cytokinins on hyperhydricity and multiplication rate of in vitro regenerated shoots of Aloe polyphylla. Plant Cell Tissue Organ Cult. 2008;92(2):227-231. doi:10.1007/s11240-007-9311-7
CrossRef - Kadota M., Niimi Y. Effects of cytokinin types and their concentrations on shoot proliferation and hyperhydricity in in vitro pear cultivar shoots. Plant Cell, Tissue and Organ Culture. 2003;72(3):261-265.
CrossRef - Sreedhar R.V., Venkatachalam L., Neelwarne B. Hyperhydricity-related morphologic and biochemical changes in vanilla (vanilla planifolia). J Plant Growth Regul. 2009;28(1):46-57. doi:10.1007/s00344-008-9073-4
CrossRef - Hammerschlag F.A. Temperate fruits and nuts. In: Tissue Culture as a Plant Production System for Horticultural Crops. In: Zimmerman R.H., Griesbach R.J., Hammerschlag F.A., Lawson R.H., eds. Martinus Nijhoff, Dorbrecht. ; 1986:221-236.
CrossRef - George E.F., Hall M.A., Klerk G.J.D. The Anatomy and Morphology of Tissue Cultured Plants. Plant Propagation by Tissue Culture. Published online 2008:465-477.
CrossRef - Evaldsson I.E., Welander N.T. The effects of medium composition on in vitro propagation and in vivo growth of Cordyline terminaliscv Atoom. J Hortic Sci. 1985; 60(4):525-530. doi:10.1080/14620316.1985.11515660
CrossRef - Declerck V., Korban S.S. Shoot regeneration from leaf tißues of phlox paniculata L. J Plant Physiol. 1995;147(3-4):441-446. doi:10.1016/s0176-1617(11)82180-2
CrossRef - Shibli R.A., Ajlouni M.M., Jaradat A., Aljanabi S., Shatnawi M. Micropropagation in wild pear (Pyrus syricca). Sci Hortic (Amsterdam). 1997;68(1-4):237-242. doi:10.1016/s0304-4238(96)00972-7
CrossRef - Devi A., Khar A., Lawande A. Genotypic response of short day garlic (Allium sativum L.) accessions to shoot multiplication. Journal of Spices and Aromatic Crops. 2007;16:15-21.
- Metwally E., El-Denary I., Dewi Y., Naidoo Y. In vitro propagation of garlic (Allium sativum L.) through adventitious shoot organogenesis. African Journal of Biotechnology. 2014;13:3892-3900.
CrossRef - Haque M., Wada T., Hattori K. Shoot regeneration and bulblet formation from shoot and root meristem of garlic cv. Bangladesh local. Asian Journal of Plant Sciences. 2003;2(1):23-27.
CrossRef

